Abstract
Owing to the constant wind generated by the vast ocean, energy production from offshore wind farms (OWFs) plays an important role in the expansion of renewable energy. However, areas close to large wind farms are often left unutilized, and aquaculture farmers find it difficult to efficiently utilize these unoccupied spaces due to limited information showing the feasibility of utilization of OWFs as potential scallop culture sites. To analyze whether the two scallop species Zhikong scallop (Chlamys farreri) and bay scallop (Argopecten irradians) can be grown at OWFs of Gochang and Buan, Jeollabuk-do, Republic of Korea, the growth characteristics of the two scallop species were analyzed and compared with those grown at the Tongyeong Megacosm Test Station. The results clearly showed that the growth of scallops at the OWF was significantly lower with respect to the shell lengths, height, width, and weight, compared to those grown at the megacosm station. However, scallops grown at the OWF still showed consistent growth in parallel with those grown at the megacosm test station. Yet, there was a species-specific mortality rate between the two sites. In addition, our results suggest that temperature may be a key determinant of the growth of C. farreri and A. irradians. Overall, this study contributes to establishing a foundation for the stable and continuous farming of marine bivalves (e.g., clams, oysters, mussels, and scallops) in OWF areas.
1. Introduction
Offshore wind farms (OWFs) produce sufficient energy for use as a renewable energy source; thus, they play a pivotal role in the expansion of renewable energy. In general, OWFs allow energy producers to secure large farm sites and mass-produce high-density energy using abiotic factors, including the speed and direction of sea wind [1,2]. In fact, OWFs produce energy much more efficiently and stably than onshore wind farms [3,4,5]. However, the development of OWFs remains challenging, largely because of their incompatibility with marine ecosystems and social acceptability among local fisheries. In addition, OWFs built in the marine environment will increase the stress on existing ecosystems that have previously been used for other purposes, such as for fisheries or shipping, or those that are yet free of human activity [6,7]. Accordingly, combining OWFs and aquaculture can be a powerful means of increasing the spatial efficiency of sea usage and supporting the livelihoods of fishers. Through this innovative approach, the scope of aquaculture activities can be expanded to enhance the efficiency of spatial utilization in marine environments [8,9]. OWFs will almost certainly gain social acceptance. Over the past few years, there have been improvements in the social acceptance of establishing OWFs, as shown in some European countries, which have generated evidence from trials to develop business models for aquaculture, tourism, fishing, and leisure. Likewise, studies on offshore aquaculture systems in the United States have identified the potential of commercial aquaculture at offshore oil fields [10]. In addition, the co-location of offshore wind power facilities with fish and shellfish farming off the coast of the United Kingdom since 2012 has been shown to positively impact the total catches and, subsequently, the socioeconomic status of fishermen [11]. Overall, OWFs reportedly bring both positive and negative impacts to the ecosystem; however, with respect to fisheries, OWFs have brought mostly positive impacts, including supporting fish communities by increasing the nursery area of key species and by the fish gathering effect to prevent fish movement toward other locations [12,13,14]. However, long-term impacts of anthropogenic activities including OWF establishment on the marine biodiversity still remain largely unknown [15].
The largest OWFs have been built in Gochang and Buan, Jeollabuk-do, Republic of Korea. This offshore wind power industry convergence facility project in the Southwest Sea of the Republic of Korea has been anticipated to have positive effects, such as improving fishing and increasing the income of fishermen, as well as demonstrating that the co-location of sea farm projects and OWFs is economically feasible [11]. However, limited biological evidence has shown that OWFs in this region have a positive impact on the shellfish community.
The Zhikong scallop (Chlamys farreri) is an epifaunal and subtidal filter-feeder species that is widely distributed across the entire coast of the Republic of Korea, northern China, and Japan [16]. This particular species is also commercially valuable and mainly inhabits pebbles and gravel at water depths of up to 10 m [17,18]. Bay scallops (Argopecten irradians) are distributed along the coastal areas of China, Taiwan, Korea, and Japan, where they mainly live on sandy and gravel-bottomed floors. It is another commercially important species [19]. The commercial size of C. farreri and A. irradians is as follows: lengths: 7–10 cm and weight: 50–110 g, with median growth of 2–3 cm for C. farreri; and lengths: 6–8 cm and weight: 40–60 g for A. irradians [20]. Recently, scallop farming has become an active industry worldwide; as larger quantities of scallops are consumed, the production increases accordingly [21]. In the Republic of Korea, most scallops sold on the market have been developed using aquaculture technology, to the extent that most scallops, including the Zhikong and bay scallops, are farm-raised. As such, wind-farm areas create new opportunities to develop aquaculture for shellfish farming, including scallops. However, the possibility of shellfish aquaculture, including scallops, has not been studied in OWF areas.
Here, to see whether there are differences in the growth rate of the two scallop species cultured at the OWF and megacosm station, we investigated and compared the growth characteristics (e.g., shell length, height, width, total weight, and body weight) of the C. farreri and A. irradians in the OWF area and Tongyeong Megacosm Test Station. The results obtained in this study suggest the possibility of scallop farming on OWFs.
2. Materials and Methods
2.1. Sampling of Scallops
C. farreri were purchased from Taegyeong Fisheries (Goseong-gun, Gyeongsangnam-do, Republic of Korea) in December 2022, and A. irradians were purchased from the same company in September 2021. The purchased scallops were kept at the Tongyeong Megacosm Test Station (Korea Institute of Ocean Science and Technology, Tongyeong-si, Gyeongsangnam-do, South Korea), and a floating shellfish farm was installed in the OWF area (Gochang-gun and Buan-gun in North Jeolla Province, Republic of Korea) at a depth of 4 m (Figure 1).
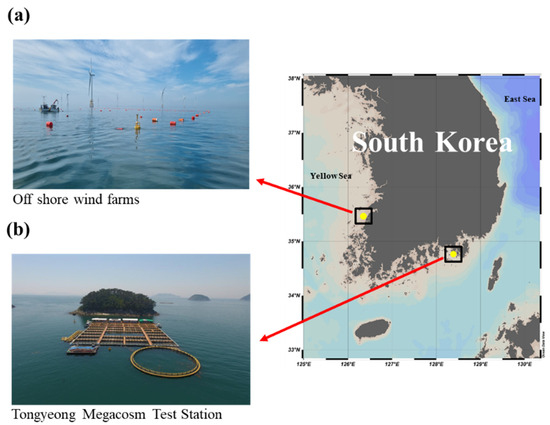
Figure 1.
Map showing (a) the offshore wind farm (Gochang-gun and Buan-gun in North Jeolla Province, Republic of Korea) and (b) Tongyeong Megacosm Test Station (Korea Institute of Ocean Science and Technology, Tongyeong-si, Gyeongsangnam-do, Republic of Korea).
2.2. Deployment of the Scallop Lantern Cages in the Longline Aquaculture Facility
On 9 December 2021, 160,000 C. farreri (shell height: 41.63 ± 3.73 mm, total weight 8.80 ± 2.58 g) were transferred into 400 scallop lantern nets and deployed on the longline aquaculture facility within the OWF area. Further, on September 2021, 90,000 A. irradians (shell height 40.14 ± 2.65 mm and shell weight 10.34 ± 1.85 g) were transferred into 300 scallop lantern nets and transplanted on the longline aquaculture facility within the OWF area.
For transplantation, they were transported by vehicle to Gochang-gun, Republic of Korea, and further transported to the OWF area using two vessels. They were nurtured in the OWF’s longline aquaculture facility along two 200 m long lines, and the spacing of each scallop lantern net was maintained at 100 cm. Four hundred 10-section scallop lantern nets with a diameter of 40 cm were used, with 40 shellfish per section. For proper immersion, 3 kg weights were attached to the bottoms of the scallop lantern nets (Figure 2).
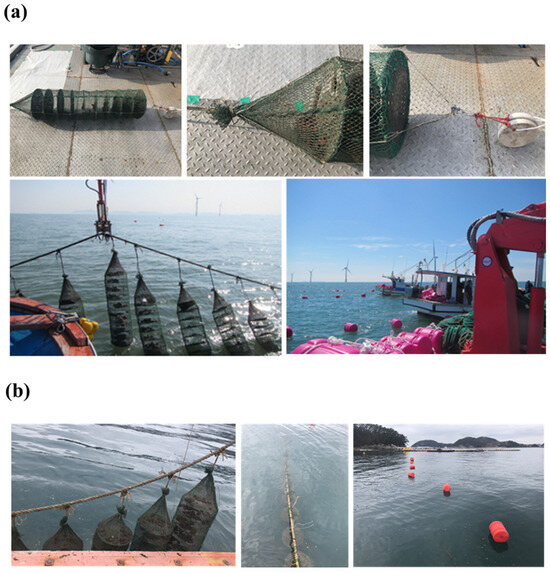
Figure 2.
Deployment of the scallop lantern cages at (a) offshore wind farm and (b) Tongyeong Megacosm Test Station.
2.3. Sampling and Measurement
The growth characteristics of the C. farreri and A. irradians were studied from December 2021 to August 2022 and from September to November 2021, respectively. Comparative studies were conducted simultaneously with more than 30 individuals once a month at the Tongyeong Megacosm Test Station and OWF area. The monthly growth of scallops was surveyed by measuring the shell length (mm), height (mm), width (mm), total weight (g), and body weight (g) for each species. We used a Vernier caliper (Mitutoyo, Absolute Digimatic Caliper 500–153-30, Kawasaki, Japan) to measure the lengths and a weighing scale (AND, FX-3000, Seoul, South Korea) to measure the weight of the samples. During the experimental period, cumulative mortality was measured by counting the number of dead scallops (Table 1).

Table 1.
Cumulative mortality of the two scallops at the two different culture sites during the experimental period.
2.4. Temperature Variation of the Study Site
Surface seawater temperature data (from September 2021 to August 2022) were obtained from offshore buoys installed in Tongyeong Yeonhwa-do (34.6672 N, 128.3847 E) and Buan Wi-do (35.6584 N, 126.2610 E), which are adjacent to Tongyeong Megacosm Test Station and the OWF area (data provided by the Real-time Information System for Aquaculture environment from National Institute of Fisheries Science, South Korea) (Figure 3).
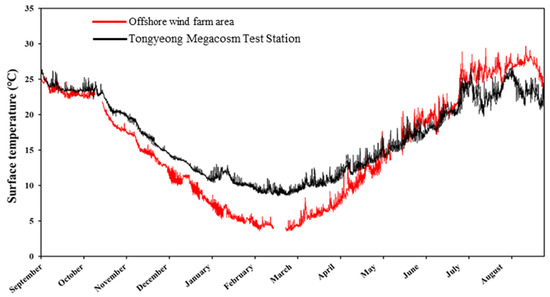
Figure 3.
Monthly change in surface seawater temperatures at the sampling sites from September 2021 to August 2022.
2.5. Statistical Analysis
The collected data were analyzed using one-way ANOVA followed by Scheffé’s post hoc test to evaluate the effect of months in the same region. The cut-off for statistical significance was set at p < 0.05. An independent-sample t-test was performed to evaluate the differences in the growth characteristics of the two scallops in the two different regions for each month. Levene’s test was used to assess the normal distribution and homogeneity of variance among the samples. Values are expressed as the mean ± standard deviation (SD). All data were analyzed using SPSS (version 27.0; IBM, Armonk, NY, USA).
3. Results and Discussion
In scallop aquaculture in general, scallops undergo an intermediate rearing process to reduce the costs arising from size selection of juvenile scallops and the consequent labor burden [22,23]. Early juvenile scallops typically range from 2 to 3 mm in size, which makes it difficult to accommodate them in the scallop lantern net; further, they lack resistance to changes in the environment (e.g., water temperature, buoyancy, dissolved oxygen). Therefore, it is essential to use scallops that have undergone an intermediate rearing process to produce healthy scallops and improve their survival rate. In addition, they must be at least 2–3 cm for controlled air exposure and long-distance transportation [20].
The comparative mortality analysis between the two scallop species showed species-specific mortality (Table 1). For C. farreri, a higher mortality was observed for those grown at Tonhyeong Megacosm Station (17.97%) compared to those grown at the OWF (0.69%) during December~August. For A. irradians cultured during September~November, a higher mortality was observed in those cultured at the OWF (26.77%) compared to the Tongyeong Megacosm Test Station (2.22%). Due to the short time frame and differences in sampling period, it is challenging and difficult to conclude that one species performs better than the other at a specific culture location, yet it could be suggested that a species-specific tolerance and optimum temperature range could contribute to the survival of the two scallops.
Previous studies have suggested that mortality and growth rates depend on differences in water depth, density, and temperature [24]. For example, increased mortality and poor growth rates in some scallops were observed as the water depth increased [25,26], whereas in other scallop species, such as Plactopecten magellanicus, the growth rate increased along with water depth [27]. In addition, scallops in longline aquaculture reportedly have the best growth rate at a water depth of 4 m [28]. Therefore, we investigated the growth rates of scallops that underwent an intermediate rearing process in the floating shellfish farm installed in the OWF area and the Tongyeong Megacosm Test Station at a depth of 4 m each.
In this study, the growth of C. farreri raised from a longline culture facility in the OWF area was compared with those raised at the Tongyeong Megacosm Test Station from December 2021 to August 2022 (Figure 4). Based on the specific growth rate (SGR) equation derived from the shell length and wet weight, C. farreri showed an SGRl of 1.46 and SGRw of 1.19 at the OWF over the 9 month period [29].
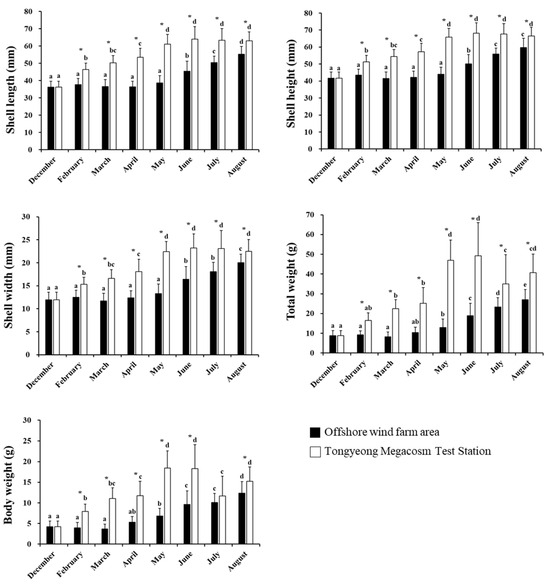
Figure 4.
Bar plots depicting the comparisons of changes in size (shell length, height, width, total weight, and body weight) of Chlamys farreri from month to month. The letters indicate a significant difference for different months in the same region (p < 0.05). The symbol “*” indicates significant differences by region in the same month (p < 0.05). All values represent mean ± standard deviation (SD).
SGR = ln ((Lf) − ln (Li))/t × 100 and SGRw = ln ((lnWf) − ln (Wi))/t × 100, where:
- Lf = final average shell length;
- Wf = wet weight at the end of the experiment;
- Li = initial average shell length;
- Wi = wet weight at the beginning of the experiment;
- ln = natural logarithm;
- t = number of days of the experimental time.
Specifically, from December 2021 to August 2022, the shell length of C. farreri cultured in the longline culture facility at the OWF area increased from 36.21 ± 3.38 mm to 55.24 ± 4.60 mm, the shell height increased from 41.63 ± 3.73 mm to 59.66 ± 5.58 mm, the shell width increased from 11.93 ± 1.69 mm to 20.06 ± 1.81 mm, the total weight increased from 8.80 ± 2.58 g to 26.99 ± 5.20 g, and the body weight increased from 4.26 ± 1.35 g to 12.34 ± 2.80 g. Overall, C. farreri cultivated in the OWF area exhibited a 1.4-times-greater shell height and 4.4-times-higher total weight, after eight months (December 2021~August 2022) of cultivation. Comparatively, those Zhikong scallops (C. farreri) reared at the Tongyeong Megacosm Test Station during the same period showed an SGRl and SGW of 1.51 and 1.35 over the same period of those cultured at the OWF, respectively. Specifically, C. farreri showed an increase in the shell length from 36.21 ± 3.38 mm to 63.05 ± 5.10 mm, the shell height increased from 41.63 ± 3.73 mm to 66.45 ± 5.12 mm, the shell width increased from 11.93 ± 1.69 mm to 22.49 ± 2.55 mm, the total weight increased from 8.80 ± 2.58 g to 40.75 ± 9. 35 g, and the body weight increased from 4.26 ± 1.35 g to 15.26 ± 3.45 g. Eight months of experimentation at the Tongyeong Megacosm Test Station indicated that the growth determinants of C. farreri increased 1.6- and 4.6-fold for shell height and total weight, respectively. Overall, the growth rate of C. farreri reared at the OWF area and Tongyeong Megacosm Test Station showed that the shell height was approximately 10 mm and the total weight was approximately 17 g higher at the Tongyeong Megacosm Test Station compared to those reared in the OWF area.
Generally, the water temperature for the growth of C. farreri ranges between 15 and 22 °C (optimal water temperature: 20 °C), with a maximum water temperature of 29 °C and minimum water temperature of 0 °C [20]. Indeed, the growth, maturation, survival, distribution and feeding, energy utilization, and metabolic activities of shellfish are known to be greatly influenced by the surrounding water temperatures [30,31]. For example, in commercial scallop Pecten fumatus (Reeve), the growth rate and survival of larvae significantly decrease in response to increasing temperature [32]. In the sea scallop Placopecten magellanicus, survival significantly increases in response to high temperatures [22,23]. Our experiments at the two different culture sites showed interesting results. In the OWF area, C. farreri showed a slower growth rate from December to May, but the growth rate slowly increased from June and continued significantly through August. In contrast, at the Tongyeong Megacosm Test Station, the growth rate of C. farreri was faster than that in the OWF area during the same months, with no significant growth changes from May to August. As previously mentioned, the growth rate is partially influenced by temperature. At the time of the growth experiment, the water temperature at the OWF area steadily decreased from December to March by up to 5 °C, and then gradually increased from June to August, reaching approximately the same temperature as the water temperature at the Tongyeong Megacosm Test Station (Figure 3).
Recently, scallop production has declined sharply due to widespread mass mortality during the summer [33,34]. Here, no mass mortality was observed in 2022, since the water temperature did not exceed 26 °C at the most; however, in the year 2021, a high-temperature period (>28 °C) resulted in the mass mortality of experimental scallops. Therefore, it was evident that the water temperature plays a crucial role in determining the growth and health of the scallops. However, the two locations naturally have different temperature ranges (i.e., the OWF area is in the west and typically has a lower temperature than the Tongyeong Macromosm station situated in the warm southern part of the Republic of Korea). Although the growth rate was not higher than that at the Tongyeong Megacosm Test Station, the results clearly show the possibility of utilizing the OWF area to cultivate C. farreri.
Generally, the optimum water temperature for the growth of A. irradians is 18–28 °C (optimal water temperature is 23 °C). The upper limit of the water temperature is 31 °C and the lower limit is 1 °C, with a growth pause below 5 °C. A. irradians take less than 10 months to grow into harvestable products of 5–7 cm in height. Their lifespan is approximately 12–16 months and does not exceed 24 months [20].
A. irradians was analyzed for growth differences at two sites (Tongyeong Megacosm Test Station and the longline culture facility in the OWF area) from September to November 2021 (Figure 5). Overall, A. irradians showed an SGRl and SGRw of 4.08 and 2.90, respectively, at the OWF over the 3 month period. Data collected from September to November 2021 at the longline culture facility within the OWF area showed increases in the shell length of the bay scallops from 34.20 ± 4.68 mm to 42.89 ± 4.90 mm, the height increased from 32.56 ± 4.49 mm to 41. 09 ± 4.84 mm, the width increased from 12.90 ± 1.78 mm to 18.18 ± 2.37 mm, the total weight increased from 6.61 ± 2.14 g to 15.51 ± 4.79 g, and the body weight increased from 2.86 ± 1.02 g to 7.00 ± 2.27 g. Three months of experimentation at the longline culture facility within the OWF area during the same period indicated that the growth determinants of the bay scallops increased 1.3- and 2.4-fold for shell length and total weight, respectively. Comparatively, those bay scallops reared at the Tongyeong Megacosm Test Station during the same period showed an SGRl and SGRw of 4.37 and 3.52. Specifically, they showed an increased shell length from 34.20 ± 4.68 mm to 54.39 ± 5.98 mm, the height increased from 32.56 ± 4.49 mm to 49.75 ± 5.42 mm, the width increased from 12.90 ± 1.78 mm to 22.63 ± 3.16 mm, the total weight increased from 6.61 ± 2.14 g to 25.65 ± 6.95 g, and the body weight increased from 2.86 ± 1.02 g to 10.21 ± 2.90 g. Three months of experimentation at the Tongyeong Megacosm Test Station indicated that the growth determinants of bay scallops increased 1.5- and 3.9-fold for shell height and total weight, respectively. Overall, the growth rate of bay scallops reared at the OWF area and the Tongyeong Megacosm Test Station showed a shell height of about 0.9 cm, with a total weight that was about 10 g higher at the Tongyeong Megacosm Test Station compared to those reared at the OWF area. Based on the preliminary experiment, the growth rates of C. farreri and A. irradians were higher at the Tongyeong Megacosm Test Station compared to the OWF area. The growth rate of C. farreri was found to be highest in summer (July to August) in the OWF area, whereas the growth rate was lowest during the same period at the Tongyeong Megacosm Test Station. Based on this short-term experiment of 8 months, we could see that temperature is indeed one of the key factors affecting the growth of C. farreri. Despite the limitation of this being a short-term experiment, the results clearly suggest the possible utilization of OWF areas as potential scallop culture locations.
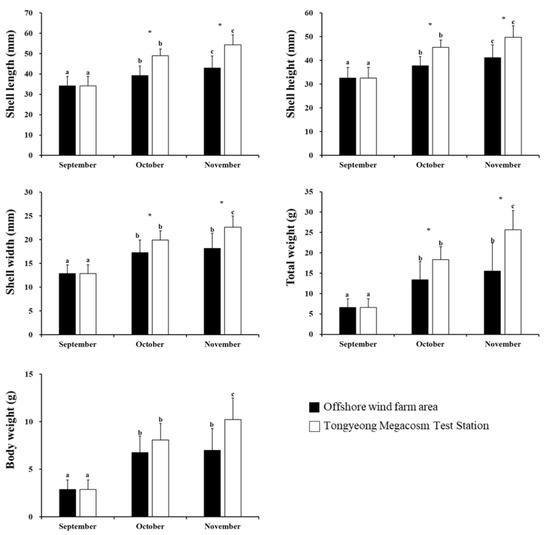
Figure 5.
Bar plots depicting the comparisons of changes in size (shell length, height, width, total weight, and body weight) of Argopecten irradians from month to month. The letters indicate a significant difference for different months in the same region (p < 0.05). The symbol “*” indicates significant differences by region in the same month (p < 0.05). All values represent mean ± standard deviation (SD).
In conclusion, based on the results obtained from 2021 and 2022, scallop farming in OWF areas is feasible. The culture of scallops in wind farm areas is not profitable but it can produce some protein that can supply protein to local and national markets and also may lead to an increase in the biodiversity of the marine ecosystem. However, more information on aquaculture should be obtained in parallel with the monitoring of environmental conditions. Regardless, this could be a step forward toward utilizing OWF areas for aquaculture in countries with limited fishing areas. Additionally, owing to poor accessibility and environmental conditions compared to the coast, further development of the facilities and management are required to establish stable aquaculture conditions for OWFs.
Author Contributions
Conceptualization, data curation, formal analysis, and writing—original draft, J.H. and J.J.C.P.; investigation, D.-W.L., Y.-H.J., H.-J.K. and D.M.C.; project administration and funding acquisition, S.-Y.O. and Y.-U.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP), and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20203030020080 and No. 20203040020130).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Experimental Committee of the Korea Institute of Ocean Science and Technology (KIOST).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are available from the data repository of KIOST. Requests for material should be made to the corresponding author.
Acknowledgments
The authors thank the Korea Institute of Energy Technology Evaluation and Planning (KETEP), Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20203030020080 and No.20203040020130). Finally, we thank the editor and the anonymous reviewers whose comments have greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyong, N.H.; Yoon, J.E.; Jang, M.S.; Jang, D.S. An assessment of offshore wind energy resources around Korean Peninsula. J. Korean Sol Energy Soc. 2003, 23, 35–41. [Google Scholar]
- Park, J.; Kim, B. An analysis of South Korea’s energy transition policy with regards to offshore wind power development. Renew. Sustain. Energy Rev. 2019, 109, 71–84. [Google Scholar] [CrossRef]
- Hevia-Koch, P.; Klinge Jacobsen, H.K. Comparing offshore and onshore wind development considering acceptance costs. Energy Policy 2019, 125, 9–19. [Google Scholar] [CrossRef]
- Chen, J.; Kim, M.-H. Review of recent offshore wind turbine research and optimization methodologies in their design. J. Mar. Sci. Eng. 2022, 10, 28. [Google Scholar] [CrossRef]
- Olczak, P.; Surma, T. Energy productivity potential of offshore wind in Poland and cooperation with onshore Wind Farm. Appl. Sci. 2023, 13, 4258. [Google Scholar] [CrossRef]
- Rehfeldt, K.; Paschedag, U.; Bömer, J. (Eds.) Offshore Wind Power Deployment in Germany; Federal Ministry for the Environment; Nature Conservation and Nuclear Safety (BMU); Offshore Wind Energy Foundation: Bonn, Germany, 2007; p. 31. [Google Scholar]
- Chaji, M.; Werner, S. Economic impacts of offshore wind farms on fishing industries: Perspectives, methods, and knowledge gaps. Mar. Coast. Fish. 2023, 15, e10237. [Google Scholar] [CrossRef]
- Buck, B.H.; Krause, G.; Michler-Cieluch, T.; Brenner, M.; Buchholz, C.M.; Busch, J.A.; Fisch, R.; Geisen, M.; Zielinski, O. Meeting the quest for spatial efficiency: Progress and prospects of extensive aquaculture within offshore wind farms. Helgol. Mar. Res. 2008, 62, 269–281. [Google Scholar] [CrossRef]
- Huang, C.T.; Afero, F.; Hung, C.W.; Chen, B.Y.; Nan, F.H.; Chiang, W.S.; Tang, H.J.; Kang, C.K. Economic feasibility assessment of cage aquaculture in offshore wind power generation areas in Changhua County, Taiwan. Aquaculture 2022, 548, 737611. [Google Scholar] [CrossRef]
- Kang, K.-S.; Jeon, I.-S.; Kwak, J.-Y. Possibilities and orientation toward co-existence of offshore wind farms. J. Wind Energy 2016, 7, 5–13. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, H.W.; Kim, J.H. An economical feasibility analysis of sea farm project using co-location in offshore wind farms. Product. Res. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Lindeboom, H.J.; Kouwenhoven, H.J.; Bergman, M.J.N.; Bouma, S.; Brasseur, S.; Daan, R.; Fijn, R.C.; De Haan, D.; Dirksen, S.; Van Hal, R.; et al. Short term ecological effects of an offshore wind farm in the Dutch coastal zone; a compliation. Environ. Res. Lett. 2011, 6, 03510. [Google Scholar] [CrossRef]
- Reubens, J.T.; Degraer, S.; Vincx, M. The ecology of benthopelagic fishes at offshore wind farms: A synthesis of 4 years of research. Hydrobiologia 2014, 727, 121–136. [Google Scholar] [CrossRef]
- Stenberg, C.; Støttrup, J.G.; Van Deurs, M.; Berg, C.W.; Dinesen, G.E.; Mosegaard, H.; Grome, T.M.; Leonhard, S.B. Long-term effects of an offshore wind farm in the North Sea on fish communities. Mar. Ecol. Prog. Ser. 2015, 528, 257–265. [Google Scholar] [CrossRef]
- Li, C.; Coolen, J.W.P.; Scherer, L.; Mogollón, J.M.; Braeckman, U.; Vanaverbeke, J.; Tukker, A.; Steubing, B. Offshore Wind Energy and Marine Biodiversity in the North Sea: Life Cycle Impact Assessment for Benthic Communities. Environ. Sci. Technol. 2023, 57, 6455–6464. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Zhang, T.; Liu, S.; Zhang, S.; Liu, Q.; Xiang, J.; Zhang, F. Influence of filtering and biodeposition by the cultured scallop Chlamys farreri on benthic-pelagic coupling in a eutrophic bay in China. Mar. Ecol. Prog. Ser. 2006, 317, 127–141. [Google Scholar] [CrossRef][Green Version]
- Park, G.J.; Yoon, S.P.; Park, Y.J.; Song, H.I. Effect of stocking density on growth and survival rate of the scallop, Chlamys farreri (Jones & Preston, 1904) cultured in hanging culture in the west coast of Korea. Korean J. Malacol. 2012, 28, 1–6. [Google Scholar] [CrossRef]
- Yoon, J.M. Genetic distances of scallop (Chlamys farreri) populations investigated by PCR procedure. Dev. Reprod. 2017, 21, 435–440. [Google Scholar] [CrossRef]
- Fay, C.W.; Neves, R.J.; Pardue, G.B. Species Profiles. Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Mid-Atlantic): Bay scallop. In Virginia Polytechnic Institute and State University Blacksburg Department of Fisheries and Wildlife Science, FWS/OBS-82/11.12; EL-82-4; U.S. Army Corps of Engineers: Washington, DC, USA, 1983; p. 17. [Google Scholar]
- NIFS (National Institute of Fisheries Science). Technical Manual for the Scallops Aquaculture; NIFS (National Institute of Fisheries Science): Busan, Republic of Korea, 2019; pp. 18–23. [Google Scholar]
- FAO. Sustainability in action (Rome). In The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Coleman, S.; Cleaver, C.; Morse, D.; Brady, D.C.; Kiffney, T. The coupled effects of stocking density and temperature on Sea Scallop (Placopecten magellanicus) growth in suspended culture. Aquacult. Rep. 2021, 20, 100684. [Google Scholar] [CrossRef]
- Coleman, S.; Morse, D.; Christian Brayden, W.; Brady, D.C. Developing a bioeconomic framework for scallop culture optimization and product development. Aquac. Econ. Manag. 2021, 27, 25–49. [Google Scholar] [CrossRef]
- Coleman, S.; Kiffney, T.; Tanaka, K.R.; Morse, D.; Brady, D.C. Meta-analysis of growth and mortality rates of net cultured sea scallops across the northwest Atlantic. Aquaculture 2022, 546, 737392. [Google Scholar] [CrossRef]
- Cano, J.; Campos, M.J.; Román, G. Growth and mortality of the king scallop grown in suspended culture in Malaga, Southern Spain. Aquacult. Int. 2000, 8, 207–225. [Google Scholar] [CrossRef]
- Yiğitkurt, S. Growth and survival performance of smooth scallop (Flexopecten glaber Linnaeus, 1758) at different depths in the Aegean Sea. Mar. Sci. Technol. Bull. 2021, 10, 278–285. [Google Scholar] [CrossRef]
- Claereboudt, M.R.; Burreau, D.; Coté, J.; Himmelman, J.H. Fouling development and its effect on the growth of juveniles giant scallop (Placopecten magallanicus) in suspended culture. Aquaculture 1994, 121, 324–342. [Google Scholar] [CrossRef]
- Sun, J.; Lin, C.; Li, P.; Jin, Y.; Zhou, L. The culture experiment of Scallop of Chlamys farreri in Nanji Islands. J. Zhejiang Coll. Fish. 1997, 16, 247–255. [Google Scholar]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Papa, L.; Denti, G.; Fanelli, G. Estimation of growth of parameters of the Black Scallop Mimachlamys Varia in the Gulf of Taranto (Ionian Sea, Southern Italy). Water 2020, 12, 3342. [Google Scholar] [CrossRef]
- Mills, D. Combined effects of temperature and algal concentration on survival, growth and feeding physiology of Pinctata maxima (Jameson) spat. J. Shellfish Res. 2000, 19, 159–166. [Google Scholar]
- Nan, X.; Wei, H.; Zhang, H.; Nie, H. Factors influencing the interannual variation in biomass of bottom-cultured Yesso scallop (Patinopecten yessoensis) in the Changhai Sea Area, China. Front. Mar. Sci. 2022, 8, 798359. [Google Scholar] [CrossRef]
- Heasman, M.P.; O’Connor, W.A.; Frazer, A.W.J. Ontogenetic changes in optimal rearing temperatures for the commercial scallop, Pecten fumatus Reeve. J. Shellfish Res. 1996, 15, 627–634. [Google Scholar]
- Xiao, J.; Ford, S.E.; Yang, H.S.; Zhang, G.F.; Zhang, F.S.; Guo, X.M. Studies on mass summer mortality of cultured Zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Han, J.C.; Jo, Q.; Park, Y.C.; Park, T.G.; Lee, D.C.; Cho, K.C. A report on the mass summer mortalities of the farmed Pacific oysters, Crassostrea gigas and Bay scallops Argopecten irradians in the local waters of Goseong Bay, Korea. Korean J. Malacol. 2013, 29, 239–244. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).