Sea Ice as a Factor of Primary Production in the European Arctic: Phytoplankton Size Classes and Carbon Fluxes
Abstract
:1. Introduction
- (i)
- How does the contribution of phytoplankton of different sizes to PP change at high and low sea ice concentrations in the European Arctic in summer?
- (ii)
- What environmental factors influence the species and size structure of phytoplankton in the European Arctic in summer?
2. Materials and Methods
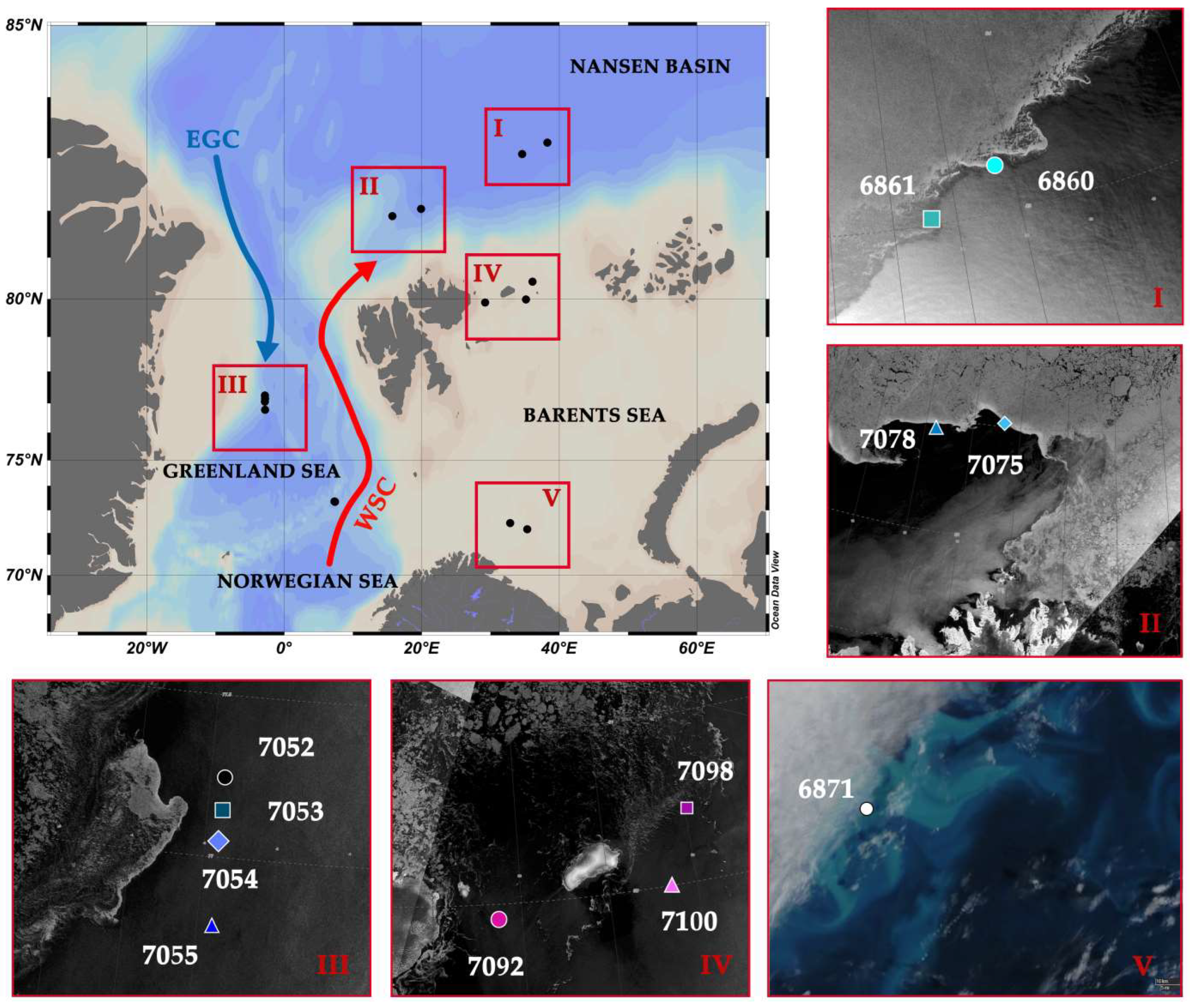
2.1. Study Area and Fieldwork Description
2.2. Sampling, Hydrography, Light Conditions and Chemical Analyses
2.3. Standing Stock of Phytoplankton
2.4. Primary Production
2.5. Analysis of the Relationship between Phytoplankton and Environmental Factors
3. Results
3.1. Sea Ice Cover in August 2020 and 2021
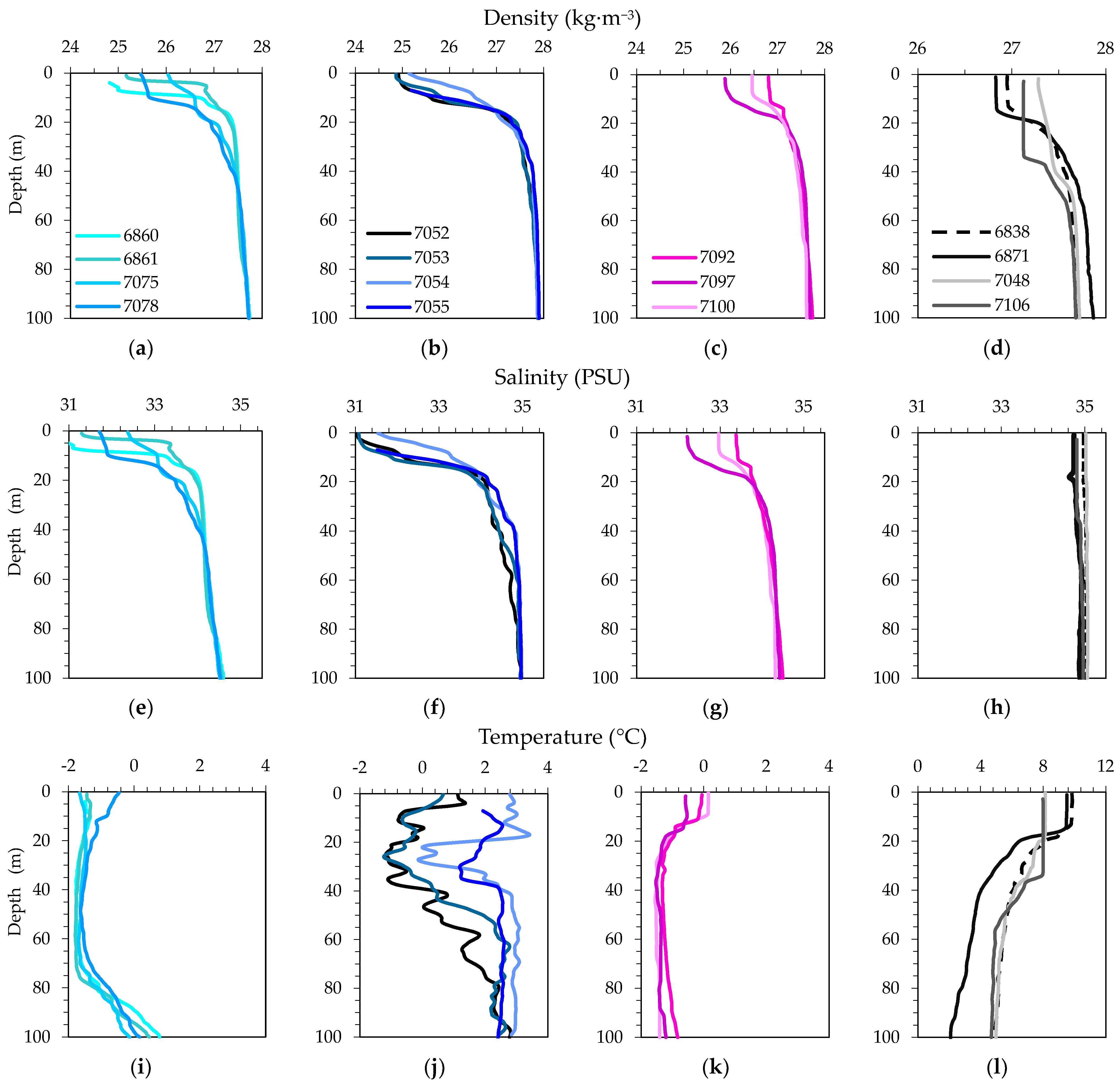
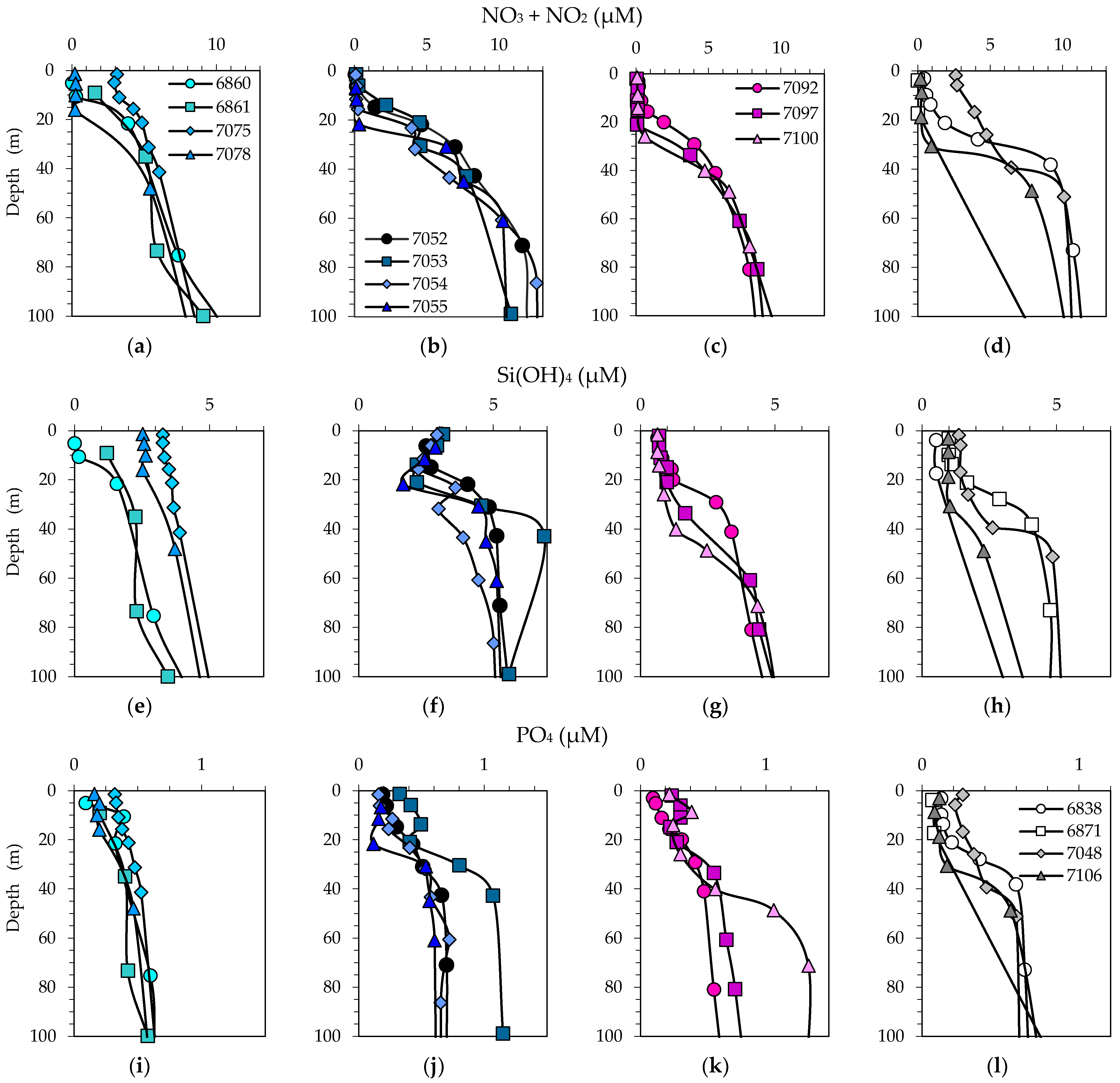
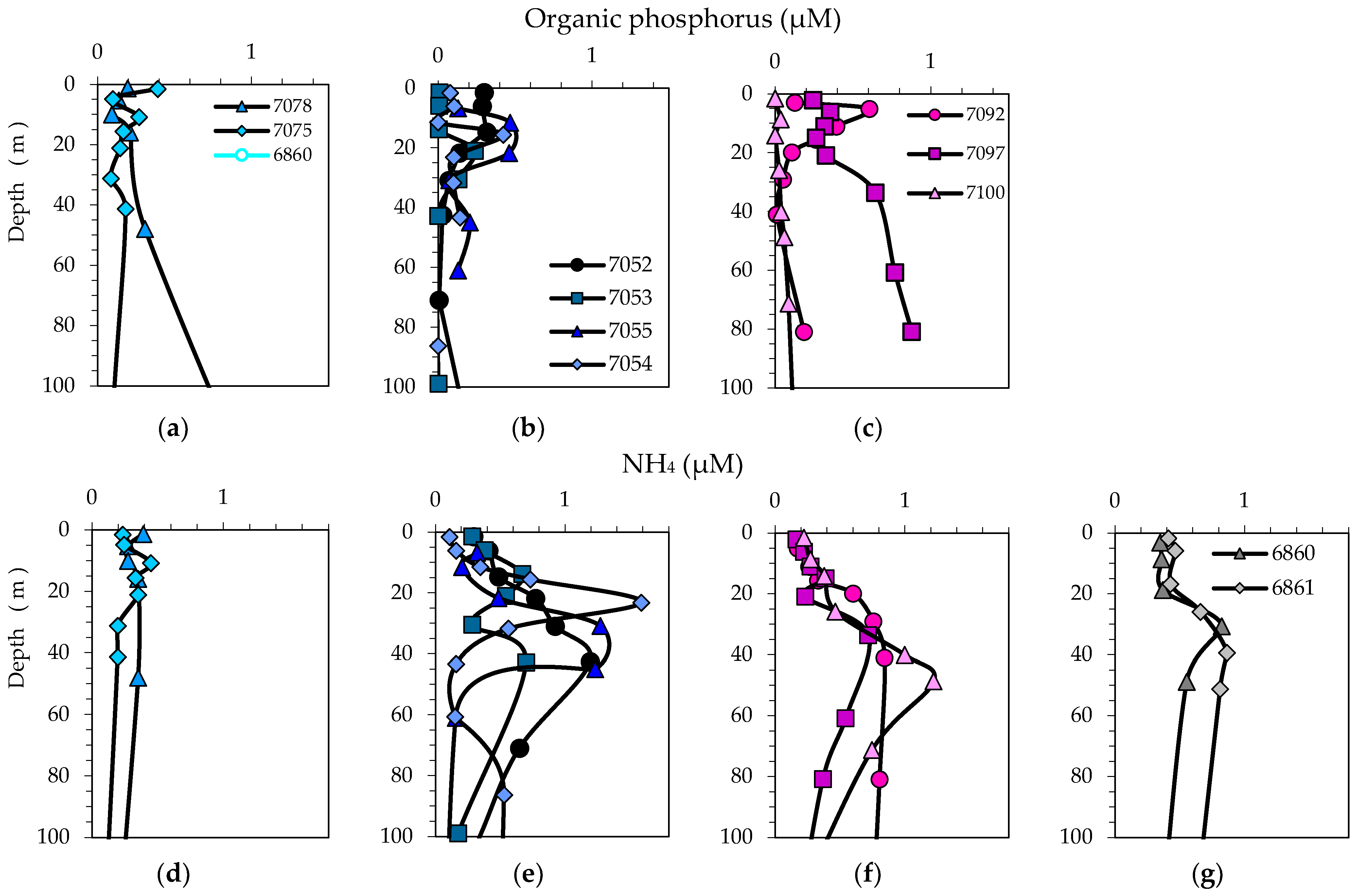
3.2. Physical, Optical and Chemical Conditions
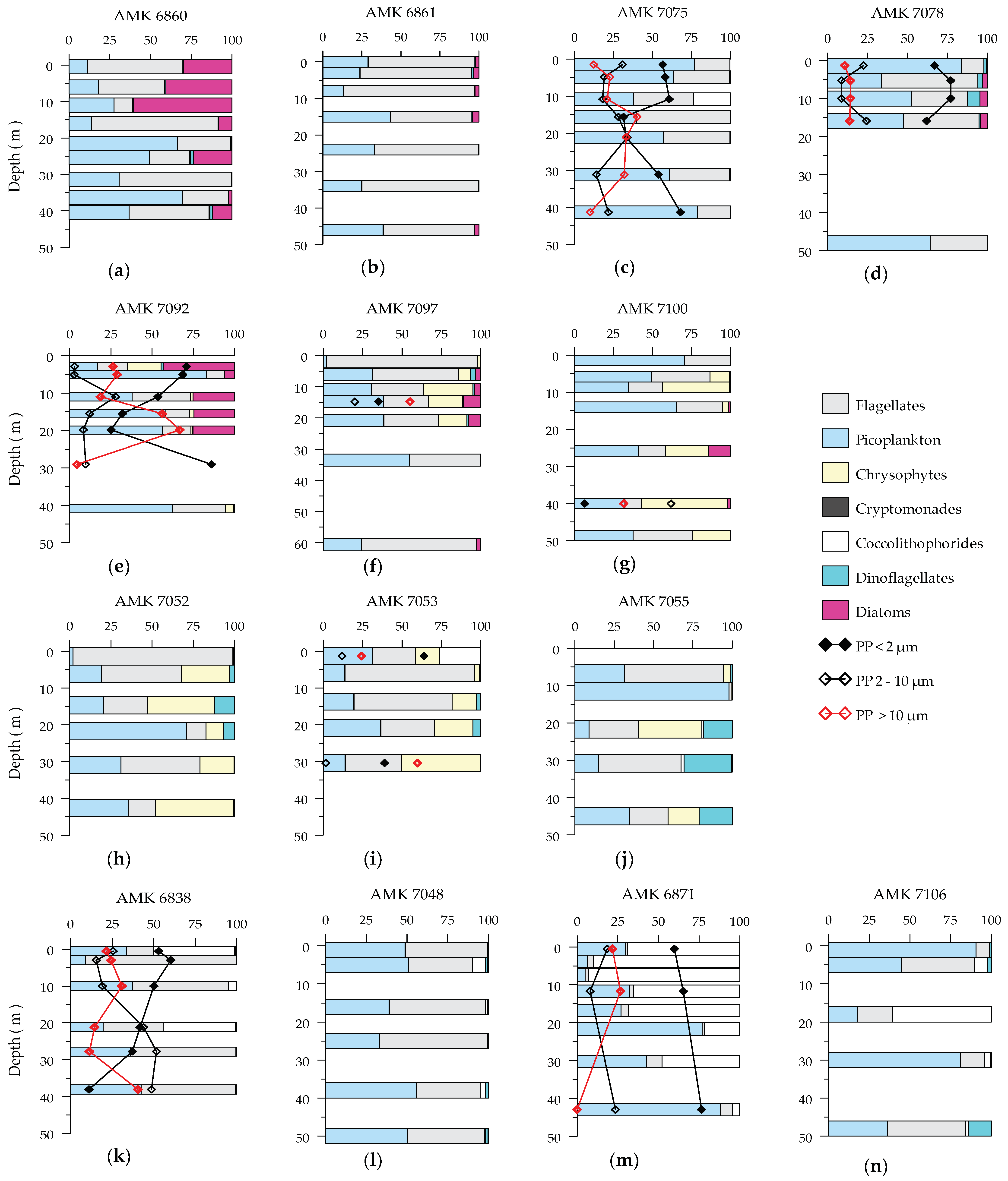
3.3. Phytoplankton Composition
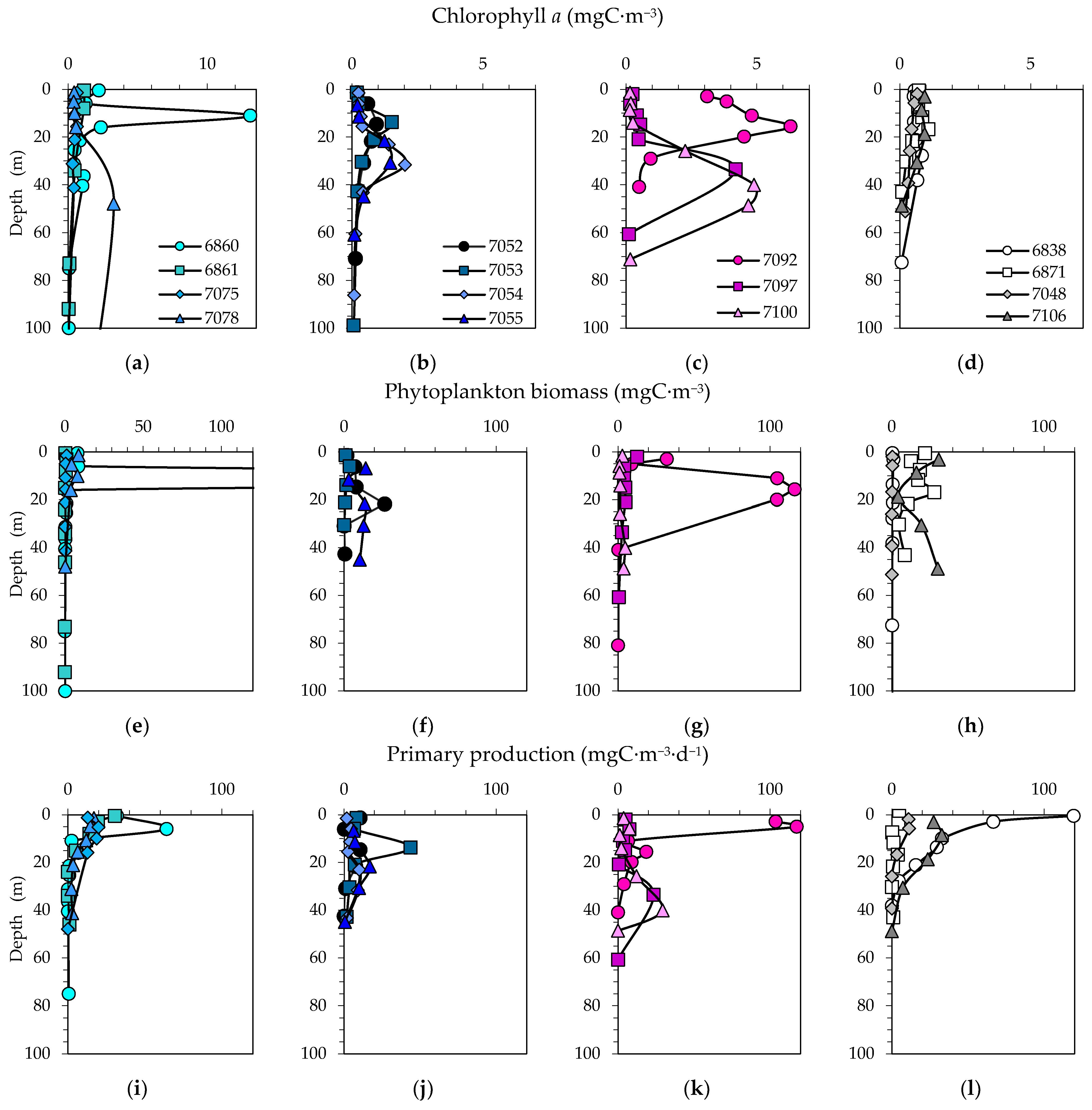
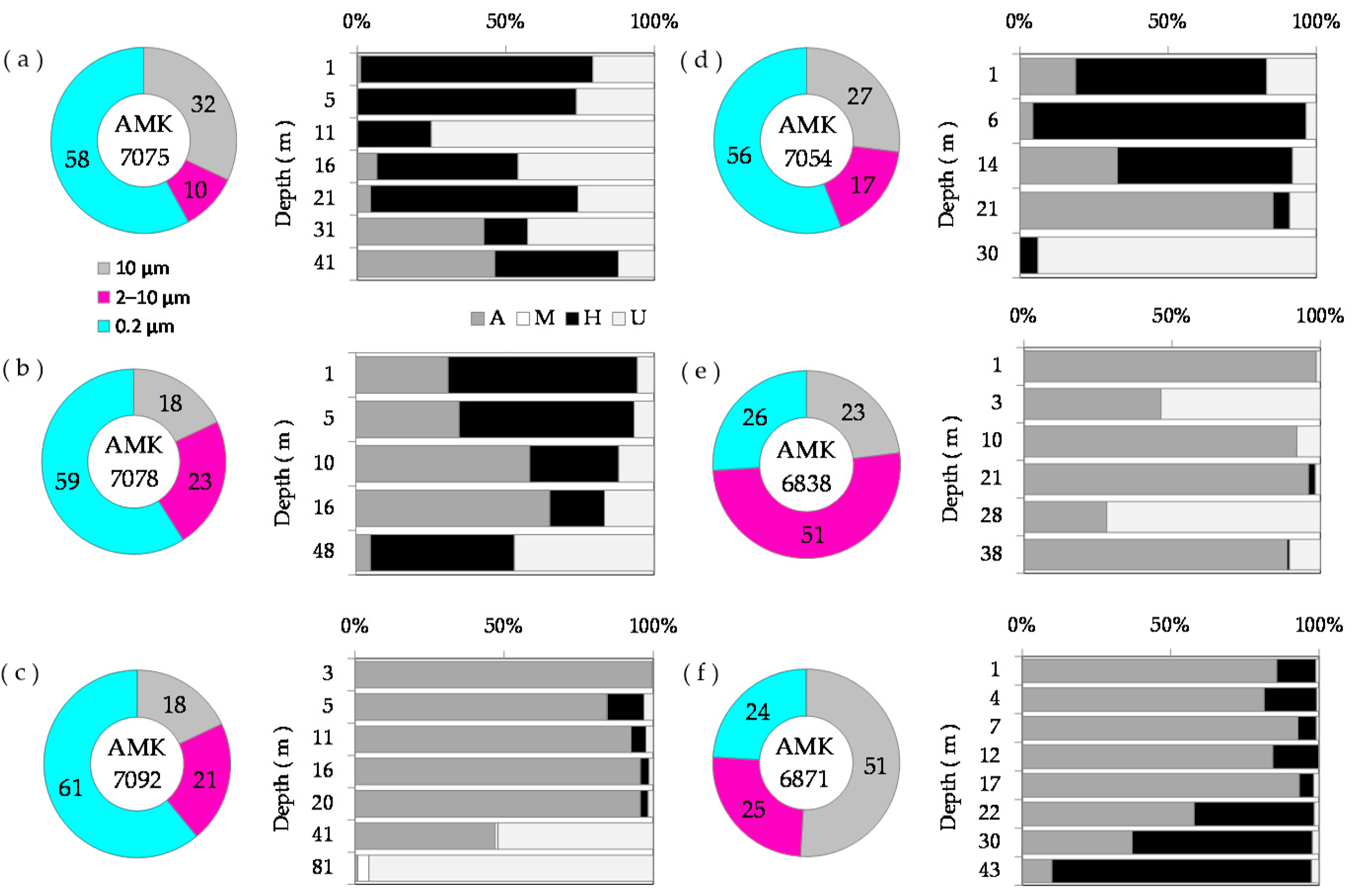
3.4. Size Distribution of Phytoplankton Carbon Biomass and Chlorophyll a
3.5. Primary Production and Grouping of Stations in Relation to Bloom Study
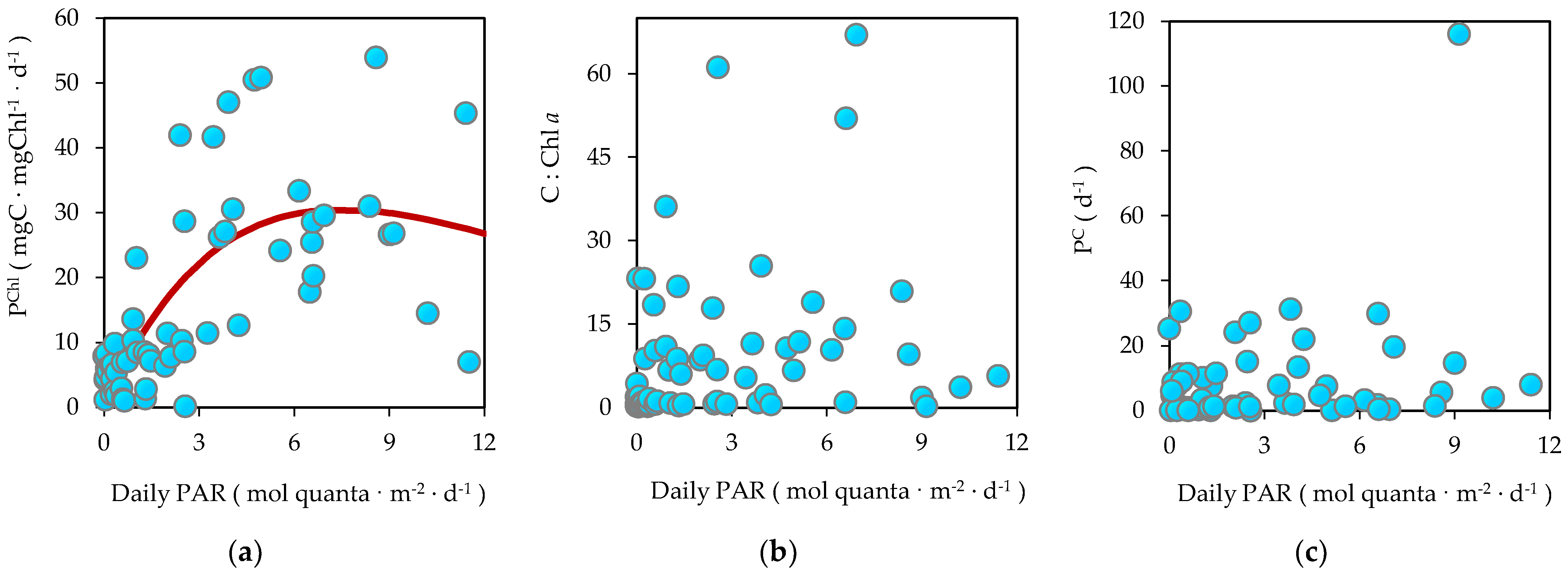
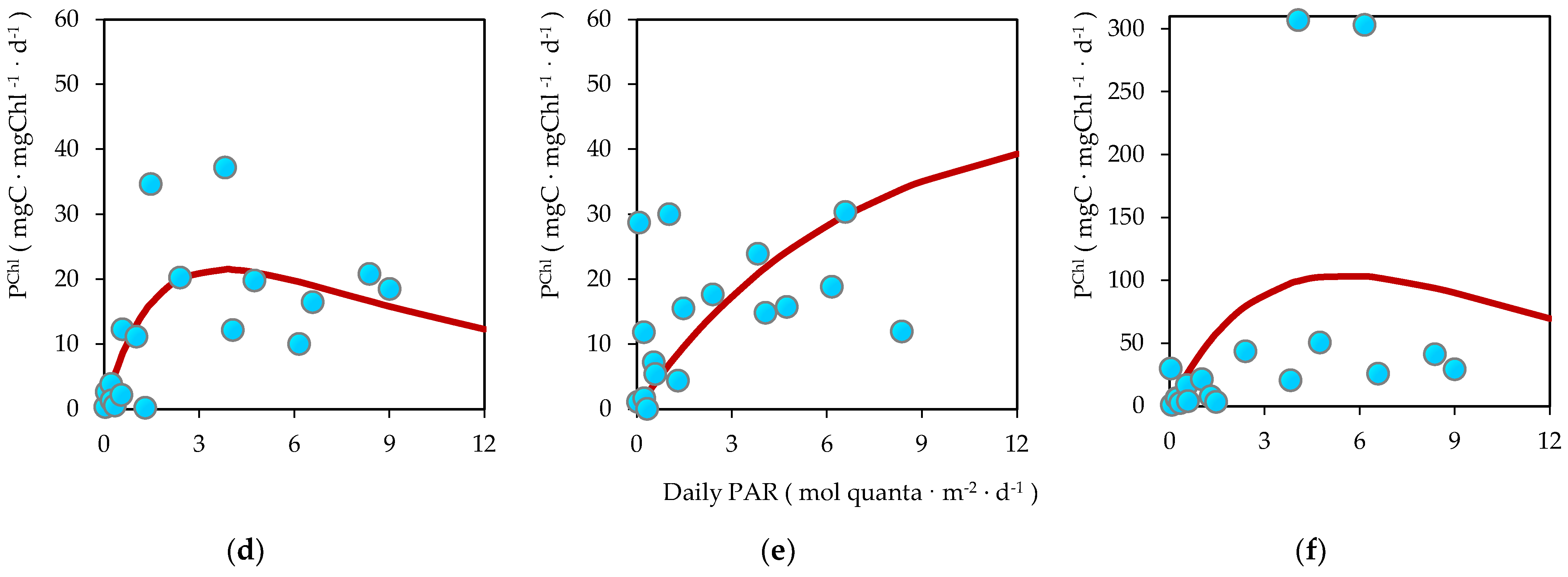
3.6. Biomass-Specific Carbon Assimilation
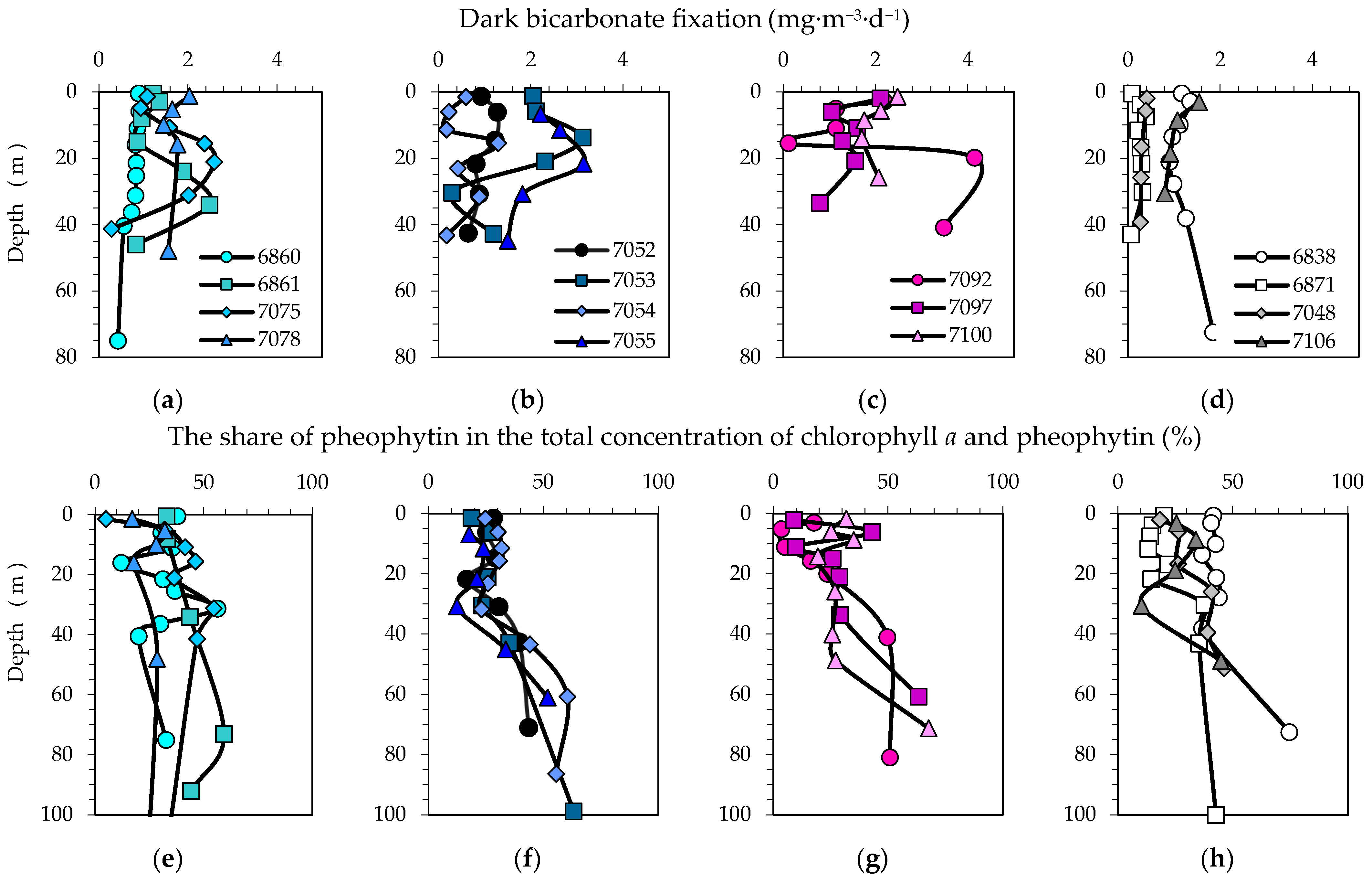
3.7. Bicarbonate Uptake in the Dark
4. Discussion
4.1. Size Distribution of Phytoplankton Chlorophyll a, Biomass and Primary Production Relative to Previous Studies
4.2. Phytoplankton Bloom Dominated by Small Phytoplankton in the MIZ
4.3. The Contribution Mixotrophs and Zooplankton to Ecosystem Dynamic
4.4. Dark Bicarbonate Fixation as a Bacterial Heterotrophic Production Indicator
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AMK | Akademik Mstislav Keldysh |
| Chl a | chlorophyll a |
| C:Chl a | carbon-to-chlorophyll a ratio |
| CTD | conductivity–temperature–depth |
| DCF | dark carbon fixation rate |
| DPM | disintegrations per minute |
| MIZ | marginal ice zone |
| PAR | photosynthetically active radiation |
| PB | phytoplankton carbon biomass |
| PChl | chlorophyll-specific particulate primary production rate at individual depths |
| PC | carbon-specific particulate primary production rate at individual depths |
| PP | particulate primary production at individual depths |
| PSU | practical salinity unit |
| SML | surface mixed layer depth |
| Zeu | euphotic zone |
References
- Kwok, R.; Rothrock, D.A. Decline in Arctic Sea ice thickness from submarine and ICES at records: 1958–2008. Geophys. Res. Lett. 2009, 36, L15501. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Pnyushkov, A.V.; Alkire, M.B.; Ashik, I.M.; Baumann, T.M.; Carmack, E.C.; Goszczko, I.; Guthrie, J.; Ivanov, V.V.; Kanzow, T.; et al. Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean. Science 2017, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R.; van Dijken, G.L. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 2015, 136, 60–70. [Google Scholar] [CrossRef]
- Hill, V.; Ardyna, M.; Lee, S.H.; Varela, D.E. Decadal trends in phytoplankton production in the Pacific Arctic Region from 1950 to 2012. Deep. Sea Res. Pt. II 2018, 152, 82–94. [Google Scholar] [CrossRef]
- Dalpadado, P.; Arrigo, K.R.; van Dijken, G.L.; Skjoldal, H.R.; Bagøien, E.; Dolgov, A.V.; Prokopchuk, I.P.; Sperfeld, E. Climate effects on temporal and spatial dynamics of phytoplankton and zooplankton in the Barents Sea. Prog. Oceanogr. 2020, 185, 102320. [Google Scholar] [CrossRef]
- Lewis, K.M.; van Dijken, G.L.; Arrigo, K.R. Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science 2020, 369, 198–202. [Google Scholar] [CrossRef]
- Ardyna, M.; Mundy, C.J.; Mayot, N.; Matthes, L.C.; Oziel, L.; Horvat, C.; Leu, E.; Assmy, P.; Hill, V.; Matrai, P.; et al. Under-Ice Phytoplankton Blooms: Shedding Light on the “Invisible” Part of Arctic Primary Production. Front. Mar. Sci. 2020, 7, 985. [Google Scholar] [CrossRef]
- Boles, E.; Provost, C.; Garçon, V.; Bertosio, C.; Athanase, M.; Koenig, Z.; Sennéchael, N. Under-ice phytoplankton blooms in the central Arctic Ocean: Insights from the first biogeochemical IAOOS platform drift in 2017. J. Geophys. Res. Oceans. 2020, 125, e2019JC015608. [Google Scholar] [CrossRef]
- Randelhoff, A.; Lacour, L.; Marec, C.; Leymarie, E.; Lagunas, J.; Xing, X.; Darnis, G.; Penkerc’h, C.; Sampei, M.; Fortier, L.; et al. Arctic mid-winter phytoplankton growth revealed by autonomous profilers. Sci. Adv. 2020, 6, eabc2678. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Hop, H.; Budgell, W.P.; Hegseth, E.N.; Korsnes, R.; Løyning, T.B.; Ørbæk, T.K.; Kawamura, T.; Shirasawa, K. Physical and ecological processes in the marginal ice zone of the northern Barents Sea during the summer melt periods. J. Mar. Syst. 2000, 27, 131–159. [Google Scholar] [CrossRef]
- Assmy, P.; Fernández-Méndez, M.; Duarte, P.; Meyer, A.; Randelhoff, A.; Mundy, C.J.; Olsen, L.M.; Kauko, H.M.; Bailey, A.; Chierici, M.; et al. Leads in Arctic pack ice enable early phytoplankton blooms below snow-covered sea ice. Sci. Rep. 2017, 7, 40850. [Google Scholar] [CrossRef]
- Strass, V.H.; Nöthig, E.M. Seasonal shifts in ice edge phytoplankton blooms in the Barents Sea related to the water column stability. Polar Biol. 1996, 16, 409–422. [Google Scholar] [CrossRef]
- Castellani, G.; Schaafsma, F.L.; Arndt, S.; Lange, B.A.; Peeken, I.; Ehrlich, J.; David, C.; Ricker, R.; Krumpen, T.; Hendricks, S.; et al. Large-scale variability of physical and biological sea-ice properties in polar oceans. Front. Mar. Sci. 2020, 7, 536. [Google Scholar] [CrossRef]
- Sakshaug, E.; Skjoldal, H.R. Life at the ice edge. Ambio 1989, 18, 60–67. [Google Scholar]
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Falkowski, P.G.; Laws, E.A.; Barber, R.T.; Murray, J.W. Phytoplankton and their role in primary, new, and export production. In Ocean Biogeochemistry. Global Change—The IGBP Series; Fasham, M.J.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 99–121. [Google Scholar]
- Krause, J.W.; Duarte, D.M.; Marquez, I.A.; Assmy, P.; Fernández-Méndez, M.; Wiedmann, I.; Wassman, P.; Kristiansen, S.; Agusty, S. Biogenic silica production and diatom dynamics in the Svalbard region during spring. Biogeosciences 2018, 15, 6503–6517. [Google Scholar] [CrossRef]
- Hátún, H.; Azetsu-Scott, K.; Somavilla, R.; Rey, F.; Johnson, C.; Mathis, M.; Mikolajewicz, U.; Coupel, P.; Tremblay, J.E.; Hartman, S.; et al. The subpolar gyre regulates silicate concentrations in the North Atlantic. Sci. Rep. 2017, 7, 14576. [Google Scholar] [CrossRef] [PubMed]
- Metfies, K.; von Appen, W.-J.; Kilias, E.; Nicolaus, A.; Nöthig, E.-M. Biogeography and Photosynthetic Biomass of Arctic Marine Pico-Eukaroytes during Summer of the Record Sea Ice Minimum. PLoS ONE 2012, 11, e0148512. [Google Scholar]
- Prashant, S.; Naresh, K.; Sagarika, P. Cyanobacteria in the polar regions: Diversity, adaptation, and taxonomic problems. In Understanding Present and Past Arctic Environments; Khare, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 189–212. [Google Scholar]
- Belevich, T.A.; Milyutina, I.A.; Abyzova, G.A.; Troitsky, A.V. The pico-sized Mamiellophyceae and a novel Bathycoccus clade from the summer plankton of Russian Arctic Seas and adjacent waters. FEMS Microbiol. Ecol. 2021, 97, fiaa251. [Google Scholar] [CrossRef]
- Klyuvitkin, A.A.; Politova, N.V.; Novigatsky, A.N.; Kravchishina, M.D. Studies of the European Arctic on cruise 80 of the RV Akademik Mstislav Keldysh. Oceanology 2021, 61, 139–141. [Google Scholar] [CrossRef]
- Kravchishina, M.D.; Klyuvitkin, A.A.; Volodin, V.D.; Glukhovets, D.I.; Dubinina, E.O.; Kruglinskii, I.A.; Kudryavtseva, E.A.; Matul, A.G.; Novichkova, E.A.; Politova, N.V.; et al. Systems Research of Sedimentation in European Arctic in the 84th Cruise of the Research Vessel Akademik Mstislav Keldysh. Oceanology 2022, 62, 572–574. [Google Scholar] [CrossRef]
- Grashoff, K.; Kremling, K.; Ehrhard, M. Methods of seawater analysis; Wiley-VCH Verlag GmbH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1999; p. 420. [Google Scholar]
- Hillebrand, H.; Durselen, C.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. Hels. Comm. Balt. Mar. Environ. Prot. Comm. 2000, 106, 144. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationship for dinoflagellates, diatom, and other protist plahkton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Tomas, C.R. (Ed.) Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997; p. 858. [Google Scholar]
- Throndsen, J.; Hasle, G.R.; Tangen, K. Norsk Kystplankton Flora; Almater Forlag AS (Norwegian): Oslo, Norway, 2003; p. 341. [Google Scholar]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a determination: Improvements in methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Steemann-Nielsen, E. The use of radio-active carbon (14C) for measuring organic production in the sea. J. Du Cons. Cons. Perm. Int. Pour L’exploration De La Mer 1952, 18, 117–140. [Google Scholar] [CrossRef]
- Jerlov, N.G. Marine Optics; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Kudryavtseva, E.; Aleksandrov, S.; Bukanova, T.; Dmitrieva, O.; Rusanov, I. Relationship between Seasonal Variations of Primary Production, Abiotic Factors and Phytoplankton Composition in the Coastal Zone of the South-eastern part of the Baltic Sea. Reg. Stud. Mar. Sci. 2019, 32, 100862. [Google Scholar] [CrossRef]
- Zdun, A.; Stoń-Egiert, J.; Ficek, D.; Ostrowska, M. Seasonal and Spatial Changes of Primary Production in the Baltic Sea (Europe) Based on in situ Measurements in the Period of 1993–2018. Front. Mar. Sci. 2021, 7, 604532. [Google Scholar] [CrossRef]
- Kudryavtseva, E.A.; Bukanova, T.V. Estimation of primary production for the southeastern Baltic Sea from chlorophyll a concentration and water column photosynthetic parameters. In Proceedings of the 28th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Tomsk, Russia, 4–8 July 2022; p. 123414R. [Google Scholar]
- Kirchman, D.L. Calculating microbial growth rates from data on production and standing stocks. Mar. Ecol. Prog. Ser. 2002, 233, 303–306. [Google Scholar] [CrossRef]
- Marañón, E.; Van Wambeke, F.; Uitz, J.; Boss, E.S.; Pérez-Lorenzo, M.; Dinasquet, J.; Haëntjens, N.; Dimier, C.; Taillandier, V. Deep maxima of phytoplankton biomass, primary production and bacterial production in the Mediterranean Sea during late spring. Biogeosciences 2021, 18, 1749–1767. [Google Scholar] [CrossRef]
- Boss, E.; Behrenfeld, M. In situ evaluation of the initiation of the North Atlantic phytoplankton bloom. Geophys. Res. Lett. 2010, 37, L18603. [Google Scholar] [CrossRef]
- Horvat, C.; Jones, D.R.; Iams, S.; Schroeder, D.; Flocco, D.; Feltham, D. The frequency and extent of sub-ice phytoplankton blooms in the Arctic Ocean. Sci. Adv. 2017, 3, e1601191. [Google Scholar] [CrossRef]
- Kudryavtseva, E.A.; Kravchishina, M.D.; Pautova, L.A.; Rusanov, I.I.; Silkin, V.A.; Glukhovets, D.I.; Torgunova, N.I.; Netsvetaeva, O.P.; Politova, N.V.; Klyuvitkin, A.A.; et al. Size Structure of Primary Producers in the Marginal Ice Zone of the European Arctic in Summer. Dokl. Earth Sci. 2022, 507, S313–S318. [Google Scholar] [CrossRef]
- Pautova, L.; Silkin, V.; Kravchishina, M.; Klyuvitkin, A.; Kudryavtseva, E.; Glukhovets, D.; Chultsova, A.; Politova, N. Phytoplankton of the High-Latitude Arctic: Intensive Growth Large Diatoms Porosira glacialis in the Nansen Basin. J. Mar. Sci. Eng. 2023, 11, 453. [Google Scholar] [CrossRef]
- Sundström, B.G. Observations on Rhizosolenia clevei Ostenfeld (Bacillariophyceae) and Richelia intracellularis Schmidt (Cyanophyceae). Bot. Mar. 1984, 27, 345–355. [Google Scholar] [CrossRef]
- Sundström, B.G. The Masrine Genus Rhizosolenia. Ph.D. Thesis, Lund University, Lund, Sweden, 1986; p. 117.
- Padmakumar, K.B.; Menon, N.R.; Sanjeevan, V.N. Occurrence of endosymbiont Richelia intracellularis (Cyanophyta) within the diatom Rhizosolenia hebetata in Northern Arabian Sea. Int. J. Biodivers. Conserv. 2010, 2, 70–74. [Google Scholar]
- Jabir, T.; Dhanya, V.; Jesmi, Y.; Prabhakaran, M.P.; Saravanane, N.; Gupta, G.V.M.; Hatha, A.A.M. Occurrence and Distribution of a Diatom-Diazotrophic Cyanobacteria Association during a Trichodesmium Bloom in the Southeastern Arabian Sea. Int. J. Oceanogr. 2013, 2013, 350594. [Google Scholar] [CrossRef]
- Madhu, N.V.; Meenu, P.; Ullas, N.; Ashwini, R.; Rehitha, T.V. Occurrence of cyanobacteria (Richelia intracellularis)-diatom (Rhizosolenia hebetata) consortium in the Palk Bay, southeast coast of India. Indian J. Geo Mar. Sci. 2013, 42, 453–457. [Google Scholar]
- Platt, T.; Gallegos, C.L. Modelling primary production. In Primary Productivity in the Sea; Falkowski, P.G., Ed.; Plenum Press: New York, NY, USA, 1980; pp. 339–351. [Google Scholar]
- Hodal, H.; Kristiansen, S. The importance of small-celled phytoplankton in spring blooms at the marginal ice zone in the northern Barents Sea. Deep Sea Res. II 2008, 55, 2176–2185. [Google Scholar] [CrossRef]
- Smith, W.O.; Bauman, M.E.M.; Wilson, D.L.; Aletsee, L. Phytoplankton biomass and productivity in the Marginal Ice Zone of the Fram Strait during Summer 1984. J. Geophys. Res. 1987, 92, 6777–6786. [Google Scholar] [CrossRef]
- Vernet, M.; Matrai, P.A.; Andreassen, I. Synthesis of particulate and extracellular carbon by phytoplankton at the marginal ice zone in the Barents Sea. J. Geophys. Res. Ocean. 1999, 103, 1023–1037. [Google Scholar] [CrossRef]
- Makarevich, P.R.; Larionov, V.V.; Vodopyanova, V.V.; Bulavina, A.S.; Ishkulova, T.G.; Venger, M.P.; Pastukhov, I.A.; Vashchenko, A.V. Phytoplankton of the Barents Sea at the Polar Front in Spring. Oceanology 2021, 61, 930–943. [Google Scholar] [CrossRef]
- Croteau, D.; Guérin, S.; Bruyant, F.; Ferland, J.; Campbell, D.A.; Babin, M.; Lavaud, J. Contrasting nonphotochemical quenching patterns under high light and darkness aligns with light niche occupancy in Arctic diatoms. Limnol. Oceanogr. 2020, 66, S231–S245. [Google Scholar] [CrossRef]
- Mei, Z.P.; Legendre, L.; Gratton, Y.; Tremblay, J.E.; LeBlanc, B.; Klein, B.; Gosselin, M. Phytoplankton production in the North Water Polynya: Size-fractions and carbon fluxes, April–July 1998. Mar. Ecol. Prog. Ser. 2003, 256, 13–27. [Google Scholar] [CrossRef]
- Spilling, K.; Markager, S. Ecophysiological growth characteristics and modeling of the onset of the spring bloom in the Baltic Sea. J. Mar. Syst. 2008, 73, 323–337. [Google Scholar] [CrossRef]
- Marañón, E. Cell Size as a Key Determinant of Phytoplankton Metabolism and Community Structure. Annu. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef]
- Okolodkov, Y. An ice-bound planktonic dinoflagellate Peridiniella catenata (Levander) Balech: Morphology, ecology and distribution. Bot. Mar. 1999, 42, 333–341. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Li, A.; Coats, D.W.; Gustafson, D.E.; Nannen, M.K. Mixotrophy in the dinoflagellate Prorocentrum minimum. Mar. Ecol. Prog. Ser. 1997, 152, 1–12. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.; Glibert, P.M.; Ahn, S.H.S. A three-dimensional mechanistic model of Prorocentrum minimum blooms in eutrophic Chesapeake Bay. Sci. Total Environ. 2021, 769, 144528. [Google Scholar] [CrossRef]
- Ahme, A.; Von Jackowski, A.; McPherson, R.A.; Wolf, K.K.E.; Hoppmann, M.; Neuhaus, S.; John, U. Winners and Losers of Atlantification: The Degree of Ocean Warming Affects the Structure of Arctic Microbial Communities. Genes 2023, 14, 623. [Google Scholar] [CrossRef]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 41063–41085. [Google Scholar]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Krisch, S.; Hopwood, M.J.; Roig, S.; Gerringa, L.J.A.; Middag, R.; Rutgers van der Loeff, M.M.; Petrova, M.V.; Lodeiro, P.; Colombo, M.; Cullen, J.N.; et al. Arctic—Atlantic Exchange of the Dissolved Micronutrients Iron, Manganese, Cobalt, Nickel, Copper and Zinc with a Focus on Fram Strait. Glob. Biogeochem. Cycles 2022, 36, e2021GB007191. [Google Scholar] [CrossRef]
- Krause, J.; Hopwood, M.J.; Höfer, J.; Krisch, S.; Achterberg, E.P.; Alarcón, E.; Carroll, D.; González, H.E.; Juul-Pedersen, T.; Liu, T.; et al. Trace Element (Fe, Co, Ni and Cu) Dynamics across the Salinity Gradient in Arctic and Antarctic Glacier Fjords. Front. Earth Sci. 2012, 9, 725279. [Google Scholar] [CrossRef]
- Joli, N.; Ardyna, M. Need for focus on microbial species following ice melt and changing freshwater regimes in a Janus Arctic Gateway. Sci. Rep. 2018, 8, 9405. [Google Scholar] [CrossRef]
- Brzezinski, M.A.; Closset, I.; Jones, J.L.; de Souza, G.F.; Maden, C. New Constraints on the Physical and Biological Controls on the Silicon Isotopic Composition of the Arctic Ocean. Front. Mar. Sci. 2021, 8, 699762. [Google Scholar] [CrossRef]
- Krisch, S.; Browning, T.G.; Graeve, M.; Ludwichowski, K.U.; Lodeiro, P.; Hopwood, M.J.; Roig, S.; Yong, J.C.; Kanzow, T.; Achterberg, E.P. The influence of Arctic Fe and Atlantic fixed N on summertime primary production in Fram Strait, North Greenland Sea. Sci. Rep. 2020, 10, 15230. [Google Scholar] [CrossRef]
- Duarte, P.; Meyer, A.; Moreau, S. Nutrients in water masses in the Atlantic sector of the Arctic Ocean: Temporal trends, mixing and links with primary production. J. Geophys. Res. Ocean. 2021, 126, e2021JC017413. [Google Scholar] [CrossRef]
- Chappell, P.; Whitney, L.A.; Haddock, T.; Menden-Deuer, S.; Roy, E.; Wells, M.; Jenkins, B. Thalassiosira, Iron, temperature, Haida Eddy, community composition, ARISA. Front. Microbiol. 2013, 4, 273. [Google Scholar]
- Polukhin, A.; Makkaveev, P.; Miroshnikov, A.; Borisenko, G.; Khlebopashev, P. Leaching of inorganic carbon and nutrients from rocks of the Arctic archipelagos (Novaya Zemlya and Svalbard), Russ. J. Earth. Sci. 2021, 21, ES4002. [Google Scholar] [CrossRef]
- Marson, J.M.; Myers, P.G.; Hu, X.; Le Sommer, J. Using vertically integrated ocean fields to characterize Greenland icebergs’ distribution and lifetime. Geophys. Res. Lett. 2018, 45, 4208–4217. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Thomsen, H. Dictyocha speculum (Silicoflagellata, Dictyochophyceae), studies on armoured and unarmoured stages. Biol. Ski. 1990, 37, 1–56. [Google Scholar]
- Henriksen, P.; Knipschildt, F.; Moestrup, Ø.; Thomsen, H. Autecology, life history and toxicology of the silicoflagellate Dictyocha speculum (Silicoflagellata, Dictyochophyceae). Artic. Phycol. 1993, 32, 29–39. [Google Scholar] [CrossRef]
- Grill, E.V.; Richards, F.A. Nutrient regeneration from phytoplankton decomposing in seawater. J. Mar. Res. 1964, 22, 51–69. [Google Scholar]
- Vernet, M.; Ellingsen, I.H.; Seuthe, L.; Slagstad, D.; Cape, M.R.; Matrai, P.A. Influence of Phytoplankton Advection on the Productivity along the Atlantic Water Inflow to the Arctic Ocean. Front. Mar. Sci. 2019, 6, 583. [Google Scholar] [CrossRef]
- Wietz, M.; Bienhold, C.; Metfies, K.; Torres-Valdés, S.; von Appen, W.J.; Salter, I.; Boetius, A. The polar night shift: Seasonal dynamics and drivers of Arctic Ocean microbiomes revealed by autonomous sampling. ISME Commun. 2021, 1, 76. [Google Scholar] [CrossRef]
- Orkney, A.; Davidson, K.; Mitchell, E.; Henley, S.F.; Bouman, H.A. Different Observational Methods and the Detection of Seasonal and Atlantic Influence upon Phytoplankton Communities in the Western Barents Sea. Front. Mar. Sci. 2022, 9, 860773. [Google Scholar] [CrossRef]
- Zhang, F.; He, J.; Lin, L.; Jin, H. Dominance of picophytoplankton in the newly open surface water of the central Arctic Ocean. Polar Biol. 2015, 38, 1081–1089. [Google Scholar] [CrossRef]
- Reynolds, C. The Ecology of Phytoplankton (Ecology, Biodiversity and Conservation); Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Spilling, K.; Fuentes-Lema, A.; Quemaliños, D.; Klais, R.; Sobrino, K. Primary production, carbon release, and respiration during spring bloom in the Baltic Sea. Limnol. Oceanogr. 2019, 64, 1779–1789. [Google Scholar] [CrossRef]
- Morel, A.; Bricaud, A. Theoretical results concerning light-absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep. Sea Res. I 1981, 28, 1375–1393. [Google Scholar] [CrossRef]
- Glover, H.E.; Keller, M.D.; Guillard, R.R.L. Light quality and oceanic ultraphytoplankters. Nature 1986, 319, 142–143. [Google Scholar] [CrossRef]
- Hegseth, E.N. Photoadaptation in marine arctic diatoms. Polar Biol. 1989, 9, 479–486. [Google Scholar] [CrossRef]
- McNair, H.M.; Brzezinski, M.A.; Till, C.P.; Krause, J.W. Taxon-specific contributions to silica production in natural diatom assemblages. Limnol. Oceanogr. 2018, 63, 1056–1075. [Google Scholar] [CrossRef] [PubMed]
- Freyria, N.J.; Joli, N.; Lovejoy, C. A decadal perspective on north water microbial eukaryotes as Arctic Ocean sentinels. Sci. Rep. 2021, 11, 8413. [Google Scholar] [CrossRef]
- Bachy, C.; López-García, P.; Vereshchaka, A.; Moreira, D. Diversity and vertical distribution of microbial eukaryotes in the snow, sea ice and seawater near the North Pole at the end of the polar night. Front. Microbiol. 2011, 2, 106. [Google Scholar] [CrossRef]
- Tragin, M.; Vaulot, D. Novel diversity within marine Mamiellophyceae (Chlorophyta) unveiled by metabarcoding. Sci. Rep. 2019, 9, 5190. [Google Scholar] [CrossRef]
- Hillebrand, H.; Acevedo-Trejos, E.; Moorthi, S.D.; Ryabov, A.; Striebel, M.; Thomas, P.K.; Schneider, M.-L. Cell size as driver and sentinel of phytoplankton community structure and functioning. Funct. Ecol. 2022, 36, 276–293. [Google Scholar] [CrossRef]
- Dabrowska, A.M.; Wiktor, J.M., Jr.; Merchel, M.; Wiktor, J.M. Planktonic Protists of the Eastern Nordic Seas and the Fram Strait: Spatial Changes Related to Hydrography during Early Summer. Front. Mar. Sci. 2020, 7, 557. [Google Scholar] [CrossRef]
- Iversen, K.; Seuthe, L. Seasonal microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): I. Heterotrophic bacteria, picoplankton and nanofagellates. Polar Biol. 2011, 34, 731–749. [Google Scholar] [CrossRef]
- Hodal, H.; Falk-Petersen, S.; Hop, H.; Kristiansen, S. Marit Reigstad Spring bloom dynamics in Kongsfjorden, Svalbard: Nutrients, phytoplankton, protozoans and primary production. Polar Biol. 2012, 35, 191–203. [Google Scholar] [CrossRef]
- Verity, P.G.; Wassmann, P.; Frischer, M.E.; Howard-Jones, M.H.; Allen, A.E. Grazing of phytoplankton by microzooplankton in the Barents Sea during early summer. J. Mar. Syst. 2002, 38, 109–123. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Eriksen, E.; Gjøsæter, H. Size-fractioned zooplankton biomass in the Barents Sea: Spatial patterns and temporal variations during three decades of warming and strong fluctuations of the capelin stock (1989–2020). Prog. Oceanogr. 2022, 206, 102852. [Google Scholar] [CrossRef]
- Hasle, G.R. Some Thalassiosira species with one central process (Bacillariophyceae). Norw. J. Bot. 1978, 25, 77–110. [Google Scholar]
- Sar, E.A.; Sunesen, I.; Lavigne, A.S.; Lofeudo, S. Thalassiosira rotula, a heterotypic synonym of Thalassiosira gravida: Morphological evidence. Diatom Res. 2011, 26, 109. [Google Scholar] [CrossRef]
- Druzhkova, E.I.; Ishkulova, T.G.; Pastukhov, I.A. Features of summer ice-edge bloom in the Barents Sea. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 539, p. 012186. [Google Scholar]
- Crawford, D.W. Mesodinium rubrum: The phytoplankter that wasn’t. In Marine Ecology Progress Series; Oldendorff Carriers: Lübeck, Germany, 1989; Volume 58, pp. 161–174. [Google Scholar]
- Trudnowska, E.; Dąbrowska, A.M.; Boehnke, R.; Zajączkowski, M.; Blachowiak-Samolyk, K. Particles, protists, and zooplankton in glacier-influenced coastal Svalbard waters. Estuar. Coast. Shelf Sci. 2020, 242, 106842. [Google Scholar] [CrossRef]
- Johnson, M.D.; Beaudoin, D.J. The genetic diversity of plastids associated with mixotrophic oligotrich ciliates. Limnol. Oceanogr. 2019, 64, 2187–2201. [Google Scholar] [CrossRef]
- Johnson, M.D.; Tengs, T.; Oldach, D.; Stoecker, D.K. Sequestration, performance and functional control of cryptophyte plastids in the ciliate Myrionecta rubra (CILIOPHORA). J. Phycol. 2006, 42, 1235–1246. [Google Scholar] [CrossRef]
- Raj, R.P.; Johannessen, J.A.; Eldevik, T.; Nilsen, J.E.Ø.; Halo, I. Quantifying mesoscale eddies in the Lofoten Basin. J. Geophys. Res. Ocean. 2016, 121, 4503–4521. [Google Scholar] [CrossRef]
- Skogen, M.D.; Budgell, W.P.; Rey, F. Interannual variability in Nordic seas primary production. ICES J. Mar. Sci. 2007, 64, 889–898. [Google Scholar] [CrossRef]
- Wiborg, K.F. Zooplankton in relation to hydrography in the Norwegian Sea. Rep. Norw. Fish. Mar. Investig. 1955, 11, 66. [Google Scholar]
- ICES. Working Group on the Integrated Assessments of the Norwegian Sea (WGINOR, Outputs from 2022 Meeting); ICES Scientific Reports; ICES: Copenhagen, Denmark, 2023; Volume 5, 57p, p. 15. [Google Scholar]
- Von Bodungen, B.; Antia, A.; Bauerfeind, E.; Haupt, O.; Koeve, W.; Machado, E.; Peeken, I.; Peinert, R.; Reitmeier, S.; Thomsen, C.; et al. Pelagic processes and vertical flux of particles: An overview of a long-term comparative study in the Norwegian Sea and Greenland Sea. Geol. Rundsch. 1995, 84, 11–27. [Google Scholar] [CrossRef]
- Drits, A.V.; Klyuvitkin, A.A.; Kravchishina, M.D.; Novigatsky, A.N.; Karmanov, V.A. Fluxes of sedimentary material in the Lofoten Basin of the Norwegian Sea: Seasonal dynamics and the role of zooplankton. Oceanology 2020, 60, 501–517. [Google Scholar] [CrossRef]
- Klyuvitkin, A.A.; Kravchishina, M.D.; Novigatsky, A.N.; Politova, N.V.; Bulokhov, A.V.; Gulev, S.K. First Data on Vertical Particle Fluxes and Environmental Conditions in the Northern Segment of the Mohns Ridge, Norwegian Sea. In Doklady Earth Sciences; 2023; Available online: https://link.springer.com/article/10.1134/S1028334X23601840 (accessed on 30 October 2023).
- Planque, B.; Favreau, A.; Husson, B.; Mousing, E.A.; Hansen, C.; Broms, C.; Lindstrøm, U.; Sivel, E. Quantification of trophic interactions in the Norwegian Sea pelagic food-web over multiple decades. ICES J. Mar. Sci. 2022, 79, 1815–1830. [Google Scholar] [CrossRef]
- Oziel, L.; Baudena, A.; Ardyna, M.; Massicotte, M.; Randelhoff, A.; Sallée, J.-B.; Ingvaldsen, R.B.; Devred, E.; Babin, M. Faster Atlantic currents drive poleward expansion of temperate phytoplankton in the Arctic Ocean. Nat. Commun. 2020, 11, 1705. [Google Scholar] [CrossRef] [PubMed]
- Silkin, V.; Pautova, L.; Giordano, M.; Kravchishina, M.; Artemiev, V. Interannual variability of Emiliania huxleyi blooms in the Barents Sea: In situ data 2014–2018. Mar. Poll. Bull. 2020, 158, 111392. [Google Scholar] [CrossRef] [PubMed]
- Dylmer, C.V.; Giraudeau, J.; Hanquiez, V.; Husum, K. The coccolithophores Emiliania huxleyi and Coccolithus pelagicus: Extant populations from the Norwegian–Iceland Seas and Fram Strait. Deep Sea Res. I 2015, 98, 1–9. [Google Scholar] [CrossRef]
- Piwosz, K. Weekly dynamics of abundance and size structure of specific nanophytoplankton lineages in coastal waters (Baltic Sea). Limnol. Oceanogr. 2019, 64, 2172–2186. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Efimova, K.V.; Stonik, I.V. Morphology and molecular phylogeny of Pseudohaptolina sorokinii sp. nov. (Prymnesiales, Haptophyta) from the Sea of Japan, Russia. Phycologia 2016, 55, 506–514. [Google Scholar] [CrossRef]
- Marañón, E.; Gonzalez, N. Primary production, calcification and macromolecular synthesis in a bloom of the coccolithophore Emiliania huxleyi in the North Sea. Mar. Ecol. Progr. Ser. 1997, 157, 61–77. [Google Scholar] [CrossRef]
- Nanninga, H.J.; Tyrrell, T. Importance of light for the formation of algal blooms of Emiliania huxleyi. Mar. Ecol. Prog. Ser. 1996, 136, 195–203. [Google Scholar] [CrossRef]
- Poulton, A.J.; Painter, S.C.; Young, J.R.; Bates, N.R.; Bowler, B.; Drapeau, D.; Lyczsckowski, E.; Balch, W.M. The 2008 Emiliania huxleyi bloom along the patagonian shelf: Ecology, biogeochemistry, and cellular calcification. Glob. Biogeochem. Cycles 2013, 27, 1023–1033. [Google Scholar] [CrossRef]
- Harris, R.P. Zooplankton grazing on the coccolithophore Emiliania huxleyi and its role in inorganic carbon flux. Mar. Biol. 1994, 119, 431–439. [Google Scholar] [CrossRef]
- Mayers, K.M.J.; Poulton, A.J.; Bidle, K.; Thamatrakoln, K.; Schieler, B.; Giering, S.L.C.; Wells, S.R.; Tarran, G.A.; Widdicombe, C.E.; Mayor, D.J.; et al. Growth and mortality of coccolithophores during spring in a temperate Shelf Sea (Celtic Sea, April 2015). Prog. Oceanogr. 2019, 177, 101928. [Google Scholar] [CrossRef]
- Mayor, D.; Johnson, M.; Riebesell, U.; Larsen, A.; Vardi, A.; Harvey, E.L. The Possession of Coccoliths Fails to Deter Microzooplankton. Grazers. Front. Mar. Sci. 2020, 7, 569896. [Google Scholar]
- Cole, J.J.; Findlay, S.; Pace, M.L. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Mar. Ecol. Prog. Ser. 1988, 43, 1–10. [Google Scholar] [CrossRef]
- Alonso-Sáez, L.; Galand, P.E.; Casamayor, E.O.; Alio, C.P.; Bertilsson, S. High bicarbonate assimilation in the dark by Arctic bacteria. ISME J. 2010, 4, 1581–1590. [Google Scholar] [CrossRef]
- Viviani, D.A.; Church, M.J. Decoupling between bacterial production and primary production over multiple time scales in the North Pacific Subtropical Gyre. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 121, 132–142. [Google Scholar] [CrossRef]
- Savvichev, A.S.; Rusanov, I.I.; Pimenov, N.V.; Mitskevich, I.N.; Bairamov, I.T.; Lein, A.Y.; Ivanov, M.V. Microbiological explorations in the northern part of the Barents Sea in early winter. Microbiology 2000, 6, 698–708. [Google Scholar] [CrossRef]
- Cota, G.F.; Kottmeier, S.T.; Robinson, D.H.; Smith, W.O.; Sullivan, C.W. Bacterioplankton in the marginal ice zone of the Weddell Sea: Biomass, production and metabolic activities during austral autumn. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1145–1167. [Google Scholar] [CrossRef]
- Spilling, K.; Camarena-Gómez, M.T.; Lipsewers, T.; Martínez, A.; Díaz, F.; Eronen-Rasimus, E.; Silva, N.; von Dassow, P.; Montecino, V. Impacts of reduced inorganic N:P ratio on three distinct plankton communities in the Humboldt upwelling system. Mar. Biol. 2019, 166, 114. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtseva, E.; Kravchishina, M.; Pautova, L.; Rusanov, I.; Glukhovets, D.; Shchuka, A.; Zamyatin, I.; Torgunova, N.; Chultsova, A.; Politova, N.; et al. Sea Ice as a Factor of Primary Production in the European Arctic: Phytoplankton Size Classes and Carbon Fluxes. J. Mar. Sci. Eng. 2023, 11, 2131. https://doi.org/10.3390/jmse11112131
Kudryavtseva E, Kravchishina M, Pautova L, Rusanov I, Glukhovets D, Shchuka A, Zamyatin I, Torgunova N, Chultsova A, Politova N, et al. Sea Ice as a Factor of Primary Production in the European Arctic: Phytoplankton Size Classes and Carbon Fluxes. Journal of Marine Science and Engineering. 2023; 11(11):2131. https://doi.org/10.3390/jmse11112131
Chicago/Turabian StyleKudryavtseva, Elena, Marina Kravchishina, Larisa Pautova, Igor Rusanov, Dmitry Glukhovets, Alexander Shchuka, Ivan Zamyatin, Nadezhda Torgunova, Anna Chultsova, Nadezhda Politova, and et al. 2023. "Sea Ice as a Factor of Primary Production in the European Arctic: Phytoplankton Size Classes and Carbon Fluxes" Journal of Marine Science and Engineering 11, no. 11: 2131. https://doi.org/10.3390/jmse11112131
APA StyleKudryavtseva, E., Kravchishina, M., Pautova, L., Rusanov, I., Glukhovets, D., Shchuka, A., Zamyatin, I., Torgunova, N., Chultsova, A., Politova, N., & Savvichev, A. (2023). Sea Ice as a Factor of Primary Production in the European Arctic: Phytoplankton Size Classes and Carbon Fluxes. Journal of Marine Science and Engineering, 11(11), 2131. https://doi.org/10.3390/jmse11112131








