Molecular Dynamics Investigation of the Effect of Shear during Extrusion on the Permeation Behavior of CH4 in High-Density Polyethylene
Abstract
:1. Introduction
2. Numerical Theory
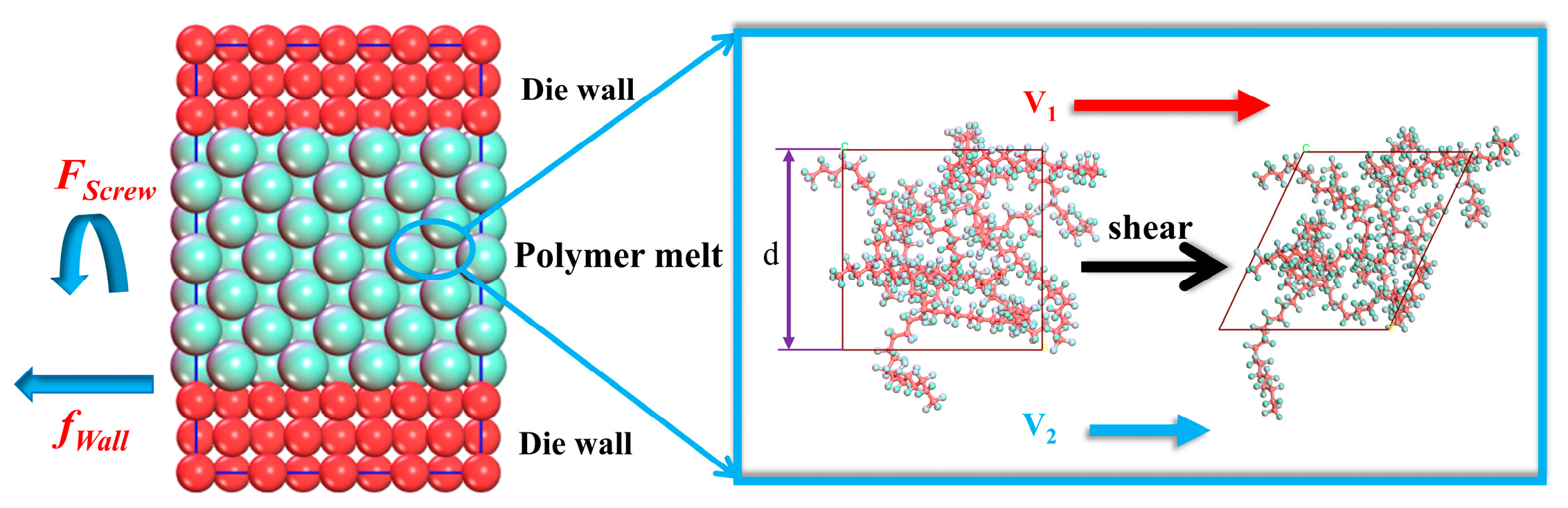
2.1. Shear Action
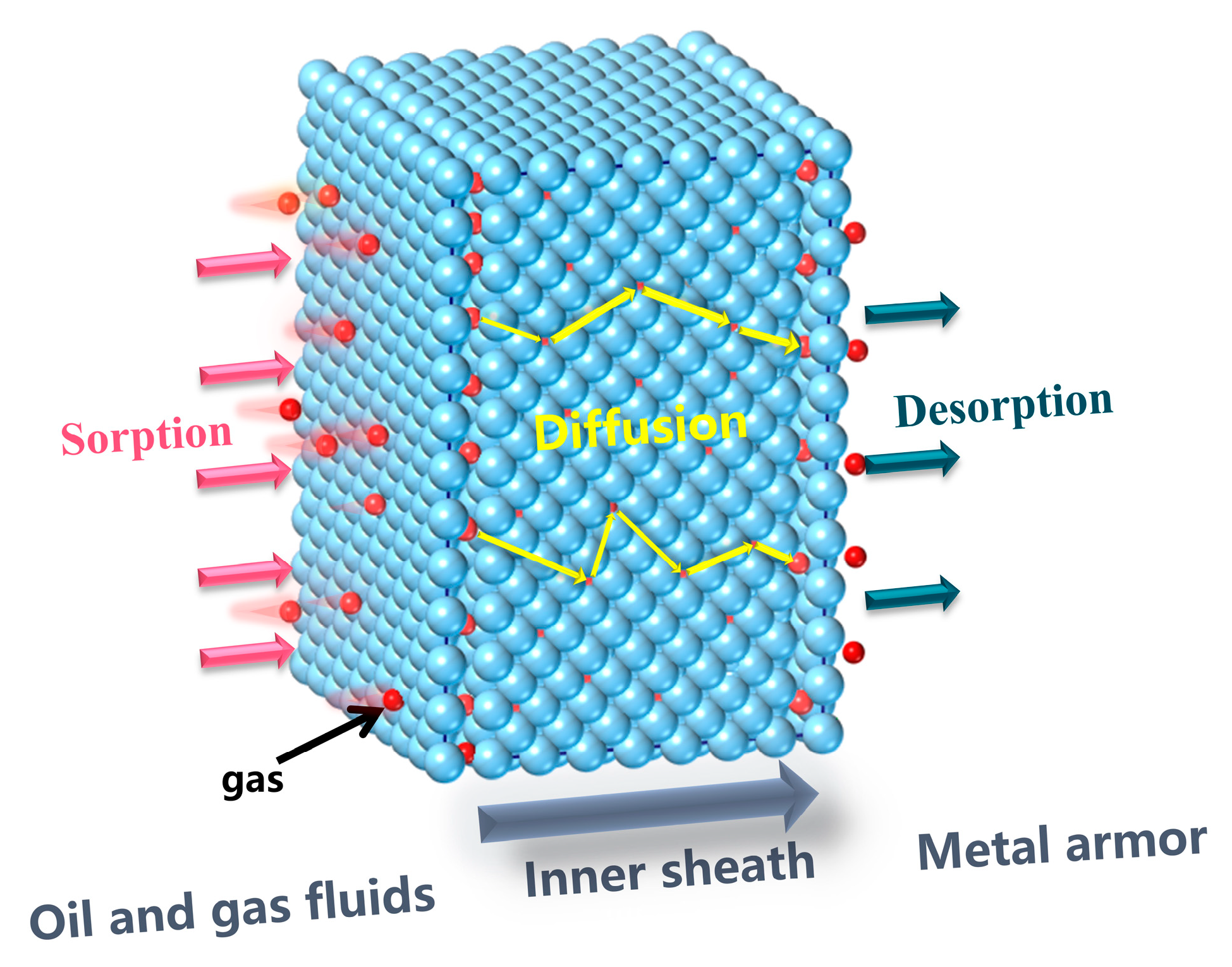
2.2. Gas Permeation
3. Model and Simulation Methodology
3.1. Model Building and Optimization
3.2. Simulation of HDPE Shear Process
3.3. Simulation of Gas Sorption in HDPE
3.4. Simulation of Gas Diffusion in HDPE
4. Results and Discussion
4.1. The Influence Law of Shear Rate on Material Micro-Morphology
4.1.1. Results of Shear Action
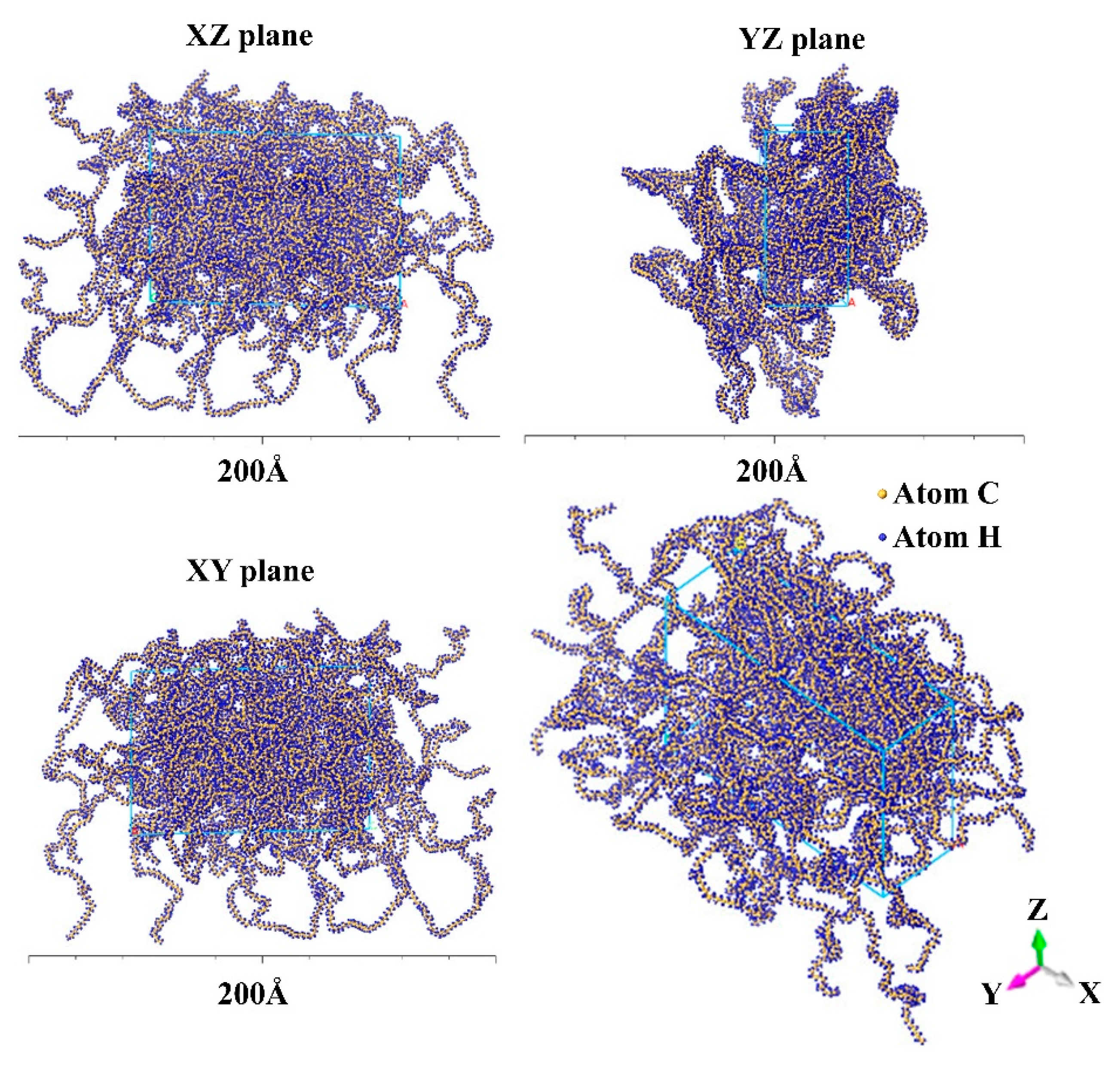
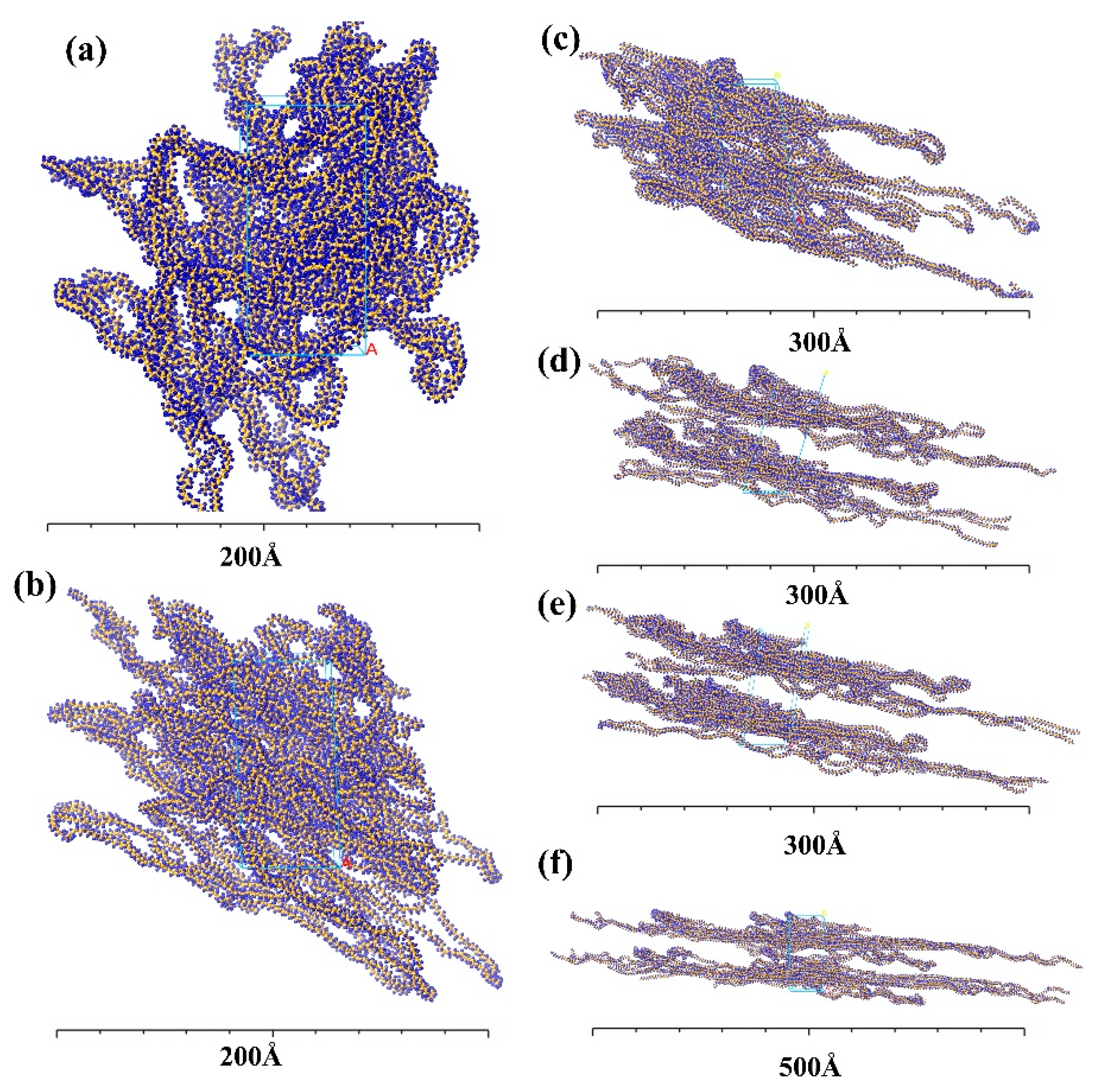
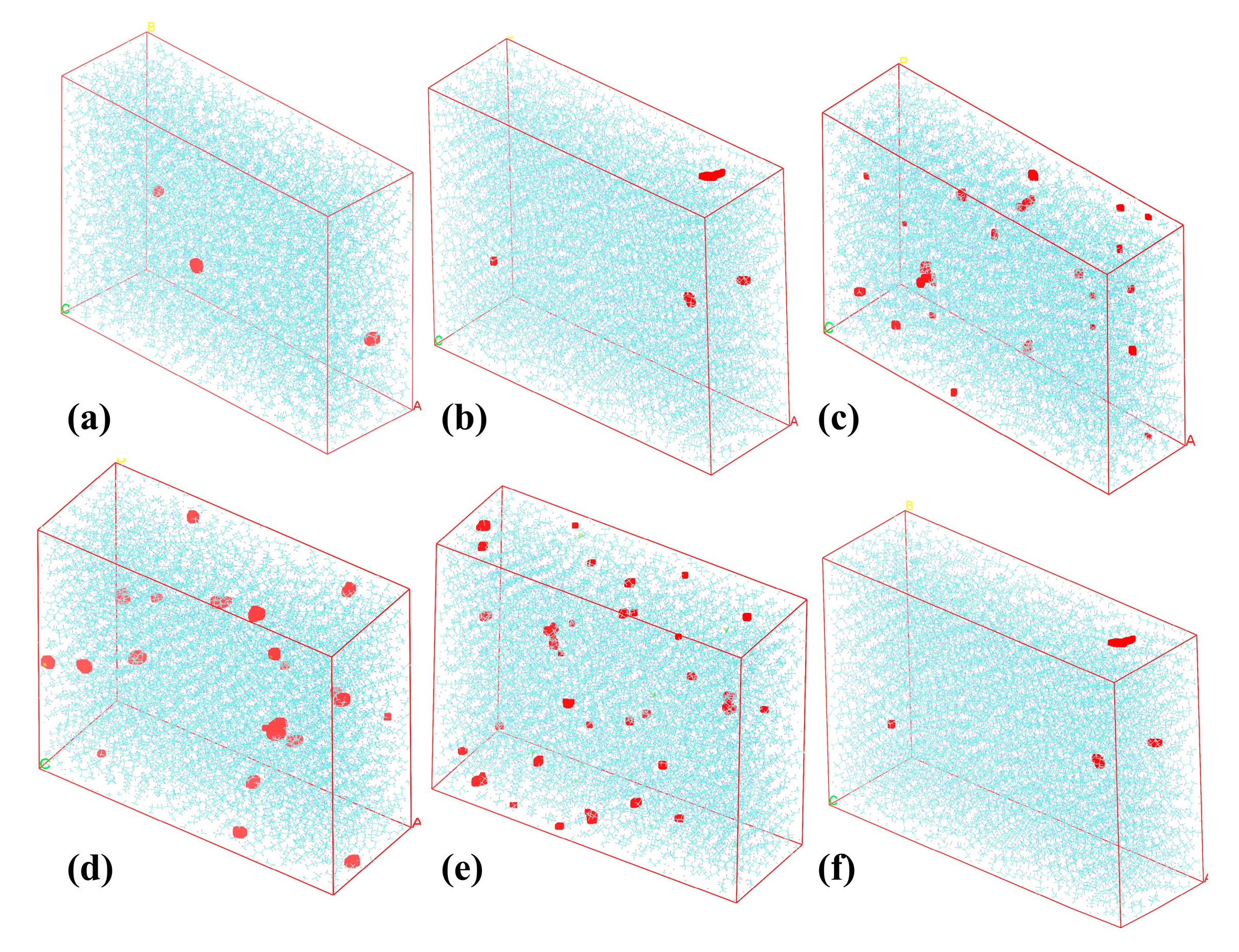
4.1.2. The Evolution of HDPE Micro-Morphology
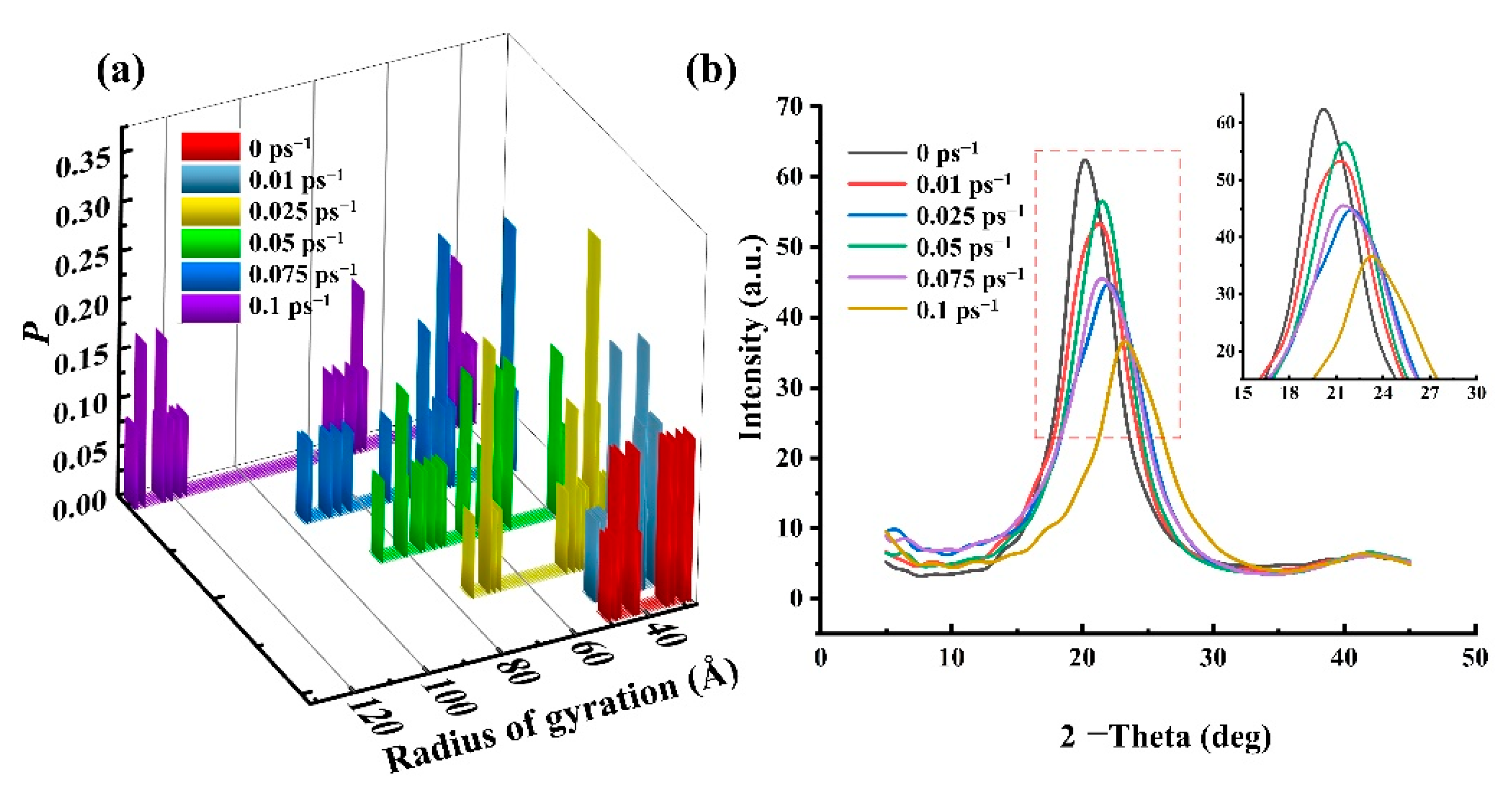
4.1.3. The Radius of Gyration of HDPE
4.1.4. D-Spacing of HDPE
4.2. Adsorption of CH4 in HDPE under Different Shear Effects
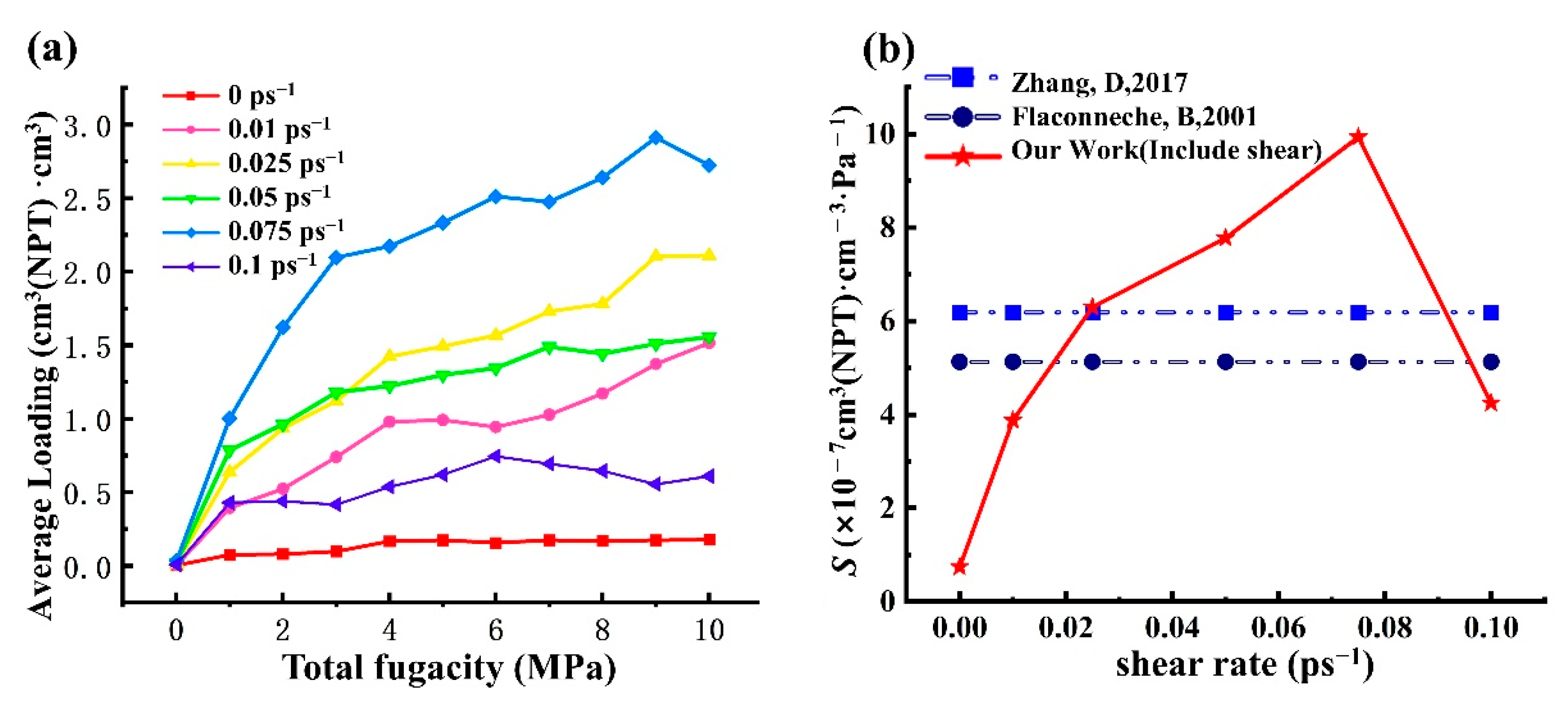
4.2.1. Gas Adsorption Isotherm and the Solubility Coefficient S
4.2.2. The Isosteric Heat and Langmuir-Type Sites
4.3. Diffusion of CH4 in HDPE under Different Shear Effects
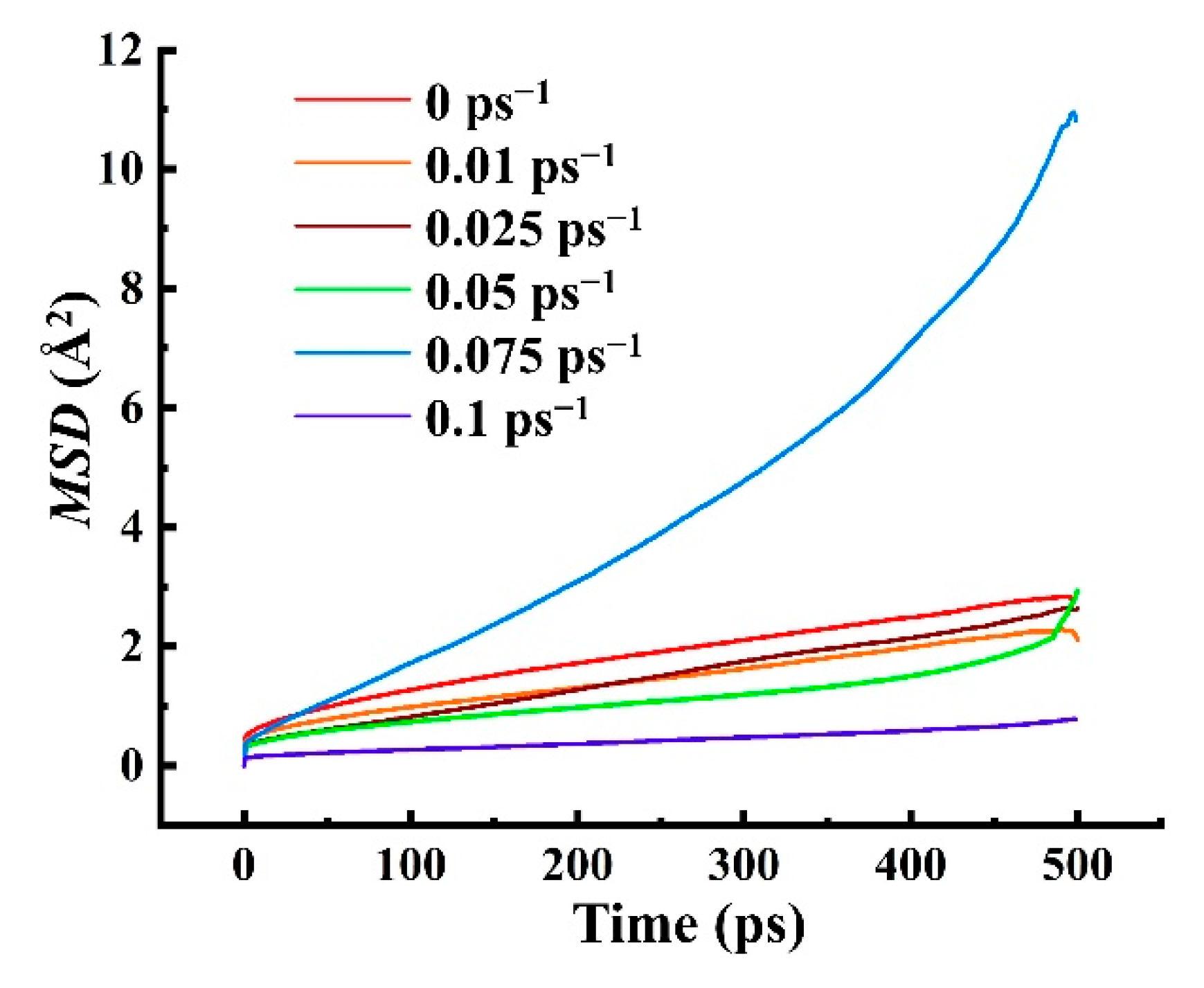
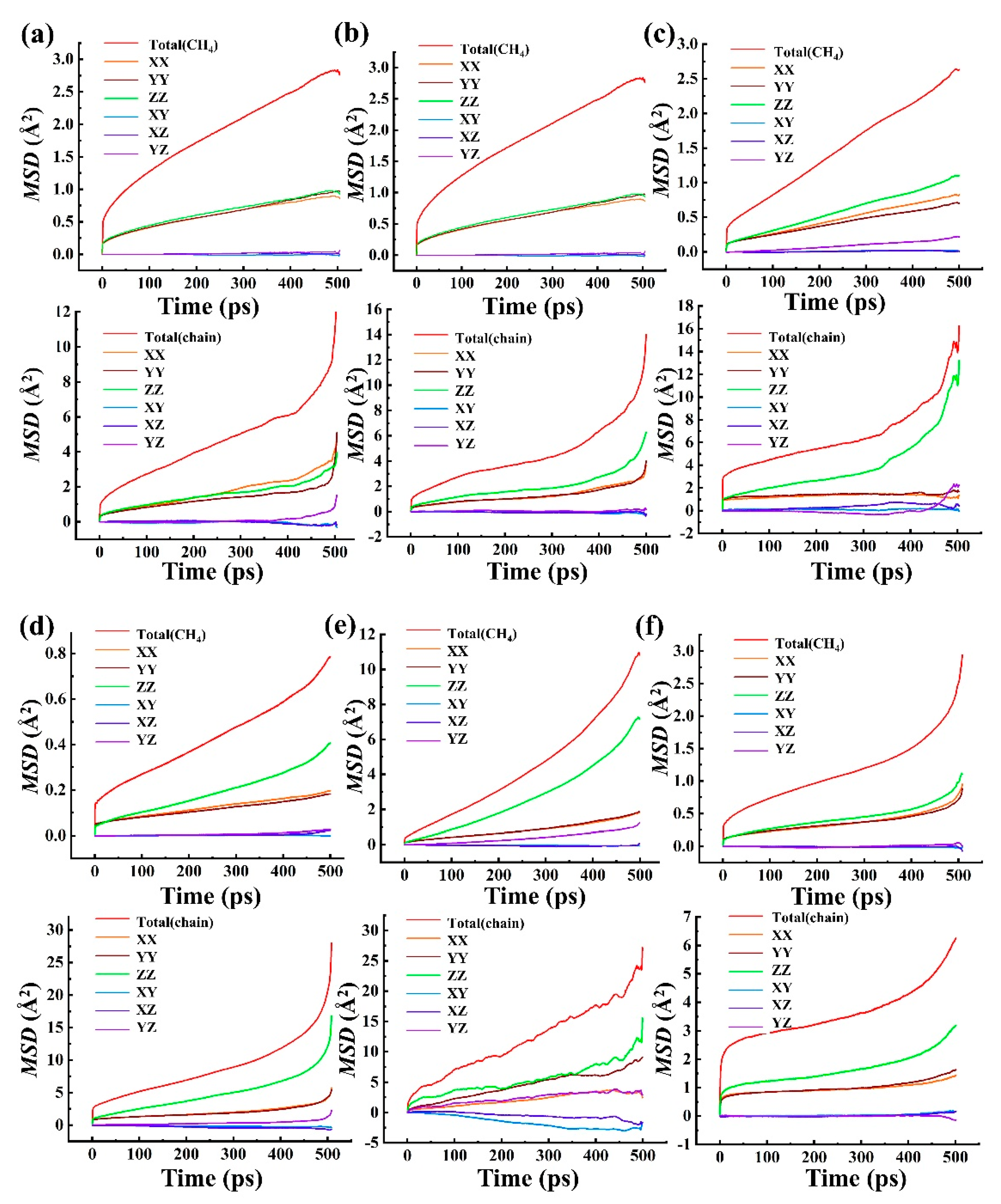
4.3.1. The Mean Square Displacement of Gas Diffusion and the Diffusion Coefficient D
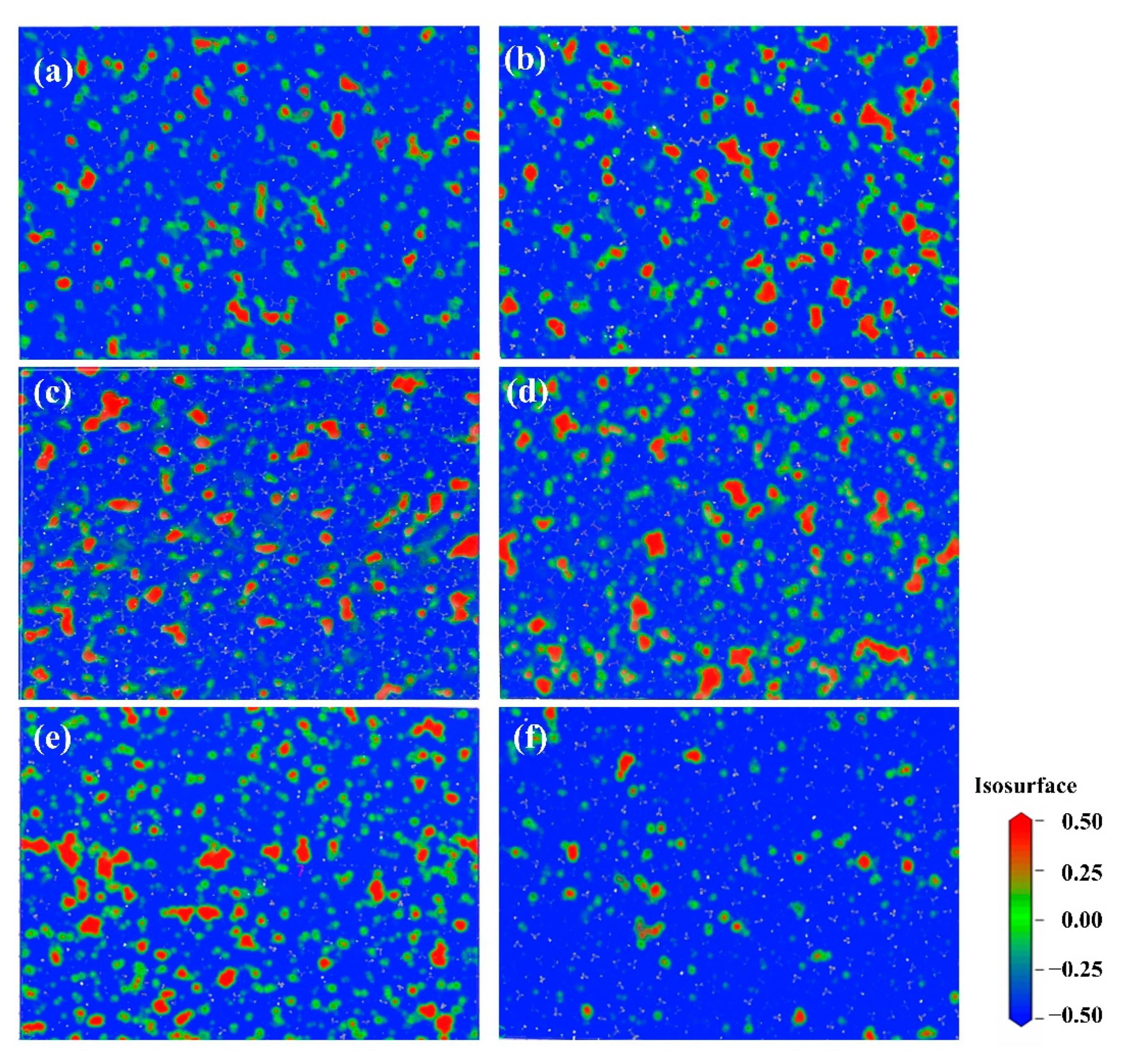
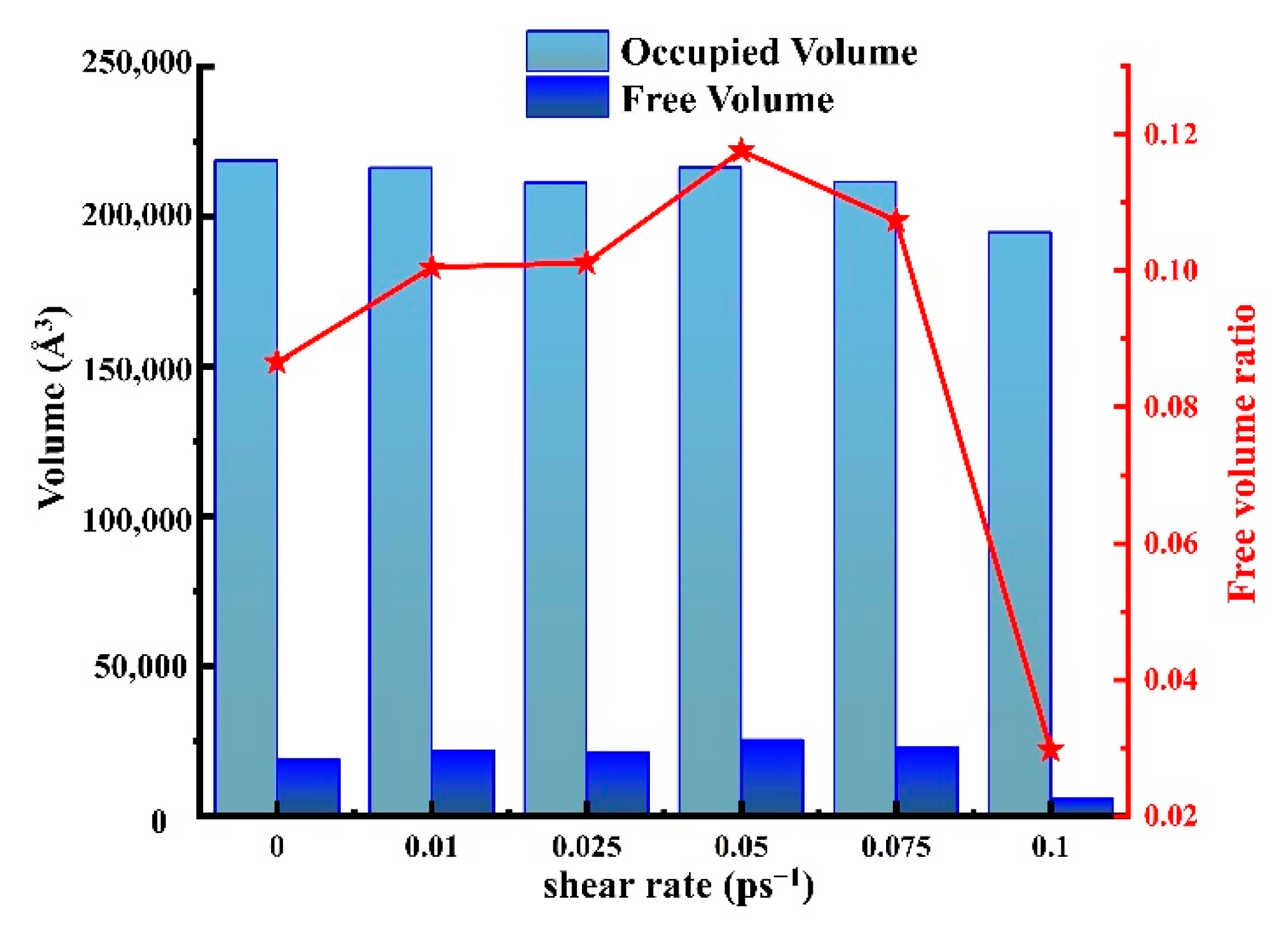
4.3.2. The Free Volume of HDPE and Motion of Polymer Chain Segments
4.4. Permeability Coefficients
5. Conclusions
- (1)
- The microstructural properties of HDPE exhibit differences across different shear rates. At lower shear rates, the chains exhibit an amorphous state. Still, when the shear rate increases, a gradual “opening-up” of structure occurs, in which the molecular chains remain entangled, with significant flexibility and consistent density. At a shear rate of 0.1 ps−1, the polymer chains untwist and form a densified flow-oriented structure with increased rigidity, density, and decreased mobility;
- (2)
- To investigate the effect of shear rate on the solubility coefficients, the sorption characteristics of CH4 in HDPE under different shear rates were conducted. The sorption of CH4 under various shear rates is still a physical sorption process. The solubility coefficients exhibit a pattern of initially increasing and subsequently decreasing with increasing shear rate. Compared with the isosteric heat, the phenomenon can be more effectively explained by considering the Langmuir-type sites, which are influenced by the microstructure of the material;
- (3)
- The diffusion coefficient of CH4 in HDPE was investigated under various shear rates. The solubility coefficients exhibit a pattern of initially increasing and subsequently decreasing with increasing shear rate as above. This analysis investigates the impact of cutting rate on the spread rate from the perspectives of free volume and chain movement. It is found that the free volume theory does not significantly influence the diffusion coefficient. However, the diffusion movement of gas molecules corresponds to the tendency of chain segments, indicating that the theory of chain movement provides a more comprehensive explanation for the process of gas dynamic diffusion compared to the free volume theory.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, M.; Zhang, Z.; Wu, B.; Chen, C.; Gu, X. Application and Technical Challenge of Flexible Pipe in Deep-sea Mining. Ocean Eng. Equip. Technol. 2019, 6, 765–770. [Google Scholar]
- Chen, H.; Yang, L. The Strategic Selection for the Exploitation and Utilization of Oil and Gas Resources under the Background of “Double Carbon” Goal and Energy Security in China; Wipro, C., Ed.; Chongqing Wipro: Chongqing, China, 2022; Volume 14, pp. 56–69. [Google Scholar]
- Matheus, F.K.; Frank, P.M.; Marcelo, F.B.; Thomas, G.R.C. RFID wireless system for detection of water in the annulus of a flexible pipe. Mar. Struct. 2020, 72, 102711–102776. [Google Scholar] [CrossRef]
- Arpin, B.; Lafleur, P.G.; Sanschagrin, B. A personal computer software program for coathanger die simulation. Polym. Eng. Sci. 1994, 34, 657–664. [Google Scholar] [CrossRef]
- Craster, B.; Jones, T.G.J. Permeation of a Range of Species through Polymer Layers under Varying Conditions of Temperature and Pressure: In Situ Measurement Methods. Polymers 2019, 11, 1056. [Google Scholar] [CrossRef]
- de Lima, M.S.C.F.; da Silva, A.H.M.d.F.T.; da Costa Neto, C.A.; Nascimento, C.R.; de Andrade, M.C.; Bertolino, L.C.; da Silva, A.L.N. Crystal morphology and non-isothermal crystallization kinetics of polyvinylidene fluoride used as a pressure barrier in flexible pipes: Nanoclay effect. Iran. Polym. J. 2022, 31, 677–690. [Google Scholar] [CrossRef]
- Nguyen-Tuong, V. Gas permeation through a high density polyethylene microwave window. J. Vac. Sci. Technol. A Vac. Surf. Film. 1993, 11, 1584–1586. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; Bozorgi, B. The effect of melt flow index, melt flow rate, and particle size on the thermal degradation of commercial high density polyethylene powder. J. Therm. Anal. Calorim. 2013, 114, 1333–1339. [Google Scholar] [CrossRef]
- Ansari, M.; Inn, Y.W.; Sukhadia, A.M.; DesLauriers, P.J.; Hatzikiriakos, S.G. Wall slip of HDPEs: Molecular weight and molecular weight distribution effects. J. Rheol. 2013, 57, 927–948. [Google Scholar] [CrossRef]
- Rabelo, M.A.; Pesce, C.P.; Santos, C.C.P.; Junior, R.R.; Franzini, G.R.; Neto, A.G. An investigation on flexible pipes birdcaging triggering. Mar. Struct. 2015, 40, 159–182. [Google Scholar] [CrossRef]
- Bernal-Lara, T.E.; Liu, R.Y.F.; Hiltner, A.; Baer, E. Structure and thermal stability of polyethylene nanolayers. Polymer 2005, 46, 3043–3055. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Alghizzi, A.G.; Suliman, M.H.; Achilias, D.S. Effect of Natural Macromolecule Filler on the Properties of High-Density Polyethylene (HDPE). In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Wang, K.; Yu, X.; Zheng, L.; Zhang, E.; He, Y. Numerical investigation of gas permeation and condensation behavior of flexible risers. J. Pet. Sci. Eng. 2021, 203, 108622. [Google Scholar] [CrossRef]
- Campos, T.; Gomes, J.A.; Seiersten, M.; Palencsar, S.; Dugstad, A. Corrosion of armor wire steel in the annulus of flexible pipes at near neutral pH. In Proceedings of the CORROSION 2019, Nashville, TN, USA, 24–28 March 2019. [Google Scholar]
- Adewole, J.K.; Jensen, L.; Al-Mubaiyedh, U.A.; von Solms, N.; Hussein, I.A. Transport properties of natural gas through polyethylene nanocomposites at high temperature and pressure. J. Polym. Res. 2012, 19, 9814. [Google Scholar] [CrossRef]
- Datta, R.; Yelash, L.; Schmid, F.; Kummer, F.; Oberlack, M.; Lukacova-Medvid’ova, M.; Virnau, P. Shear-Thinning in Oligomer Melts-Molecular Origins and Applications. Polymers 2021, 13, 2806. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Wu, J.-S.; Chang, R.-Y. Shear thinning and shear dilatancy of liquid n -hexadecane via equilibrium and nonequilibrium molecular dynamics simulations: Temperature, pressure, and density effects. J. Chem. Phys. 2008, 129, 014502. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.C.; Wu, J.S.; Chang, R.Y. Nanocontraction flows of short-chain polyethylene via molecular dynamics simulations. Mol. Simul. 2009, 35, 691–704. [Google Scholar] [CrossRef]
- Huang, L.-H.; Wu, C.-H.; Hua, C.-C.; Huang, T.-J. Multiscale simulations of coupled composition-stress-morphology of binary polymer blend. Polymer 2020, 193, 122366. [Google Scholar] [CrossRef]
- Baig, C.; Harmandaris, V.A. Quantitative Analysis on the Validity of a Coarse-Grained Model for Nonequilibrium Polymeric Liquids under Flow. Macromolecules 2010, 43, 3156–3160. [Google Scholar] [CrossRef]
- McGonigle, E.A.; Liggat, J.J.; Pethrick, R.A.; Jenkins, S.D.; Daly, J.H.; Hayward, D. Permeability of N2, Ar, He, O2, and CO2 through as-extruded amorphous and biaxially oriented polyester films: Dependence on chain mobility. J. Polym. Sci. B Polym. Phys. 2004, 42, 2916–2929. [Google Scholar] [CrossRef]
- Mozaffari, F.; Eslami, H.; Moghadasi, J. Molecular dynamics simulation of diffusion and permeation of gases in polystyrene. Polymer 2010, 51, 300–307. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S.; Mustafa, A. Asymmetric mixed matrix membrane incorporating organically modified clay particle for gas separation. Chem. Eng. J. 2014, 241, 495–503. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chu, H.; Qi, G.; Ding, H.; Gao, X.; Meng, J. Molecular Simulation on Permeation Behavior of CH4/CO2/H2S Mixture Gas in PVDF at Service Conditions. Polymers 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.J.; Xiao, S.; Ruan, Q.D.; Wu, H.; Chen, G.H.; Zhou, C.L.; Jiang, S.H.; Xi, K.; He, M.H.; Chu, P.K. Hydrogen permeation behavior and mechanism of multi-layered graphene coatings and mitigation of hydrogen embrittlement of pipe steel. Appl. Surf. Sci. 2022, 573, 151529. [Google Scholar] [CrossRef]
- Lu, T.; Xu, Y.P.; Pan, X.D.; Zhou, H.S.; Ding, F.; Yang, Z.S.; Niu, G.J.; Luo, G.N.; Li, X.C.; Gao, F. Atomistic study of hydrogen behavior around dislocations in alpha iron. J. Nucl. Mater. 2018, 510, 219–228. [Google Scholar] [CrossRef]
- Hu, S.; Yang, W.; Liang, S.-P.; Yang, B.; Yang, M.-B. Simulation of Gas-Assisted Injection Molding of High-Density Polyethylene: The Role of Rheological Properties and Physical Fields on the Crystalline Morphology. J. Macromol. Sci. Phys. 2009, 48, 1201–1211. [Google Scholar] [CrossRef]
- Wu, W.; Duan, F.; Zhao, B.; Qiang, Y.; Zhou, M.; Jiang, B. Molecular Insights into the Wall Slip Behavior of Pseudoplastic Polymer Melt in Nanochannels during Micro Injection Molding. Polymers 2022, 14, 3218. [Google Scholar] [CrossRef]
- Berro, H.; Fillot, N.; Vergne, P.; Tokumasu, T.; Ohara, T.; Kikugawa, G. Energy dissipation in non-isothermal molecular dynamics simulations of confined liquids under shear. J. Chem. Phys. 2011, 135, 134708. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chatterji, A.; Sutmann, G.; Gompper, G.; Winkler, R.G. Cell-level canonical sampling by velocity scaling for multiparticle collision dynamics simulations. J. Comput. Phys. 2010, 229, 168–177. [Google Scholar] [CrossRef]
- Khare, R.; de Pablo, J.; Yethiraj, A. Molecular simulation and continuum mechanics study of simple fluids in non-isothermal planar couette flows. J. Chem. Phys. 1997, 107, 2589–2596. [Google Scholar] [CrossRef]
- Yu, W.J.; Shen, G.C.; Zhang, Y.; Li, D.Q.; Zhou, H.M. Molecular configuration evolution model and simulation for polymer melts using a non-equilibrium irreversible thermodynamics method. Appl. Math. Model. 2021, 89, 1357–1372. [Google Scholar] [CrossRef]
- Bondar, V.I.; Kamiya, Y.; Yampol’skii, Y.P. On pressure dependence of the parameters of the dual-mode sorption model. J. Polym. Sci. B Polym. Phys. 1996, 34, 369–378. [Google Scholar] [CrossRef]
- Feng, H. Modeling of vapor sorption in glassy polymers using a new dual mode sorption model based on multilayer sorption theory. Polymer 2007, 48, 2988–3002. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Müller-Plathe, F. Permeation of polymers—A computational approach. Acta Polym. 1994, 45, 259–293. [Google Scholar] [CrossRef]
- Ninarello, A.; Berthier, L.; Coslovich, D. Models and Algorithms for the Next Generation of Glass Transition Studies. Phys. Rev. X 2017, 7, 021039. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, MI, USA, 2003; pp. 137–139. [Google Scholar]
- Yuan, C.; Fu, S.; Yang, K.; Hou, B.; Liu, Y.; Jiang, J.; Cui, Y. Crystalline C—C and C═C Bond-Linked Chiral Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 369–381. [Google Scholar] [CrossRef]
- Daldrop, J.O.; Kappler, J.; Brunig, F.N.; Netz, R.R. Butane dihedral angle dynamics in water is dominated by internal friction. Proc. Natl. Acad. Sci. USA 2018, 115, 5169–5174. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.Y.; Qin, J.Q.; Gu, Y. Molecular dynamics simulation on glass transition temperature of isomeric polyimide. Express Polym. Lett. 2009, 3, 665–675. [Google Scholar] [CrossRef]
- Boldt, R.; Leuteritz, A.; Schob, D.; Ziegenhorn, M.; Wagenknecht, U. Barrier Properties of GnP-PA-Extruded Films. Polymer 2020, 12, 669. [Google Scholar] [CrossRef]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Permeability, Diffusion and Solubility of Gases in Polyethylene, Polyamide 11 and Poly (Vinylidene Fluoride). Oil Gas Sci. Technol. 2001, 56, 261–278. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Qi, D.; Ding, N.; Shao, X.; Wei, B.; Cai, X. Gas permeation behaviors of high-density polyethylene as a liner material of flexible pipes. Nat. Gas Ind. 2017, 37, 104–110. [Google Scholar] [CrossRef]
- Yeganegi, S.; Gholampour, F. Simulation of methane adsorption and diffusion in a carbon nanotube channel. Chem. Eng. Sci. 2016, 140, 62–70. [Google Scholar] [CrossRef]
- Xiang, J.; Zeng, F.; Liang, H.; Li, B.; Song, X. Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci. China Earth Sci. 2014, 57, 1749–1759. [Google Scholar] [CrossRef]
- McGonigle, E.A.; Liggat, J.J.; Pethrick, R.A.; Jenkins, S.D.; Daly, J.H.; Hayward, D. Permeability of N2, Ar, He, O2 and CO2 through biaxially oriented polyester films—Dependence on free volume. Polymer 2001, 42, 2413–2426. [Google Scholar] [CrossRef]
- Siebel, D.; Scharfer, P.; Schabel, W. Prediction of diffusion in a ternary solvent-solvent-polymer blend by means of binary diffusion data: Comparison of experimental data and simulative results. J. Appl. Polym. Sci. 2016, 133, 43899. [Google Scholar] [CrossRef]
- Sharma, J.; Tewari, K.; Arya, R.K. Diffusion in polymeric systems–A review on free volume theory. Prog. Org. Coat. 2017, 111, 83–92. [Google Scholar] [CrossRef]
- Fried, J.R.; Sadat-Akhavi, M.; Mark, J.E. Molecular simulation of gas permeability: Poly(2,6-dimethyl-1,4-phenylene oxide). J. Membr. Sci. 1998, 149, 115–126. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer-solvent systems. II. A predictive theory for dependence of diffusion-coefficients on temperature, concentration, and molecular-weight. J. Polym. Sci. Part B-Polym. Phys. 1977, 15, 417–439. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer—Solvent systems. I. Re-examination of free-volume theory. J. Polym. Sci. Part B-Polym. Phys. 1977, 15, 403–416. [Google Scholar] [CrossRef]
- Offord, G.T.; Armstrong, S.R.; Freeman, B.D.; Baer, E.; Hiltner, A.; Paul, D.R. Influence of processing strategies on porosity and permeability of β nucleated isotactic polypropylene stretched films. Polymer 2013, 54, 2796–2807. [Google Scholar] [CrossRef]
- Yu, Y.-W.; Liu, T.-J.; Hsu, C.-L.; Yang, Y.-S. A hybrid 3D/2D finite element technique for polymer processing operations. Polym. Eng. Sci. 1999, 39, 44–54. [Google Scholar] [CrossRef]
- Lang, U.; Michaeli, W. Development of a Mathematical Model for the Calculation of the Pressure Drop in Extrusion Dies. J. Reinf. Plast. Compos. 1998, 17, 1110–1118. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, T.; Guo, X.; Wu, Y.; Yan, J.; Lu, Q. Molecular Dynamics Investigation of the Effect of Shear during Extrusion on the Permeation Behavior of CH4 in High-Density Polyethylene. J. Mar. Sci. Eng. 2023, 11, 1876. https://doi.org/10.3390/jmse11101876
Li H, Yang T, Guo X, Wu Y, Yan J, Lu Q. Molecular Dynamics Investigation of the Effect of Shear during Extrusion on the Permeation Behavior of CH4 in High-Density Polyethylene. Journal of Marine Science and Engineering. 2023; 11(10):1876. https://doi.org/10.3390/jmse11101876
Chicago/Turabian StyleLi, Hongxia, Tianyang Yang, Xuewen Guo, Yupeng Wu, Jun Yan, and Qingzhen Lu. 2023. "Molecular Dynamics Investigation of the Effect of Shear during Extrusion on the Permeation Behavior of CH4 in High-Density Polyethylene" Journal of Marine Science and Engineering 11, no. 10: 1876. https://doi.org/10.3390/jmse11101876





