Experimental Study on High-Efficiency Cyclic CO2 Capture from Marine Exhaust by Transition-Metal-Modified CaO/Y2O3 Adsorbent
Abstract
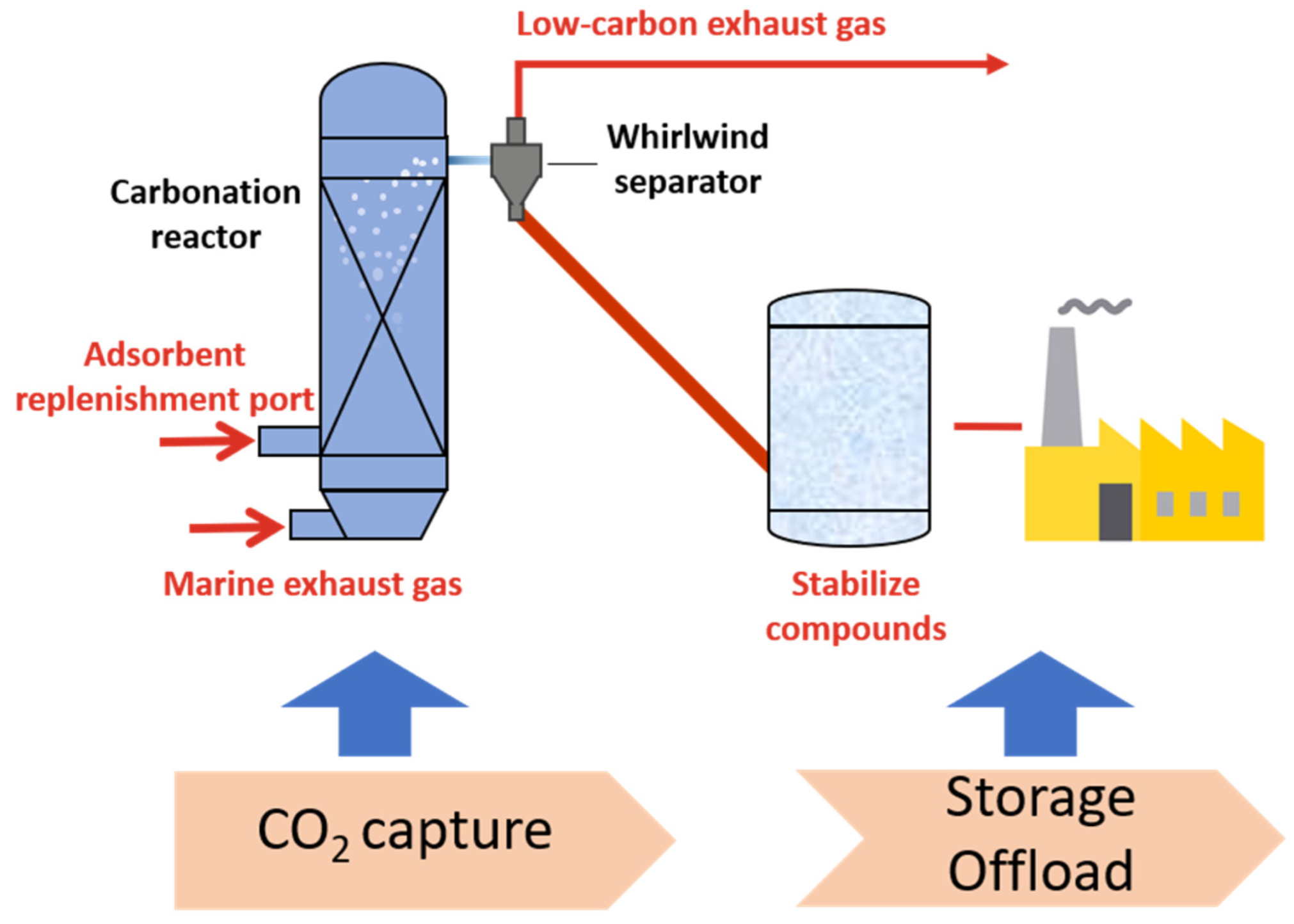
:1. Introduction
2. Experiment
2.1. Preparation of Adsorbent
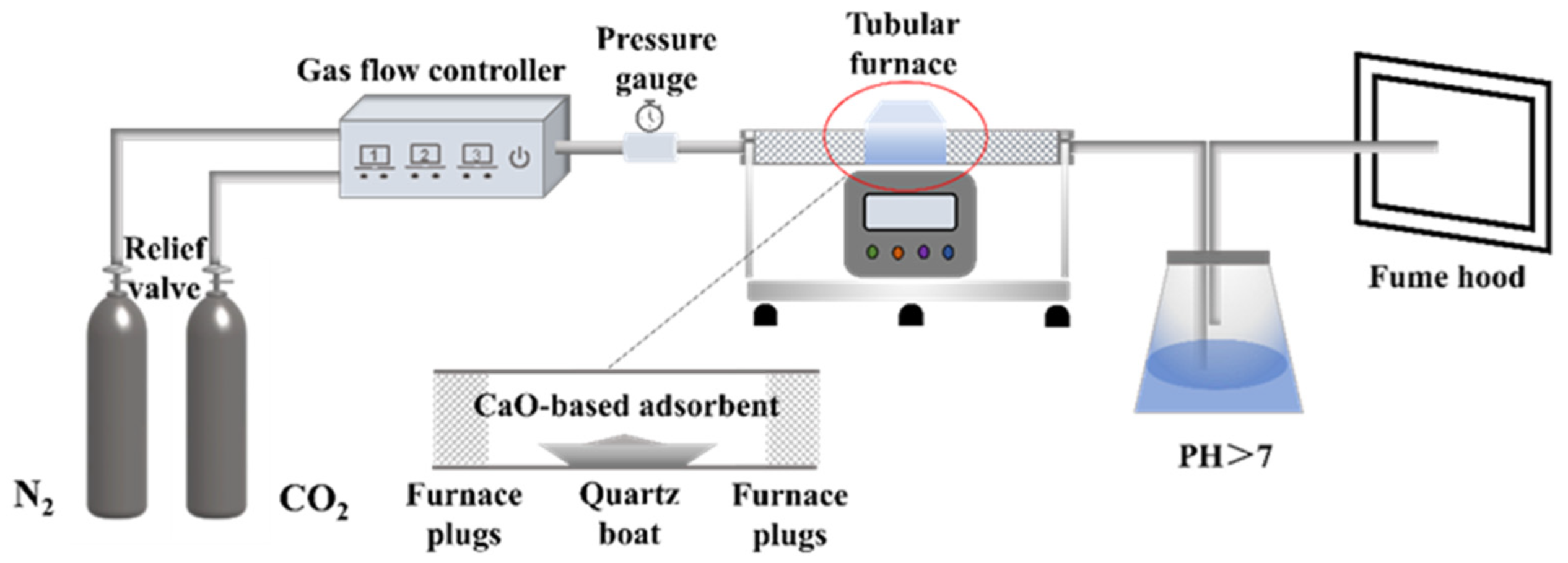
2.2. CO2 Cyclic Adsorption Experiment
2.3. Methods of Characterization of Adsorbents
3. Results and Discussion
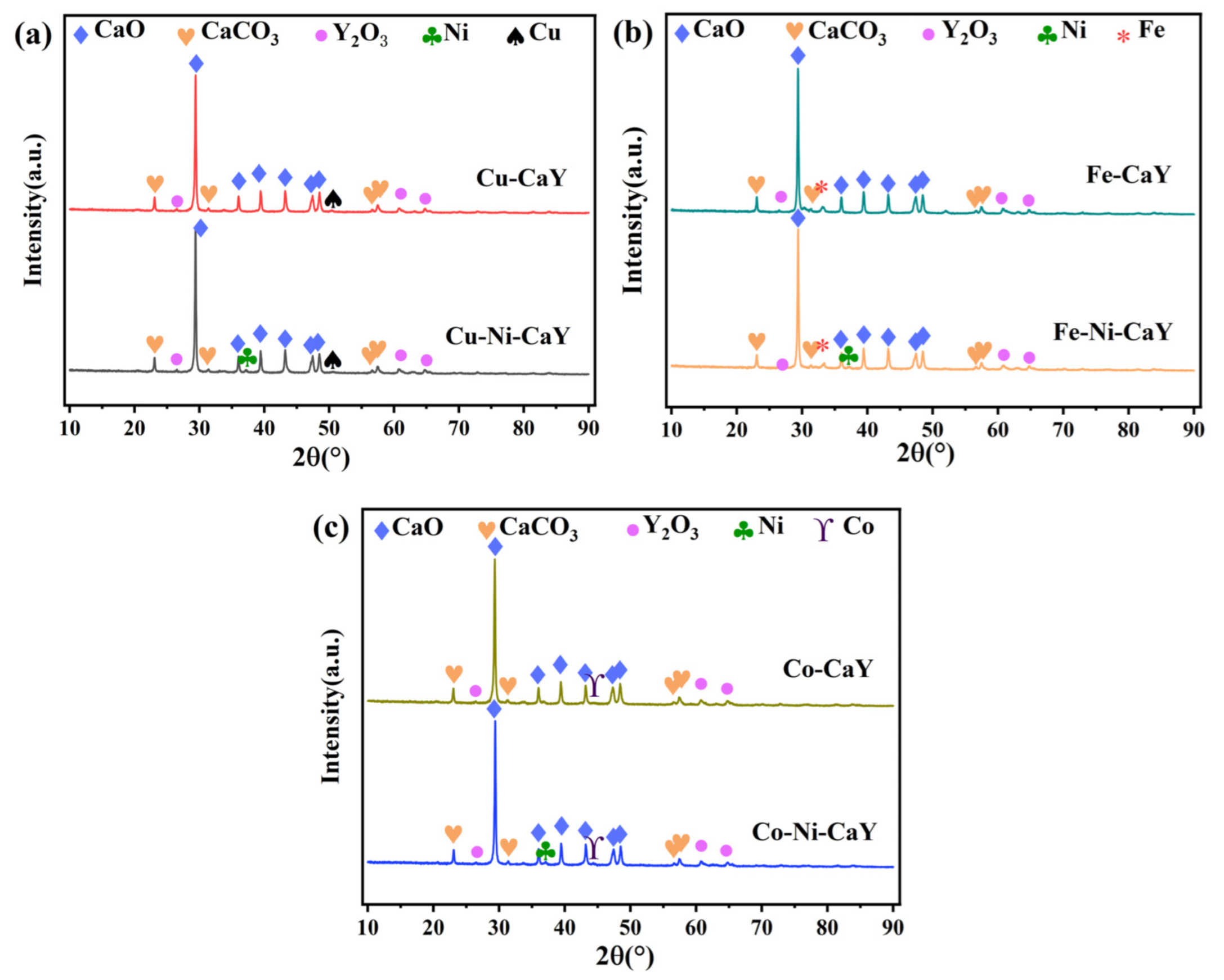
3.1. XRD Analysis
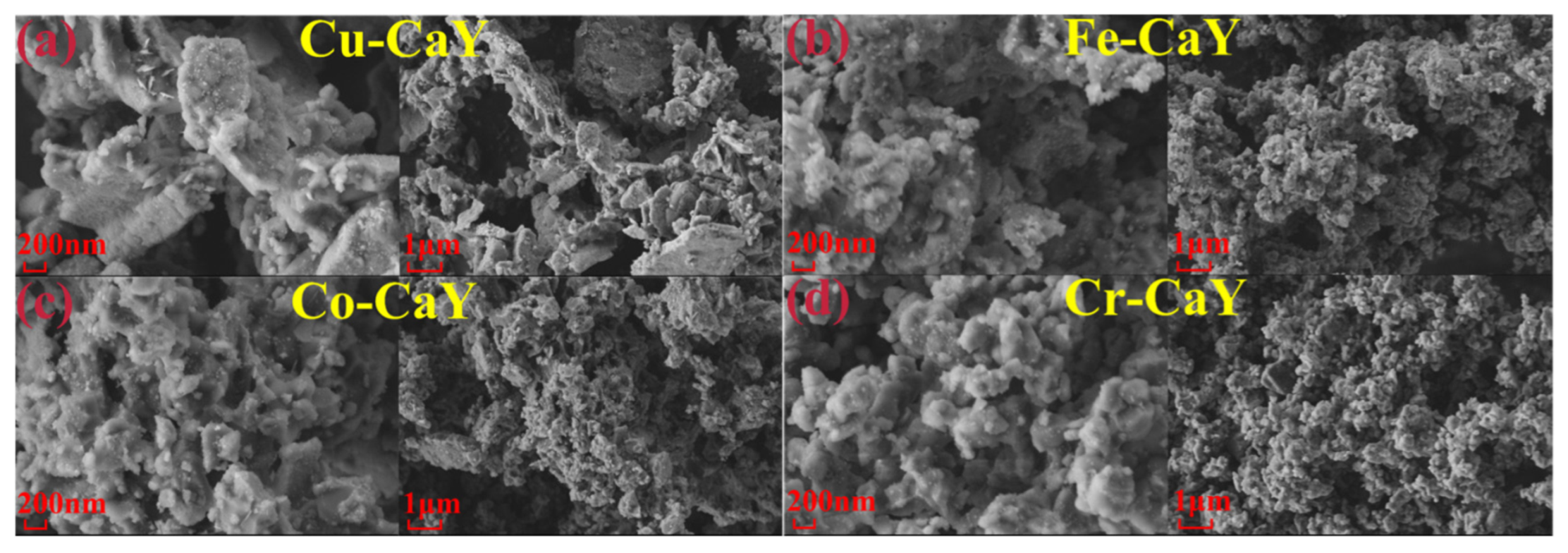
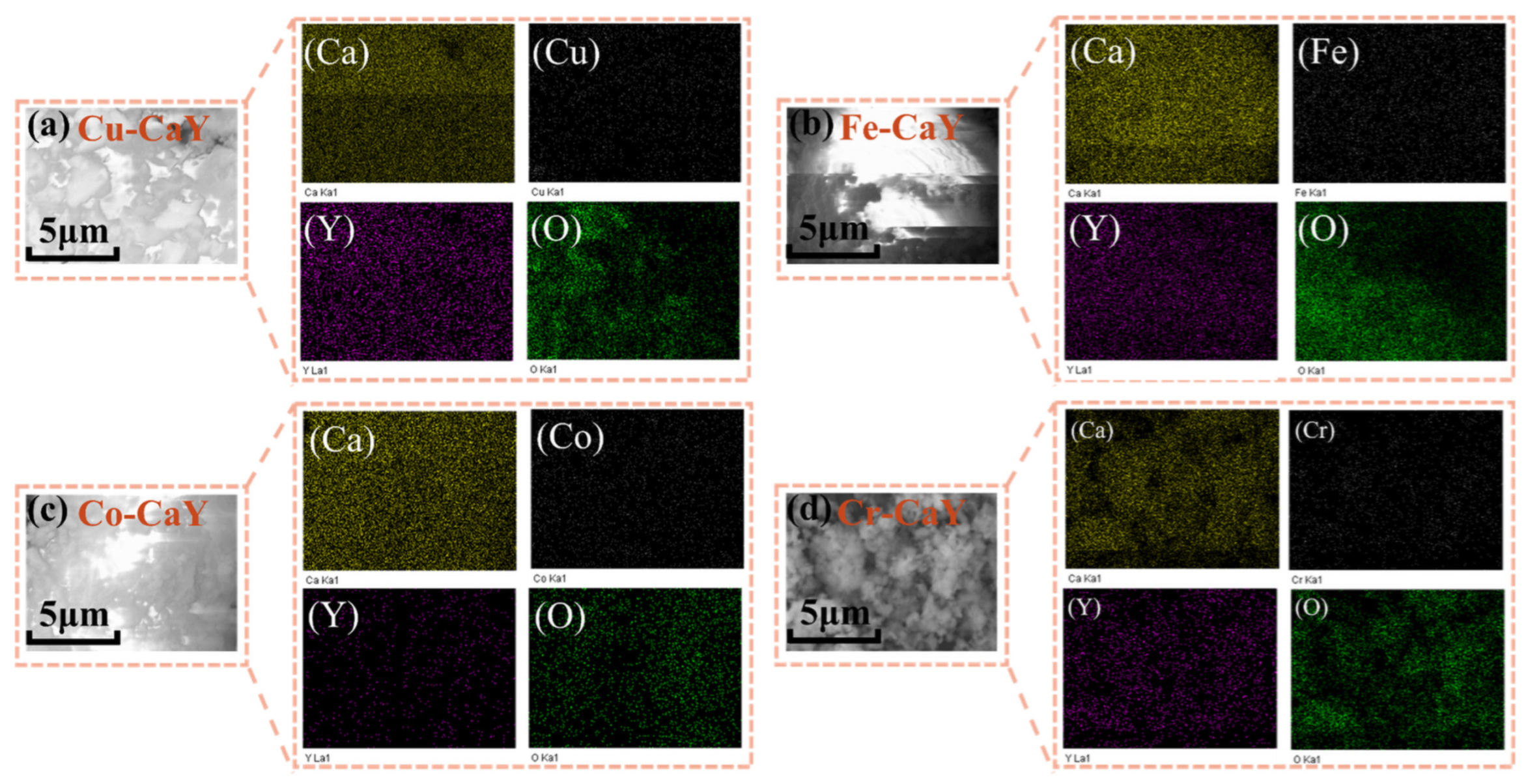
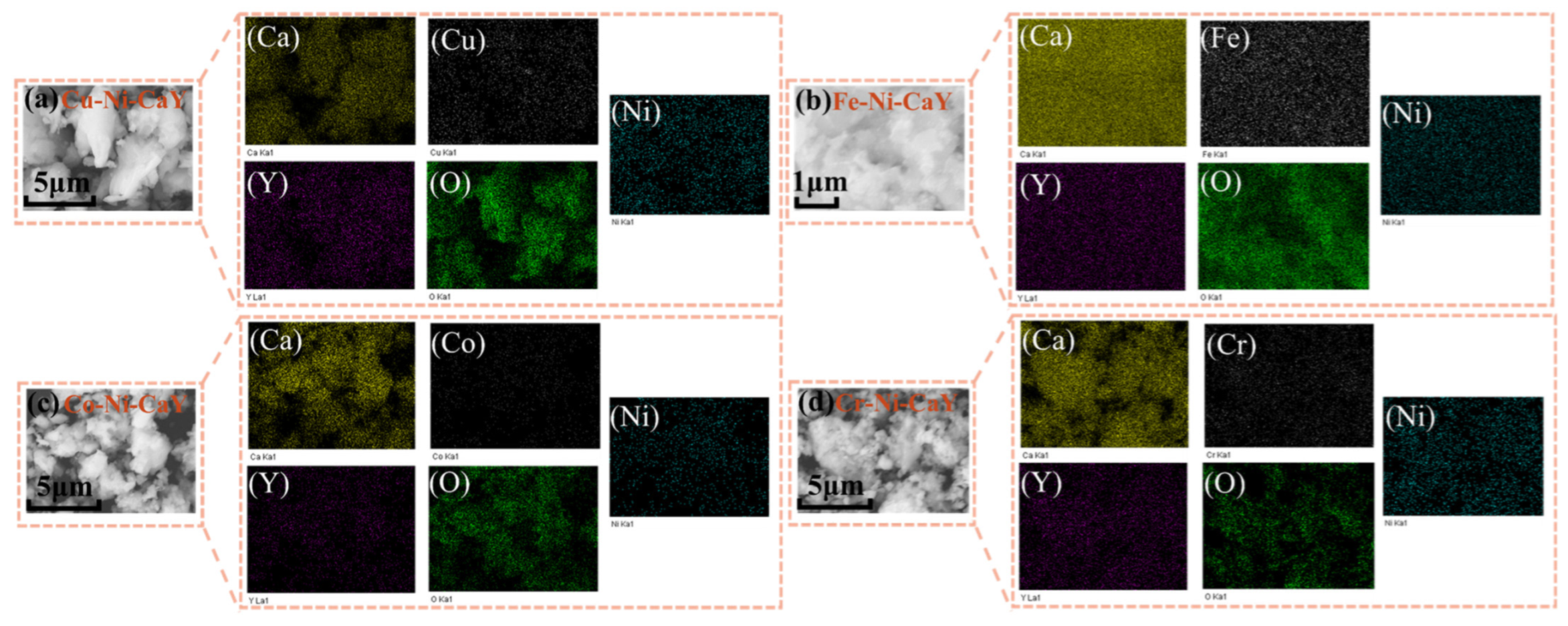
3.2. SEM and EDS Analysis
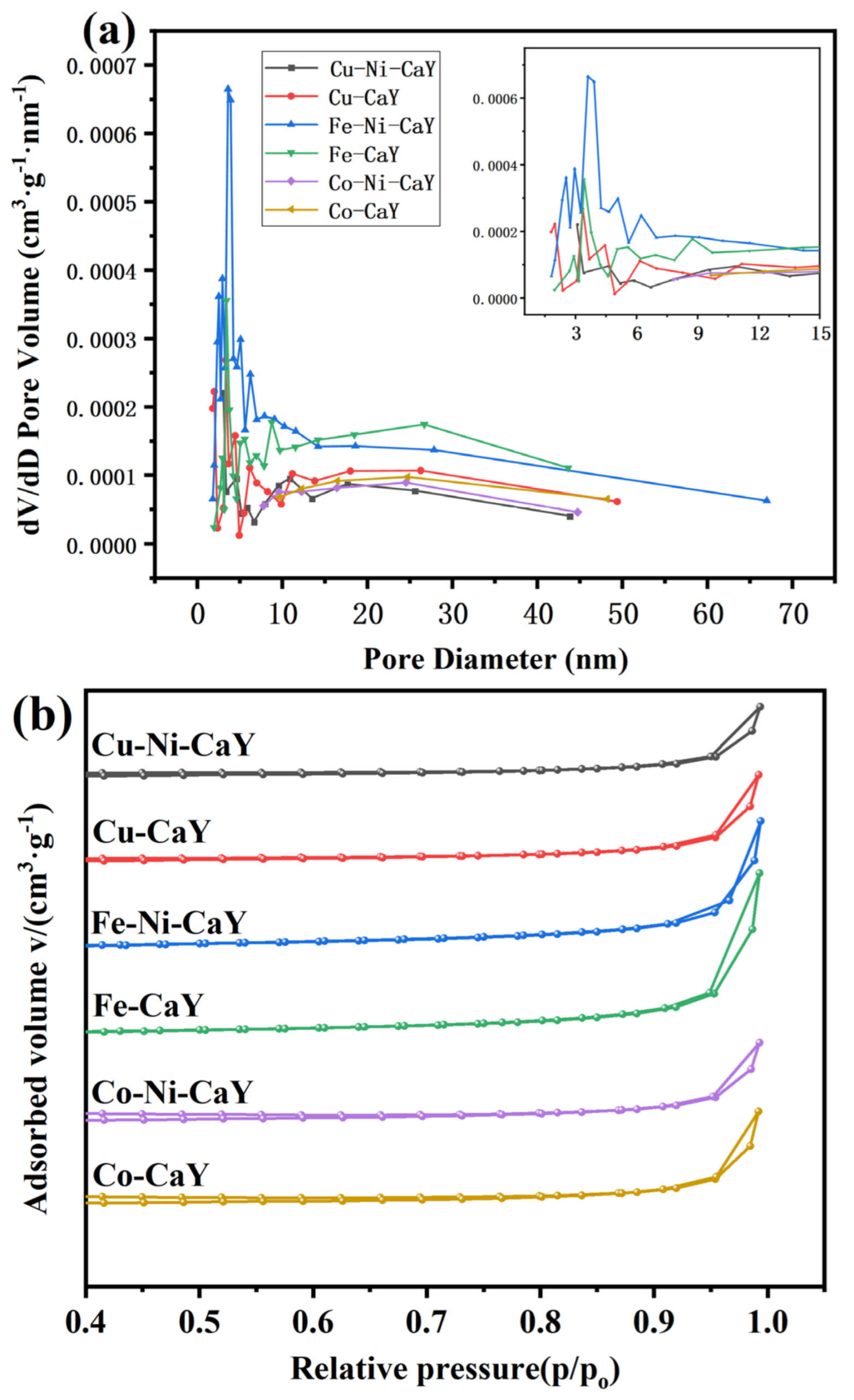
3.3. BET Analysis
4. CO2 Adsorption Performance
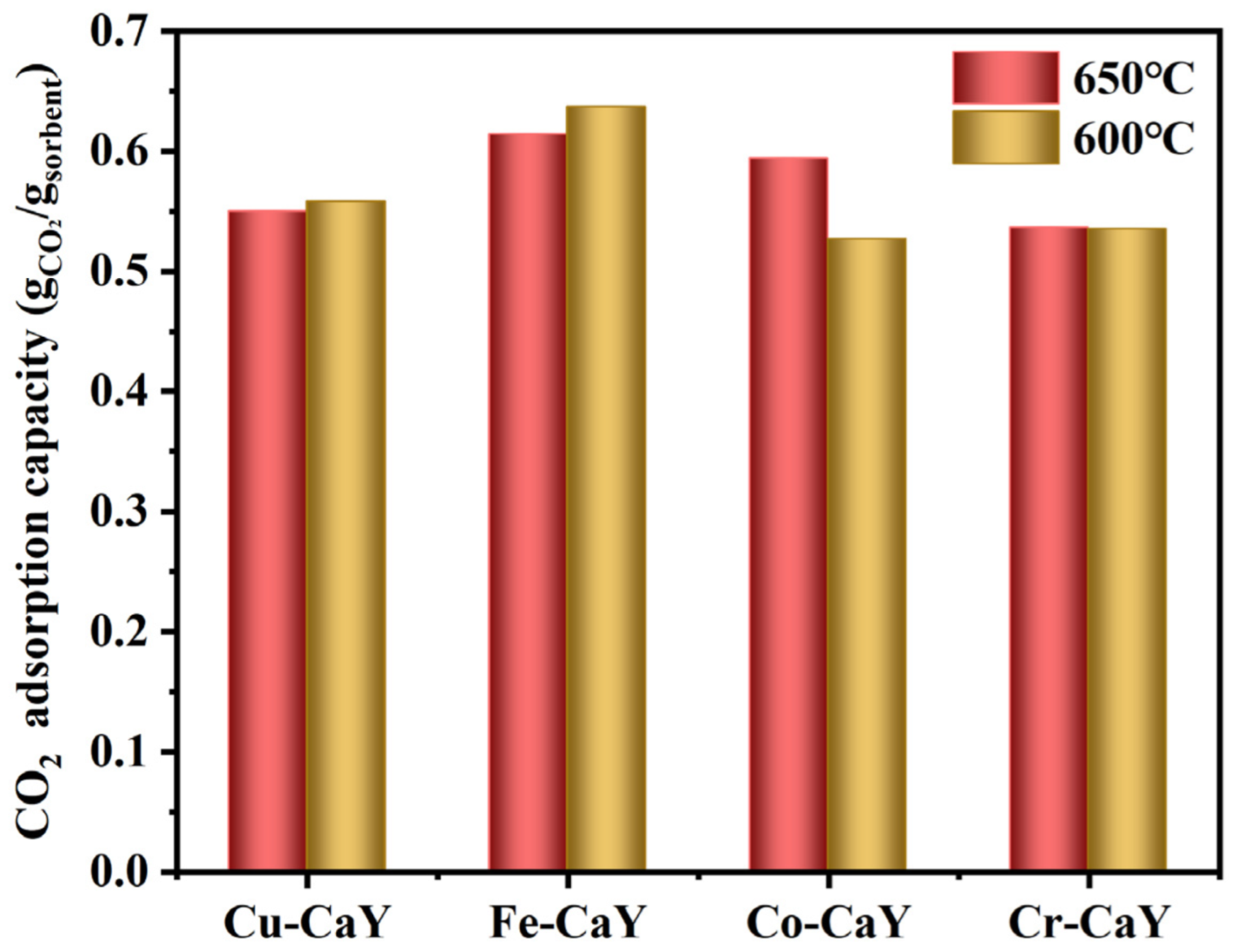
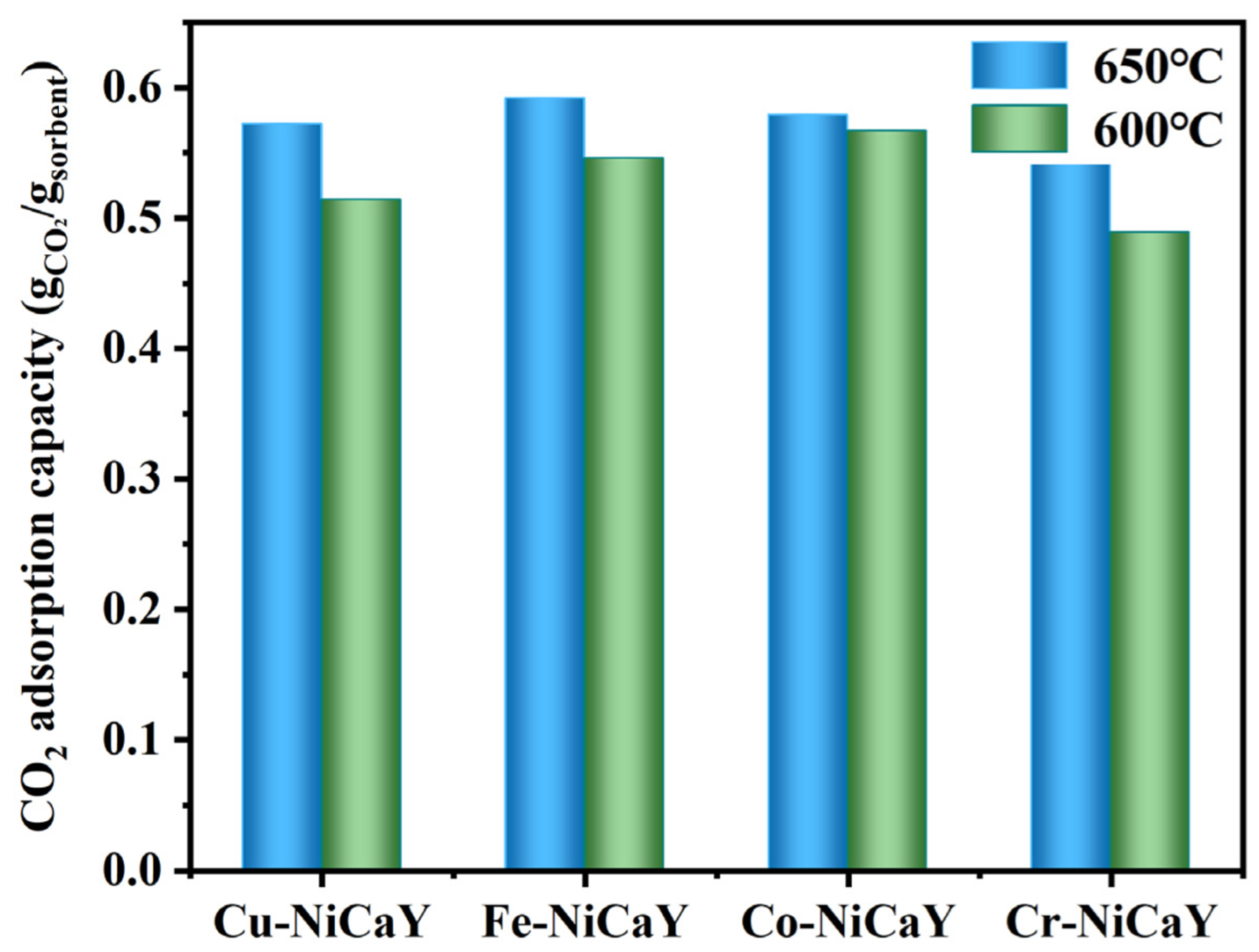
4.1. Monometallic Doped CaO-Based Adsorbent
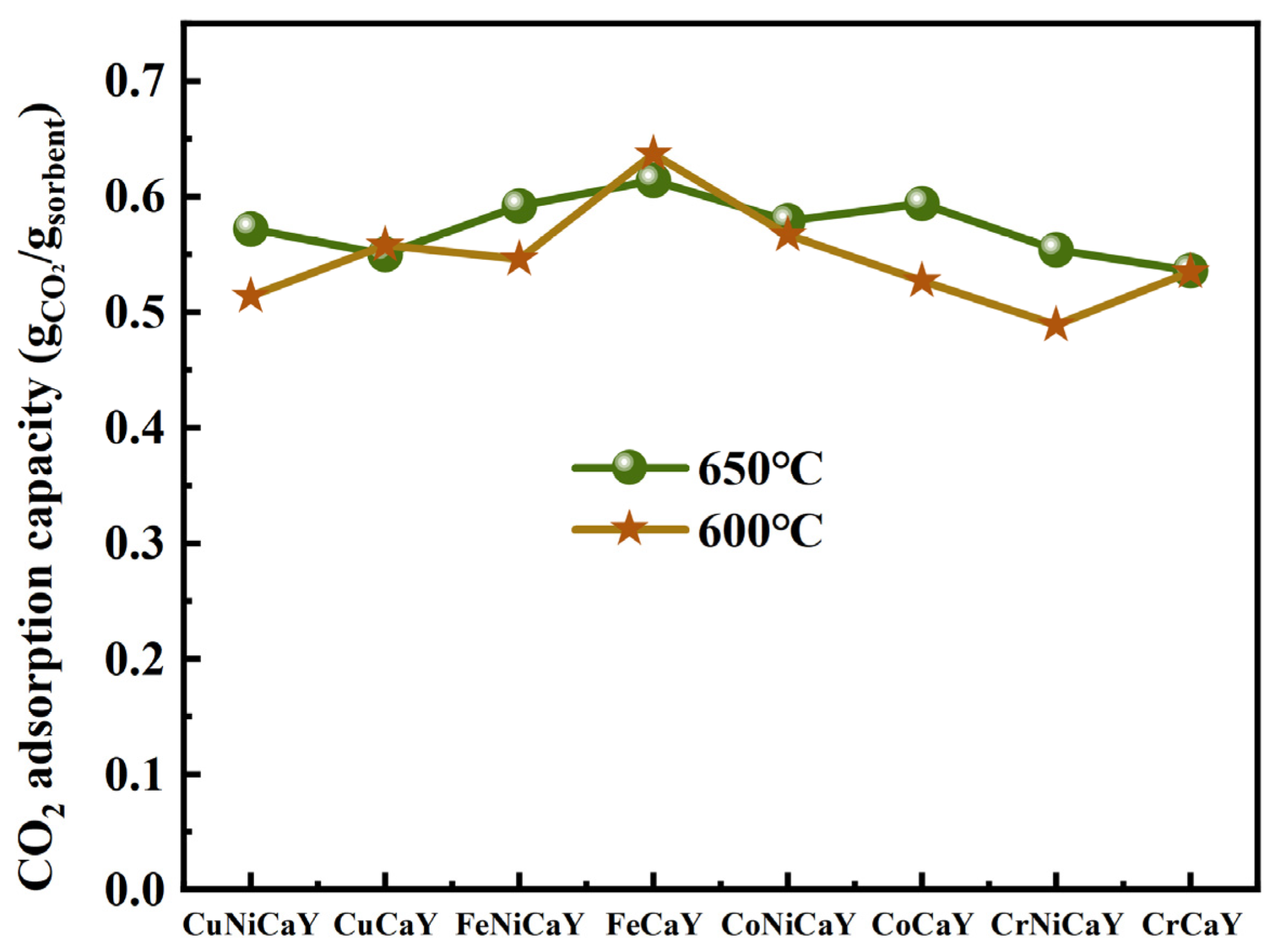
4.2. Bimetallic Doped CaO-Based Adsorbents
4.3. Effect of Temperature on Cyclic Adsorption Performance of Fe-CaY Adsorbent
5. Conclusions
- (1)
- Among monometallic doped CaO-based adsorbents, the adsorption capacity of the Fe-CaY adsorbent is the highest. Compared to the CaO-Y2O3 adsorbent, Fe doping optimizes the adsorption performance of the adsorbent. The initial adsorption capacity of Fe-CaY was 0.62 g/g at 650 °C; the adsorption capacity was 0.59 g/g at the 25th cycle, and the experimental calcination temperature was 750 °C. The Fe-CaY adsorbent with the largest pore size and specific surface area of 22.91 nm and 27.11m2/g, respectively, showed the best adsorption performance due to the contribution of macropores to prevent sintering. The loose and porous structure of the adsorbent is conducive to more CO2 entering the adsorbent particles to react with the active CaO in the adsorbent particles; thus, Fe doping can greatly improve the CO2 adsorption capacity and cycle stability of the adsorbent, and also reduce the CaO-based adsorbent cycle temperature.
- (2)
- The results show that Ni addition had a different impact on the CO2 adsorption capacity of the adsorbent. At 600 °C, the addition of Ni mainly inhibited the adsorption properties of the samples. On the contrary, at 650 °C, the adsorption ability of CaO-based adsorbents modified with Cu and Cr was enhanced by adding Ni. Among the Fe-modified CaO-based adsorbents, the adsorption capacity of Fe-CaY was higher than that of Fe-Ni-CaY. The addition of Ni destroyed the rich pore structure between Fe-Ca-Y and the stability of the adsorbent particle structure, leading to the aggregation of CaO crystals and reducing the CO2 adsorption capacity of the adsorbent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, C.; Stapersma, D.; Visser, K.; de Vos, P.; Ding, Y. Energy effectiveness of ocean-going cargo ship under various operating conditions. Ocean Eng. 2019, 190, 106473. [Google Scholar] [CrossRef]
- Bouman, E.A.; Lindstad, E.; Rialland, A.I.; Strømman, A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping–A review. Transp. Res. Part D Transp. Environ. 2017, 52, 408–421. [Google Scholar] [CrossRef]
- Carapellucci, R.; Milazzo, A. Membrane systems for CO2 capture and their integration with gas turbine plants. Proc. Inst. Mech. Eng. Part A J. Power Energy 2003, 217, 505–517. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Wang, Z. Reviews on Current Carbon Emission Reduction Technologies and Projects and their Feasibilities on Ships. Marine Sci. Appl. 2017, 16, 129–136. [Google Scholar] [CrossRef]
- Akker, J. Carbon Capture Onboard LNG-fueled Vessels: A Feasibility Study; Delft University of Technology: Delft, The Netherlands, 2017. [Google Scholar]
- Feenstra, M.; Monteiro, J.; van den Akker, J.T.; Abu-Zahra, M.R.; Gilling, E.; Goetheer, E. Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M. Study of solvent- based carbon capture for cargo ships through process modelling and simulation. Appl. Energy 2017, 195, 402–413. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Acharya, B.; Dutta, A. CaO-based CO2 sorbents: A review on screening, enhancement, cyclic stability, regeneration and kinetics modelling. J. CO2 Util. 2018, 23, 179–199. [Google Scholar] [CrossRef]
- Corte’s, C.G.; Tzimas, E.; Peteves, S.D. Technologies for coal based hydrogen and electronics co-production power plants with CO2 capture. JRC Sci. Tech. Rep. 2009. [Google Scholar]
- Moene, R.; Schoon, L.; van Straelen, J.; Geuzebroek, F. Prepitating carbonate process for energy efficient post-combustion CO2 capture. Energy Procedia 2013, 37, 1881–1887. [Google Scholar] [CrossRef]
- Singh, D.V.; Pedersen, E. A review of waste heat recovery technologies for maritime applications. Energy Convers. Manag. 2016, 111, 315–328. [Google Scholar] [CrossRef]
- Su, C.; Duan, L.; Donat, F.; Anthony, E.J. From waste to high value utilization of spent bleaching clay in synthesizing high-performance calculation-based sorbent for CO2 capture. Appl. Energy 2018, 210, 117–126. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Yan, Y.F.; Yang, Z.Q.; Guo, M.N. Effect of Ce, Zr on cyclic performance of CaO-based CO2 sorbents. CIESC J. 2015, 66, 612–617. [Google Scholar]
- Li, H.; Qu, M.; Yang, Y.; Hu, Y.; Liu, W. One-step synthesis of spherical CaO pellets via novel graphics-casting method for cyclic CO2 capture. Chem. Eng. J. 2019, 374, 619–625. [Google Scholar] [CrossRef]
- Soleimanisalim, A.H.; Sedghkerdar, M.H.; Karami, D.; Mahinpey, N. Pelletizing and coating of synthetic zirconia stabilized calculation-based sorbents for application in calculation looping CO2 capture. Ind. Eng. Chem. Res. 2017, 56, 5395–5402. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C. CO2 capture capacity of CaO in long series of carbonation/calculation cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Guo, H.; Wang, S.; Li, C.; Zhao, Y.; Sun, Q.; Ma, X. Incorporation of Zr into calendar oxide for CO2 capture by a simple and facile sol-gel method. Ind. Eng. Chem. Res. 2016, 55, 7873–7879. [Google Scholar] [CrossRef]
- Kuang, S.D.; Chen, W.; Lin, W.S.; Wang, A.; Liu, S.Y.; Tian, C. Enhanced adsorption of CO2 by Mg/Y modified Ca-based adsorbents. Clean Coal Technol. 2022, 28. [Google Scholar]
- Zhang, X.; Li, Z.; Peng, Y.; Su, W.; Sun, X.; Li, J. Investigation on a novel CaO-Y2O3 sorbent for efficient CO2 mitization. Chem. Eng. J. 2014, 243, 297–304. [Google Scholar] [CrossRef]
- Han, R.; Xing, S.; Wu, X.; Pang, C.; Lu, S.; Su, Y.; Liu, Q.; Song, C.; Gao, J. Relevant influence of alkali carbonate doping on the thermochemical energy storage of Ca-based natural minerals during CaO/CaCO3 cycles. Renew. Energy 2022, 181, 267–277. [Google Scholar] [CrossRef]
- Liu, W.; Low NW, L.; Feng, B.; Wang, G.; Diniz da Costa, J.C. Calcium precursors for the production of CaO sorbents for multicycle CO2 capture. Environ. Sci. Technol. 2010, 44, 841–847. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Ding, N.; Wu, Q.L.; Zheng, C.G. SGCS-made ultrafine CaO/Al2O3 sorbent for cyclic CO2 capture. Chin. Chem. Lett. 2011, 22, 615–618. [Google Scholar] [CrossRef]
- Shen, Q.; Shao, Z.; Li, S.; Yang, G.; Yuan, J.; Pan, X. Experimental Study of O2-Enriched CO2 Production by BaCo0.8B0.2O3−δ (B = Ce, Al, Fe, Cu) Perovskites Sorbent for Marine Exhaust CO2 Capture Application. J. Mar. Sci. Eng. 2021, 9, 661. [Google Scholar] [CrossRef]
- Lu, H.; Reddy, E.P.; Smirniotis, P.G. Calcium oxide based sorbents for capture of carbon dioxide at high temperatures. Ind. Eng. Chem. Res. 2006, 45, 3944–3949. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Wang, H.; Cui, W.; Li, M.; Xie, W. Double-exchange-induced effective increased CO2 capture of CaO by doping bimetallic oxides with variable value state. Chem. Eng. J. 2022, 433, 134490. [Google Scholar] [CrossRef]
- Qin, C.; Yin, J.; Liu, W.; An, H.; Feng, B. Behavior of CaO/CuO based composite in a combined calendar and copper chemical looping process. Ind. Eng. Chem. Res. 2012, 51, 12274–12281. [Google Scholar]
- Zamboni, I.; Courson, C.; Kiennemann, A. Fe-Ca interactions in Fe-based/CaO catalyst/sorbent for CO2 sorption and hydrogen production from toluene steam reforming. Appl. Catal. B Environ. 2017, 203, 154–165. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Chen, J.; Wei, W.; Chirawatkul, P.; Galvita, V.V.; Kawi, S. Tandem distributing Ni into CaO framework for isothermal integration of CO2 capture and conversion. Chem. Eng. J. 2023, 452, 139460. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.; Ma, S.; Feng, Y. Understanding the synergistic effect of Ni/Al2O3 on CO2 adsorption and sintering properties of CaO in sorption-enhanced steam reforming process. Fuel 2023, 341, 127766. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Cai, N. Understanding the enhancement effect of high-temperature steam on the bonation reaction of CaO with CO2. Fuel 2014, 127, 88–93. [Google Scholar] [CrossRef]


| Serial Number | Sample Named | Mass Ratio |
|---|---|---|
| 1 | Cu-CaY | Cu/Fe/Co/Cr:CaO:Y2O3 = 7:85:8 |
| 2 | Fe-CaY | |
| 3 | Co-CaY | |
| 4 | Cr-CaY | |
| 5 | Cu-Ni-CaY | Cu/Fe/Co/Cr:Ni:CaO:Y2O3 = 5:5:85:5 |
| 6 | Fe-Ni-CaY | |
| 7 | Co-Ni-CaY | |
| 8 | Cr-Ni-CaY |
| Adsorbent | Pore Size (nm) | Pore Volume (cm3/g) | SBET (m2/g) |
|---|---|---|---|
| Cu-Ni-CaY | 19.9588 | 0.0166 | 11.0622 |
| Cu-CaY | 19.6457 | 0.0129 | 7.3152 |
| Fe-Ni-CaY | 21.6570 | 0.0251 | 25.9717 |
| Fe-CaY | 22.9176 | 0.0317 | 27.1188 |
| Co-Ni-CaY | 7.0618 | 0.0030 | 4.1180 |
| Co-CaY | 5.9285 | 0.0025 | 4.0900 |
| Adsorbent Property | Ca-Y2O3 | Fe-CaY |
|---|---|---|
| Reaction temperature (°C) | 700 | 650 |
| Desorption temperature (°C) | 850 | 750 |
| Initial adsorption capacity (g/g) | 0.58 | 0.62 |
| Adsorption capacity after 15 cycles (g/g) | 0.52 | 0.61 |
| Adsorption capacity after 25 cycles (g/g) | -- | 0.59 |
| References | [18] | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shen, Q.; Zhu, K.; Chen, G.; Yang, G.; Li, S. Experimental Study on High-Efficiency Cyclic CO2 Capture from Marine Exhaust by Transition-Metal-Modified CaO/Y2O3 Adsorbent. J. Mar. Sci. Eng. 2023, 11, 2229. https://doi.org/10.3390/jmse11122229
Zhang X, Shen Q, Zhu K, Chen G, Yang G, Li S. Experimental Study on High-Efficiency Cyclic CO2 Capture from Marine Exhaust by Transition-Metal-Modified CaO/Y2O3 Adsorbent. Journal of Marine Science and Engineering. 2023; 11(12):2229. https://doi.org/10.3390/jmse11122229
Chicago/Turabian StyleZhang, Xin, Qiuwan Shen, Kuanyu Zhu, Gaokui Chen, Guogang Yang, and Shian Li. 2023. "Experimental Study on High-Efficiency Cyclic CO2 Capture from Marine Exhaust by Transition-Metal-Modified CaO/Y2O3 Adsorbent" Journal of Marine Science and Engineering 11, no. 12: 2229. https://doi.org/10.3390/jmse11122229





