The Biotoxic Effects of Ag Nanoparticles (AgNPs) on Skeletonema costatum, a Typical Bloom Alga Species in Coastal Areas
Abstract
:1. Introduction
2. Methods and Materials
2.1. Characteristics of AgNPs
2.2. Determination of Cytotoxic Effects
2.2.1. The Relative Growth Rates
2.2.2. Ag Accumulation in the Algal Cell
2.2.3. The Damage to the Cell Membrane
2.2.4. Determination of the Chl-a
2.3. Determination of Antioxidant Stress
2.3.1. Preparation of Protease Extract
2.3.2. Determination of Protein Content, SOD, CAT, and MDA
2.4. Statistical Analyses
3. Results and Discussion
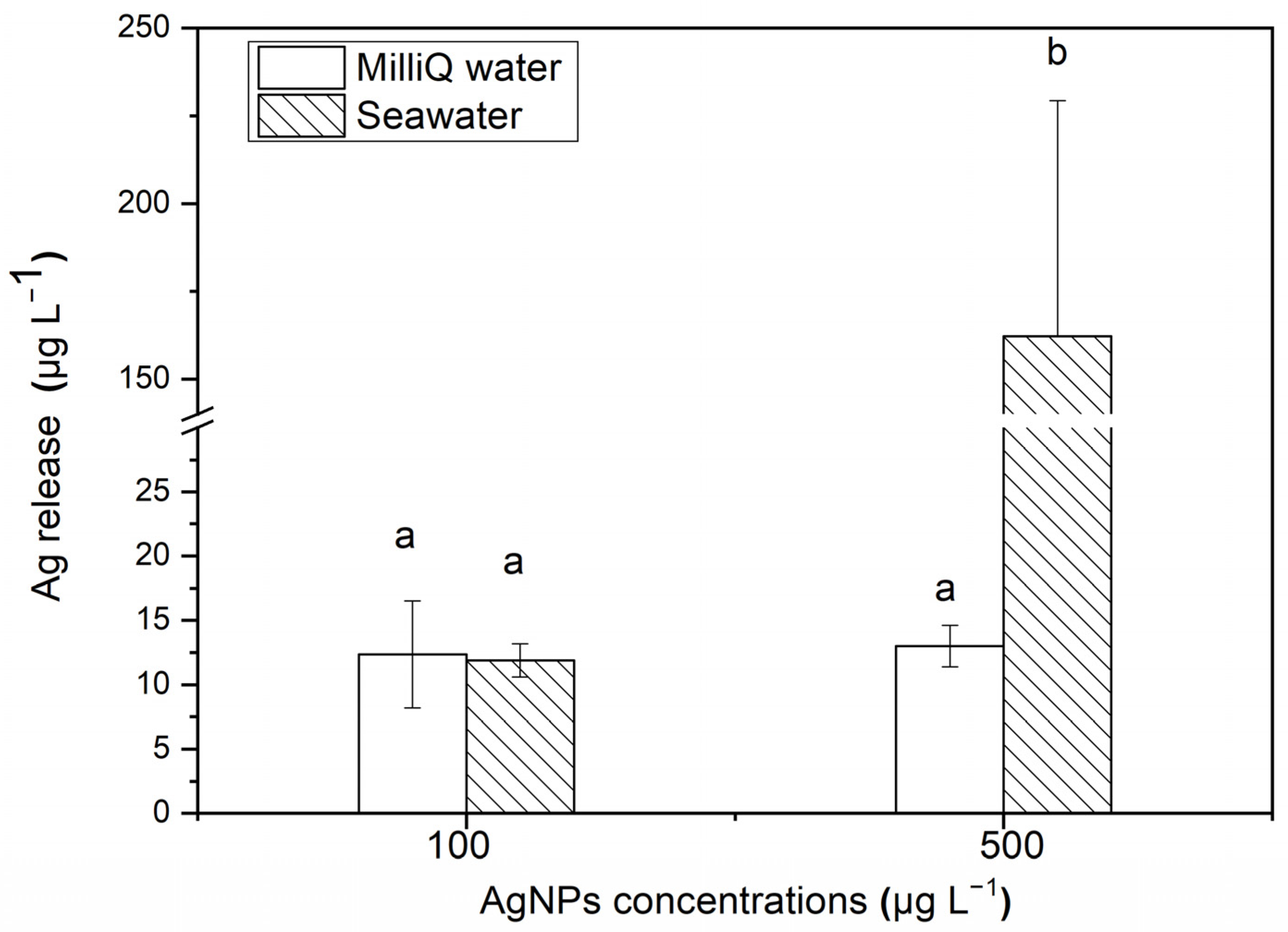
3.1. Characteristics of AgNPs
3.2. Biological Effects of AgNPs on S. costatum
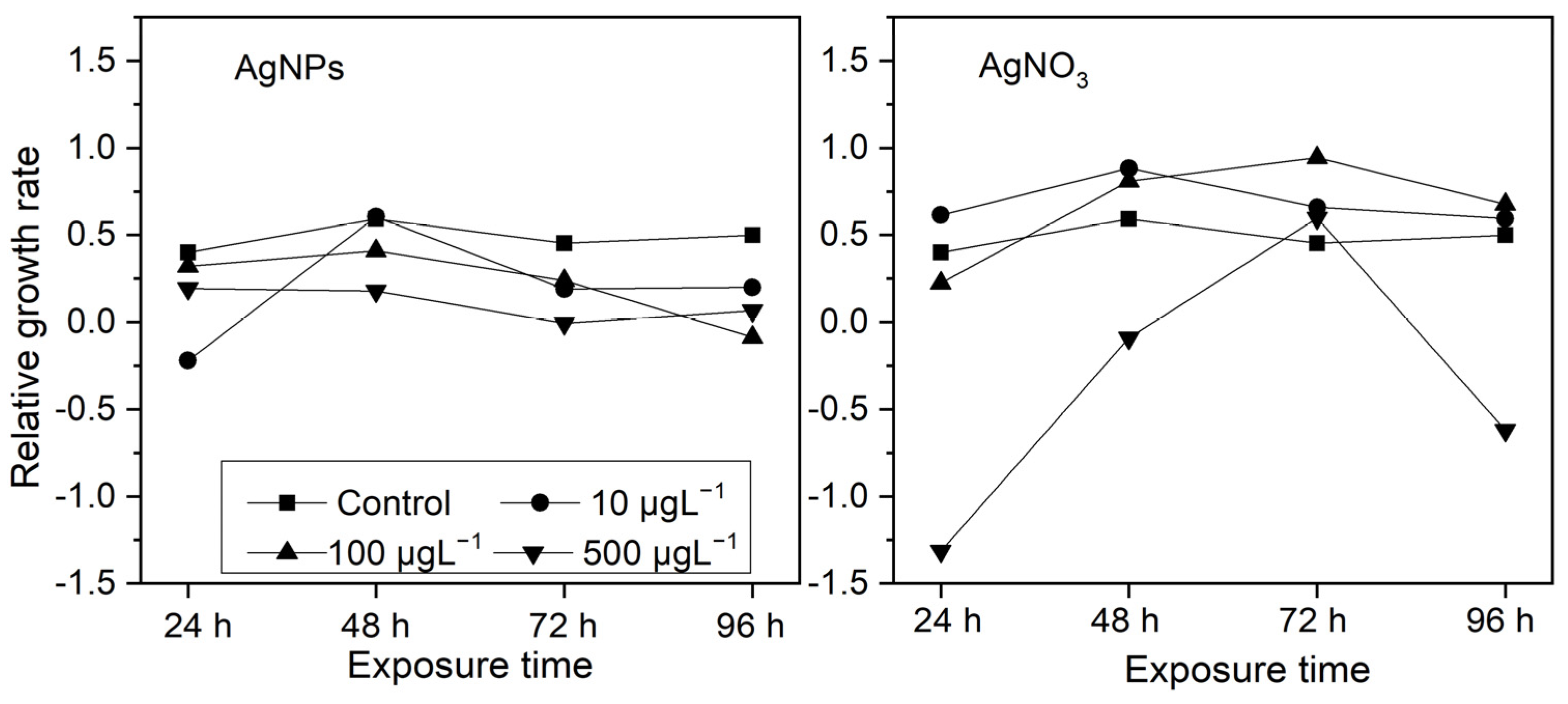
3.2.1. The Relative Growth Rate
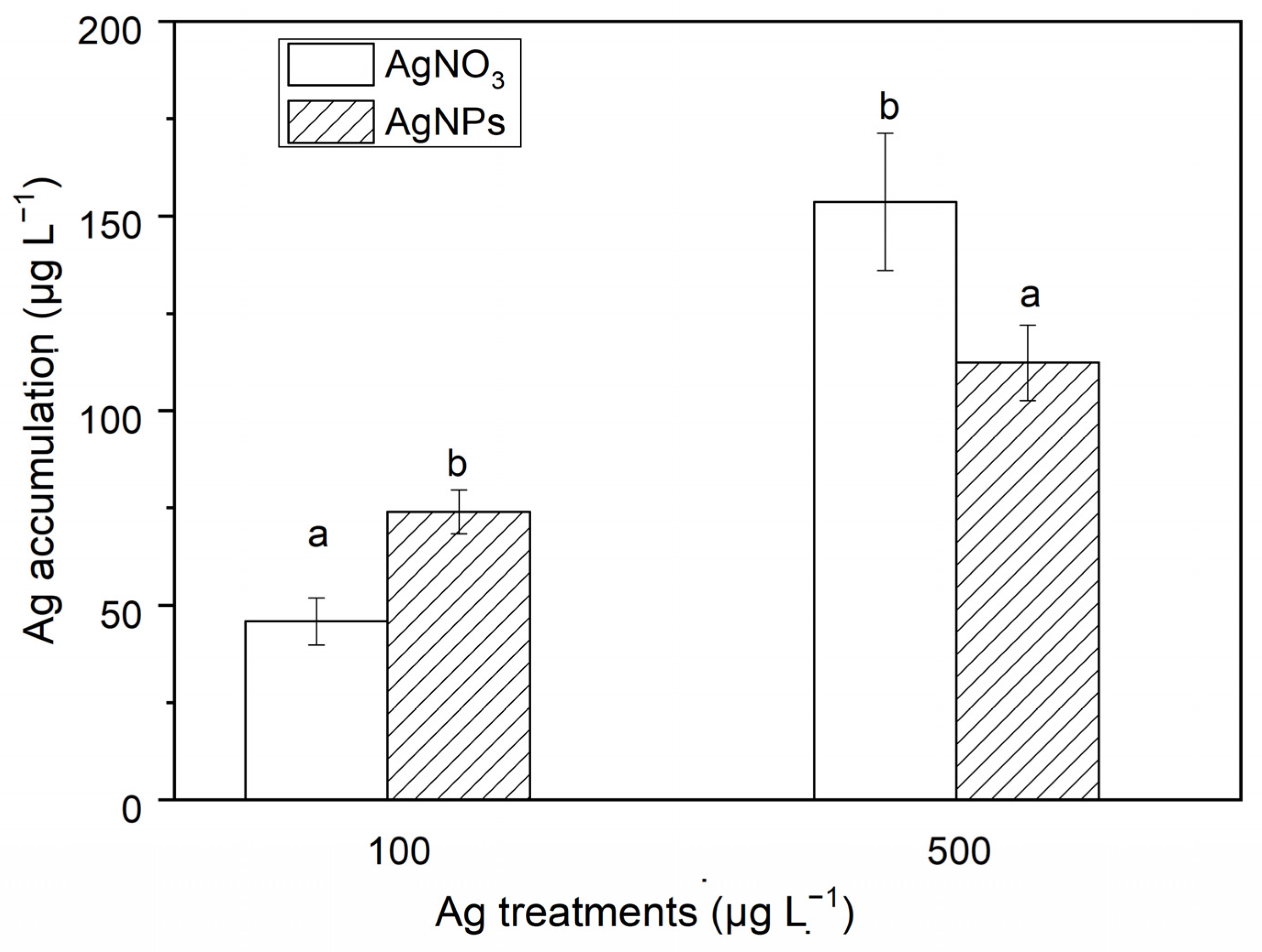
3.2.2. Ag Accumulation
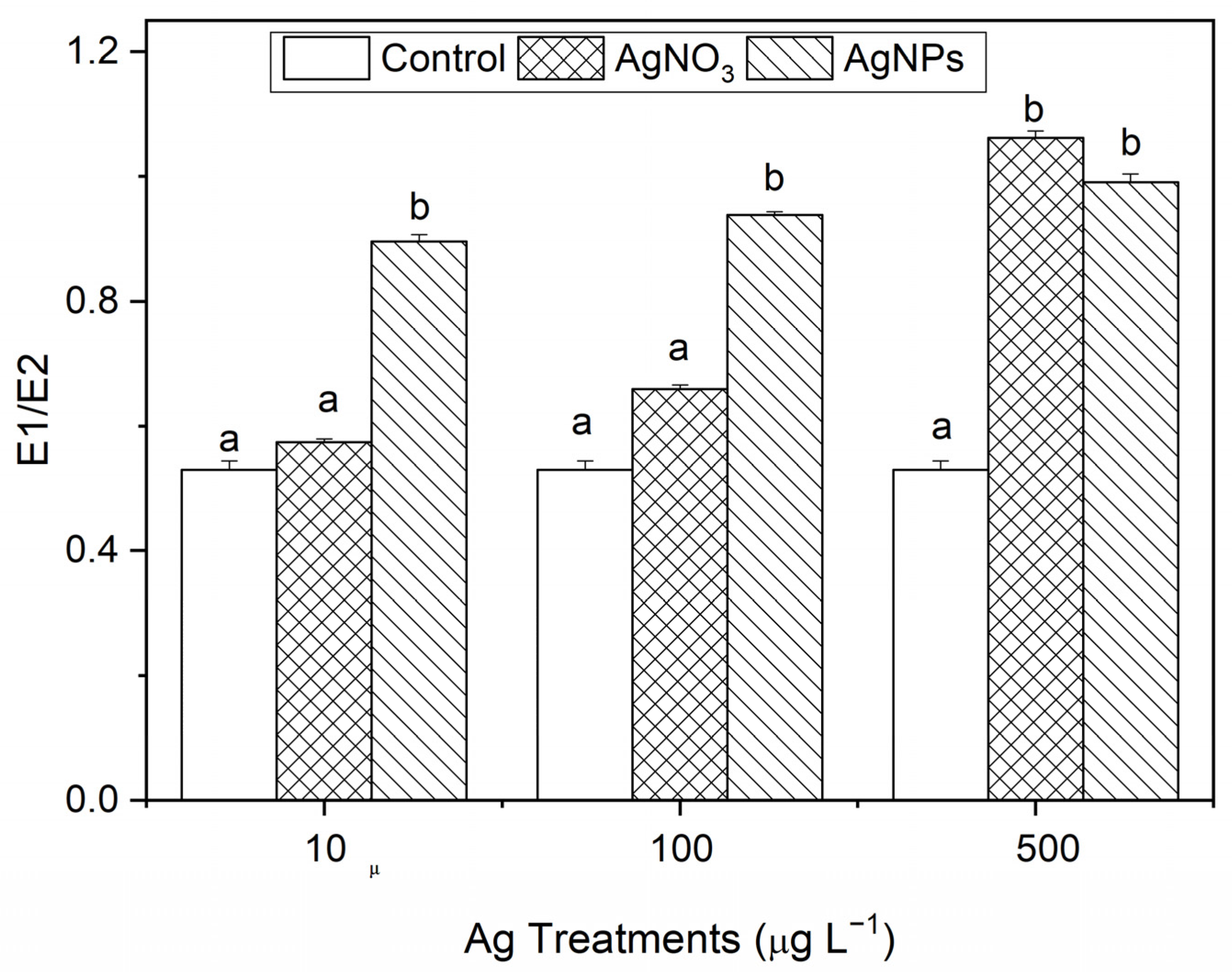
3.2.3. Destruction of Membrane Stability of S. costatum by AgNPs
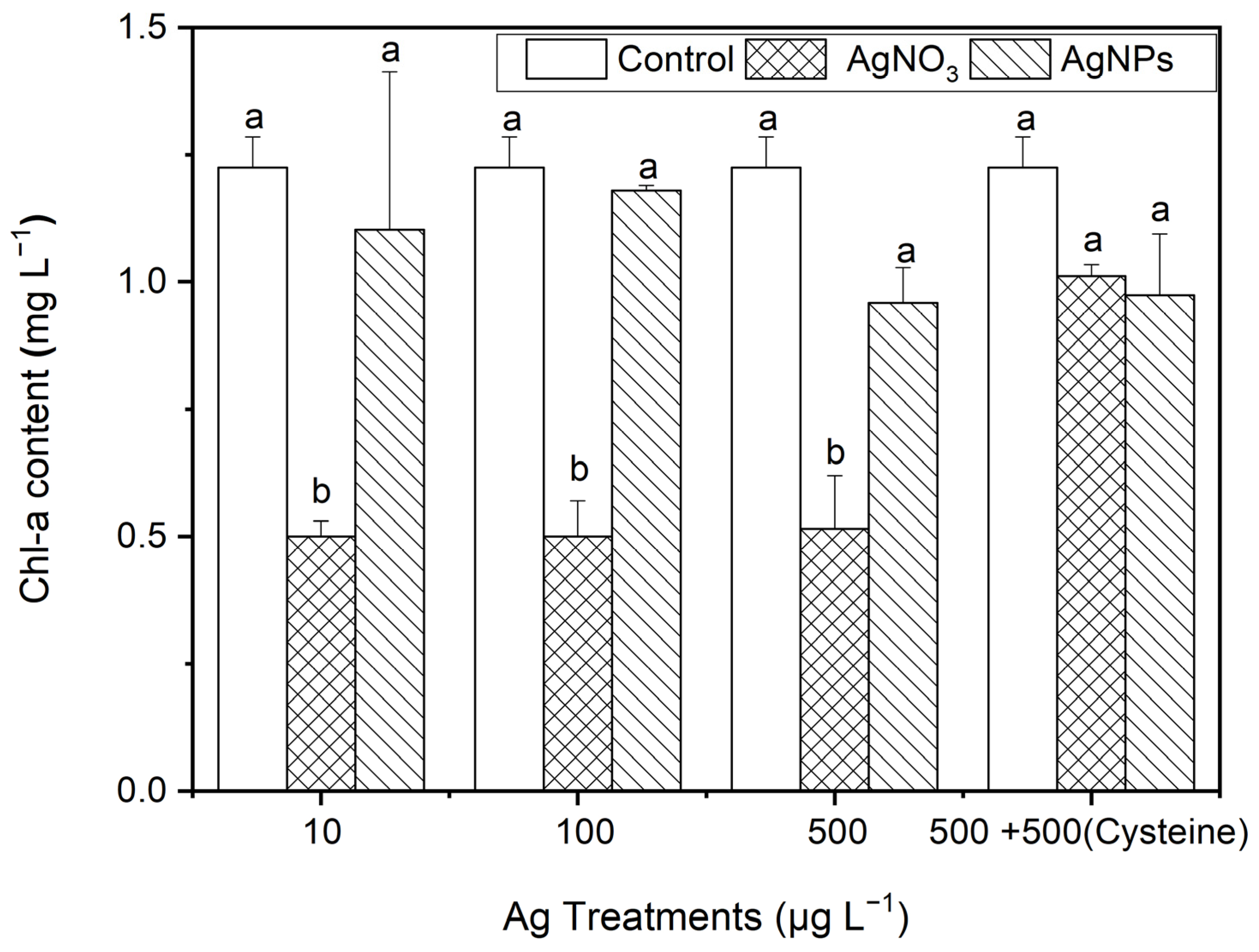
3.2.4. The Effect of AgNPs on Chl-a Contents in S. costatum
3.3. The Effect of AgNPs on the Antioxidant System of S. costatum
3.3.1. SOD
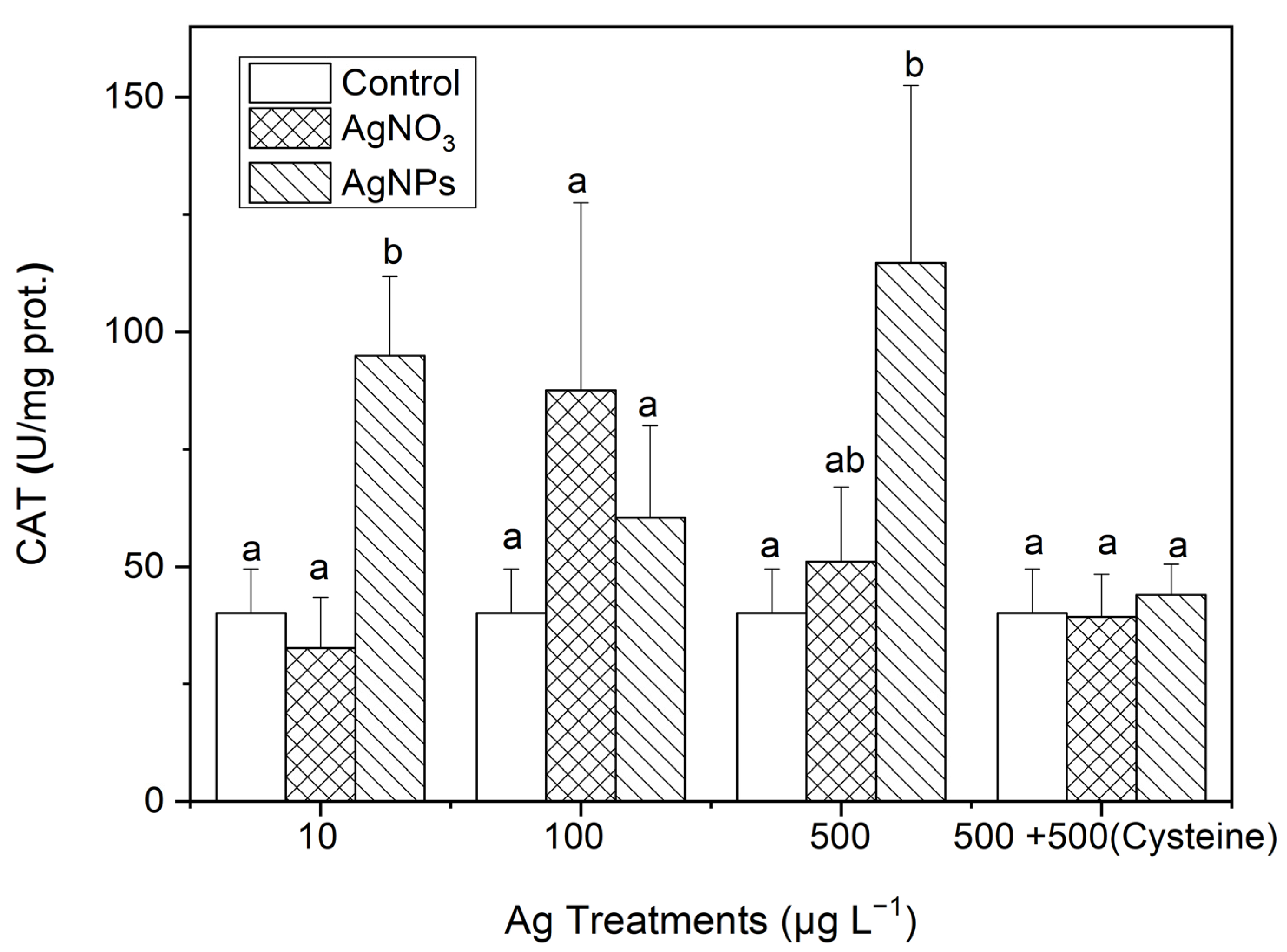
3.3.2. CAT
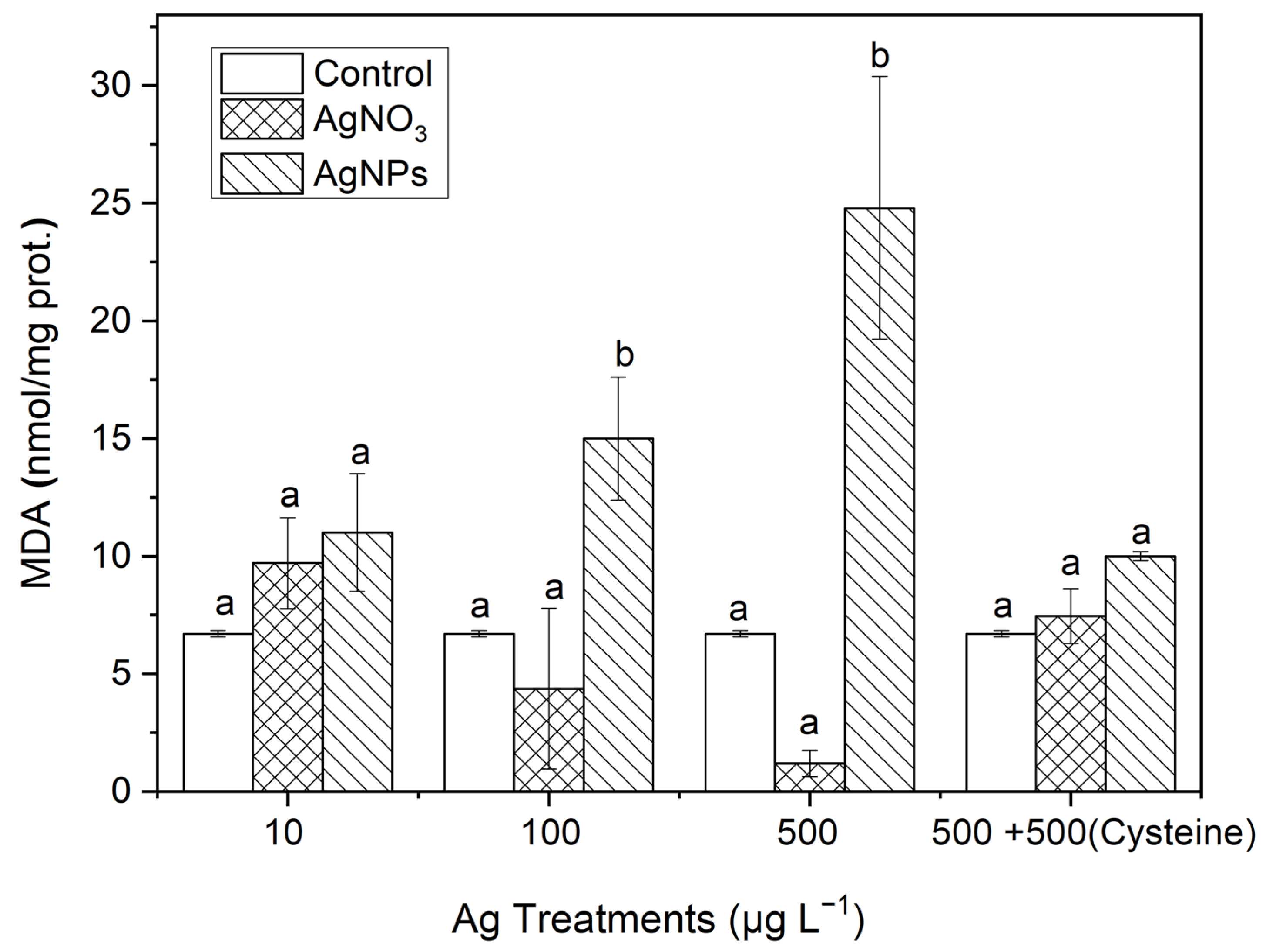
3.3.3. MDA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahjoubian, M.; Naeemi, A.S.; Moradi-Shoeili, Z.; Tyler, C.R.; Mansouri, B. Toxicity of Silver Nanoparticles in the Presence of Zinc Oxide Nanoparticles Differs for Acute and Chronic Exposures in Zebrafish. Arch. Environ. Con. Tox. 2023, 84, 1–17. [Google Scholar] [CrossRef]
- Bakr, Z.; Abdel-Wahab, M.; Thabet, A.A.; Hamed, M.; El-Aal, M.A.; Saad, E.; Faheem, M.; Sayed, A.E.-D.H. Toxicity of silver, copper oxide, and polyethylene nanoparticles on the earthworm Allolobophora caliginosa using multiple biomarkers. Appl. Soil Ecol. 2023, 181, 104681. [Google Scholar] [CrossRef]
- Kose, O.; Mantecca, P.; Costa, A.; Carrière, M. Putative adverse outcome pathways for silver nanoparticle toxicity on mammalian male reproductive system: A literature review. Part. Fibre Toxicol. 2023, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Lubick, N. Nanosilver toxicity: Ions, nanoparticles—Or both? Environ. Sci. Technol. 2008, 42, 8617. [Google Scholar] [CrossRef]
- Arora, S.; Rajwade, J.M.; Paknikar, K.M. Nanotoxicology and in vitro studies: The need of the hour. Toxicol. Appl. Pharm. 2012, 258, 151–165. [Google Scholar]
- Wang, L.-F.; Habibul, N.; He, D.-Q.; Li, W.-W.; Zhang, X.; Jiang, H.; Yu, H.-Q. Copper release from copper nanoparticles in the presence of natural organic matter. Water Res. 2015, 68, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Dei, R.C.H.; Wang, W.-X.; Guo, L. Marine diatom uptake of iron bound with natural colloids of different origins. Mar. Chem. 2003, 81, 177–189. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.X. bioavailability of natural colloid-bound iron to marine plankton: Influences of colloidal size and aging. Limnol. Oceanogr. 2001, 46, 1956–1967. [Google Scholar] [CrossRef]
- Peyrot, C.; Wilkinson, K.J.; Desrosiers, M.; Sauve, S. Effects of silver nanoparticles on soil enzyme activities with and without added organic matter. Environ. Toxicol. Chem. 2014, 33, 115–125. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2013, 204, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Kim, H.-J.; Phenrat, T.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ. Sci. Technol. 2008, 42, 3349–3355. [Google Scholar] [CrossRef]
- Buffet, P.-E.; Pan, J.-F.; Poirier, L.; Amiard-Triquet, C.; Amiard, J.-C.; Gaudin, P.; Faverney, C.R.-d.; Guibbolini, M.; Gilliland, D.; Valsami-Jones, E.; et al. Biochemical and behavioural responses of the endobenthic bivalve Scrobicularia plana to silver nanoparticles in seawater and microalgal food. Ecotox. Environ. Safe 2013, 89, 117–124. [Google Scholar] [CrossRef]
- Buffet, P.E.; Zalouk-Vergnoux, A.; Chatel, A.; Berthet, B.; Metais, I.; Perrein-Ettajani, H.; Poirier, L.; Luna-Acosta, A.; Thomas-Guyon, H.; Risso-de Faverney, C.; et al. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: The ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci. Total Environ. 2014, 470, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Garcés, M.; Cáceres, L.; Chiappetta, D.; Magnani, N.; Evelson, P. Current understanding of nanoparticle toxicity mechanisms and interactions with biological systems. New J. Chem. 2021, 45, 14328–14344. [Google Scholar] [CrossRef]
- Miao, A.-J.; Schwehr, K.A.; Xu, C.; Zhang, S.-J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Newton, K.M.; Puppala, H.L.; Kitchens, C.L.; Colvin, V.L.; Klaine, S.J. Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environ. Toxicol. Chem. 2013, 32, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.X.Z.; Radisic, M. Organ-on-a-chip platforms for evaluation of environmental nanoparticle toxicity. Bioact. Mater. 2021, 6, 2801–2819. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.-X. Silver nanoparticle toxicity to the larvae of oyster Crassostrea angulata: Contribution of in vivo dissolution. Sci. Total Environ. 2023, 858, 159965. [Google Scholar] [CrossRef]
- Ringwood, A.H.; McCarthy, M.; Bates, T.C.; Carroll, D.L. The effects of silver nanoparticles on oyster embryos. Mar. Environ. Res. 2010, 69, S49–S51. [Google Scholar] [CrossRef] [PubMed]
- Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water 2023, 15, 585. [Google Scholar]
- Liu, Z.; Malinowski, C.R.; Sepúlveda, M.S. Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere 2022, 291, 132941. [Google Scholar] [CrossRef] [PubMed]
- Padhye, L.P.; Jasemizad, T.; Bolan, S.; Tsyusko, O.V.; Unrine, J.M.; Biswal, B.K.; Balasubramanian, R.; Zhang, Y.; Zhang, T.; Zhao, J.; et al. Silver contamination and its toxicity and risk management in terrestrial and aquatic ecosystems. Sci. Total Environ. 2023, 871, 161926. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Powers, K.; Wang, Y.; Zhou, H.; Roberts, S.M.; Moudgil, B.M.; Koopman, B.; Barber, D.S. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles. Chemosphere 2012, 89, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Okuthe, G.E. Engineered nanoparticles in aquatic systems: Toxicity and mechanism of toxicity in fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Liu, D.; Sun, J.; Zou, J.; Zhang, J. Phytoplankton succession during a red tide of Skeletonema costatum in Jiaozhou Bay of China. Mar. Pollut. Bull. 2005, 50, 91–94. [Google Scholar] [CrossRef]
- Guillard, R.; Ryther, J. Studies of marine planktonic diatoms I: Cyclotella nanai Hustedt and Detonula confervacea Cleva. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- He, Y.-L.; Bai, J.; Bai, X.-Y.; Li, K.-R. Effect of Nano-Silver on Planktonic-Bacterial Activity in Qingdao Coastal Waters. P. Ocean Univ. China 2020, 50, 76–82. [Google Scholar]
- Pisani, T.; Munzi, S.; Paoli, L.; Bačkor, M.; Loppi, S. Physiological effects of arsenic in the lichen Xanthoria parietina (L.) Th. Fr. Chemosphere 2011, 82, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Pinheiro, J.P.; Cancio, I.; Bebianno, M.J. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat. Toxicol. 2012, 118, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Zou, X.; Shi, J.; Zhang, H. Coexistence of silver and titanium dioxide nanoparticles: Enhancing or reducing environmental risks? Aquat. Toxicol. 2014, 154, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, A.D.; Carvalho, R.N.; Valente, A.; Nativo, P.; Gilliland, D.; Garcìa, C.P.; Passarella, R.; Pedroni, V.; Rossi, F.; Lettieri, T. Effects of Silver Nanoparticles in Diatom Thalassiosira pseudonana and Cyanobacterium Synechococcus sp. Environ. Sci. Technol. 2012, 46, 11336–11344. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Y.; Lin, Z.; Yao, Z.; Zhang, W. The joint effects on Photobacterium phosphoreum of metal oxide nanoparticles and their most likely coexisting chemicals in the environment. Aquat. Toxicol. 2014, 154, 200–206. [Google Scholar] [CrossRef]
- Seitz, F.; Rosenfeldt, R.R.; Storm, K.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Bundschuh, M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotox Environ. Safe 2015, 111, 263–270. [Google Scholar] [CrossRef]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms to Inform. Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ji, J.; Long, Z.; Yang, K.; Wu, F. The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012, 46, 4477–4487. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Von der Kammer, F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles—Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; Oukarroum, A.; Melegari, S.P.; Matias, W.G.; Popovic, R. Polymer coating of copper oxide nanoparticles increases nanoparticles uptake and toxicity in the green alga Chlamydomonas reinhardtii. Chemosphere 2012, 87, 1388–1394. [Google Scholar] [CrossRef]
- Hull, M.S.; Vikesland, P.J.; Schultz, I.R. Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis). Aquat. Toxicol. 2013, 140–141, 89–97. [Google Scholar] [CrossRef]
- Vrieling, E.G.; Beelen, T.P.M.; van Santen, R.A.; Gieskes, W.W.C. Diatom silicon biomineralization as an inspirational source of new approaches to silica production. J. Biotechnol. 1999, 70, 39–51. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Ovečka, M.; Lang, I.; Baluška, F.; Ismail, A.; Illeš, P.; Lichtscheidl, I.K. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 2005, 226, 39–54. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotox. Environ. Safe 2012, 78, 80–85. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotox. Environ. Safe 2012, 84, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide–engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef]
- Röhder, L.A.; Brandt, T.; Sigg, L.; Behra, R. Influence of agglomeration of cerium oxide nanoparticles and speciation of cerium (III) on short term effects to the green algae Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 152, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Buffet, P.-E.; Poirier, L.; Zalouk-Vergnoux, A.; Lopes, C.; Amiard, J.-C.; Gaudin, P.; Risso-de Faverney, C.; Guibbolini, M.; Gilliland, D.; Perrein-Ettajani, H.; et al. Biochemical and behavioural responses of the marine polychaete Hediste diversicolor to cadmium sulfide quantum dots (CdS QDs): Waterborne and dietary exposure. Chemosphere 2014, 100, 63–70. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Qiu, X.-N.; Li, G.-B.; Li, W.; Yin, L.-Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Wang, W.X. Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ. Toxicol. Chem. 2011, 30, 885–892. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Yao, Y.; Xue, J.; Cheng, D.; Wang, B. The Biotoxic Effects of Ag Nanoparticles (AgNPs) on Skeletonema costatum, a Typical Bloom Alga Species in Coastal Areas. J. Mar. Sci. Eng. 2023, 11, 1941. https://doi.org/10.3390/jmse11101941
Shi K, Yao Y, Xue J, Cheng D, Wang B. The Biotoxic Effects of Ag Nanoparticles (AgNPs) on Skeletonema costatum, a Typical Bloom Alga Species in Coastal Areas. Journal of Marine Science and Engineering. 2023; 11(10):1941. https://doi.org/10.3390/jmse11101941
Chicago/Turabian StyleShi, Ke, Yuehong Yao, Jianliang Xue, Dongle Cheng, and Bo Wang. 2023. "The Biotoxic Effects of Ag Nanoparticles (AgNPs) on Skeletonema costatum, a Typical Bloom Alga Species in Coastal Areas" Journal of Marine Science and Engineering 11, no. 10: 1941. https://doi.org/10.3390/jmse11101941
APA StyleShi, K., Yao, Y., Xue, J., Cheng, D., & Wang, B. (2023). The Biotoxic Effects of Ag Nanoparticles (AgNPs) on Skeletonema costatum, a Typical Bloom Alga Species in Coastal Areas. Journal of Marine Science and Engineering, 11(10), 1941. https://doi.org/10.3390/jmse11101941






