Southern Ocean Iron Limitation of Primary Production between Past Knowledge and Future Projections
Abstract
:1. Introduction
2. Iron in the Southern Ocean
3. Iron Limitation Impact on Primary Production and Ecosystem Structure
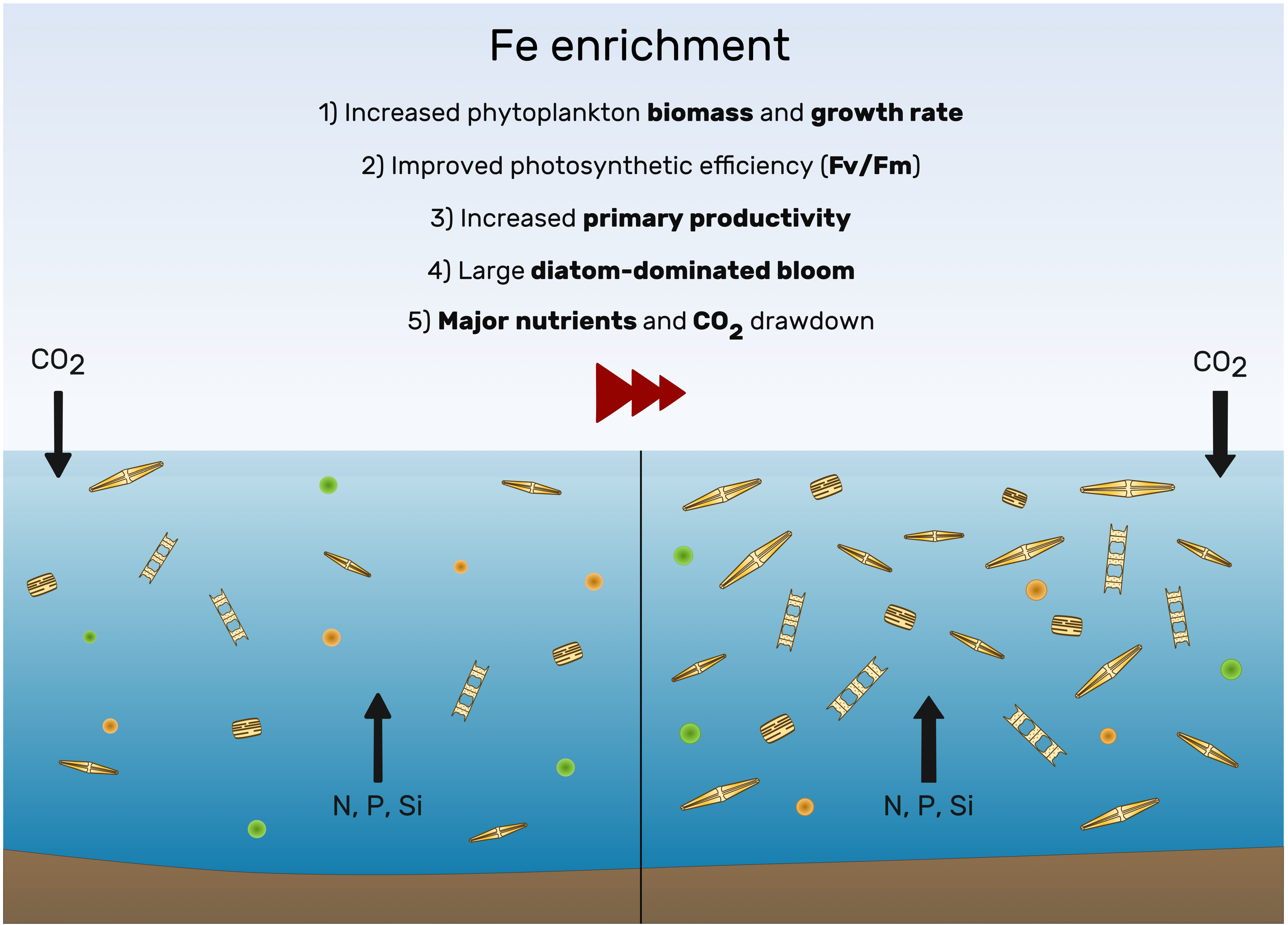
3.1. Knowledge from the Past
| Experiment Type | Area | Period | Reference(s) |
|---|---|---|---|
| Artificial Fe fertilization | SO Australian Sector | Summer (Feb) | SOIREE [52,53,54] |
| SO Atlantic Sector | Spring (Nov) | EisenEx [55] | |
| Polar waters of New Zealand, north of the Polar Front | Summer (Jan–Feb) | SOFeX-N [56] | |
| Subpolar waters of New Zealand, south of the Polar Front | Summer (Jan–Feb) | SOFeX-S [56,57] | |
| SO Atlantic Sector | Summer–Autumn (Jan–Mar) | EIFEX [58] | |
| Subpolar waters of New Zealand | Autumn (Mar–Apr) | SAGE [59] | |
| SO Australian Sector | Summer–Autumn (Jan–Mar) | LOHAFEX [60,61] | |
| Natural Fe fertilization | SO Atlantic Sector | Spring (Oct–Nov) | [13] |
| SO Pacific Sector | Autumn (Mar–Apr) | [36] | |
| Crozet Plateau | Spring–Summer (Nov–Jan) | CROZEX [37,47,62] | |
| Kerguelen Plateau | Summer (Jan–Feb) | KEOPS 1 [45,46] | |
| SO Pacific Sector | Summer (Jan–Feb) | [30] | |
| Kerguelen Plateau | Spring (Oct–Nov) | KEOPS 2 [63] | |
| In vitro Fe enrichment (bottle incubation experiments) | Weddel Sea; Scotia Sea | Spring–Summer (Nov–Dec) | [33] |
| SO Pacific Sector | Autumn (Mar–May) | [39] | |
| Subantarctic Zone (SE New Zealand) | Autumn (Apr–May) Spring (Oct–Nov) | [64] | |
| Polar Frontal Zone, Pacific Sector | Spring (Oct–Nov) Summer (Jan–Feb) | [65] | |
| Subantarctic Zone, Australian Sector | Late Summer (Mar) | [66] | |
| Subantarctic Zone, Australian Sector | Late Summer (Mar) | [67] | |
| Ross Sea | Summer (Jan) | [38] | |
| Antarctic Circumpolar Current | Spring–Summer (Oct–Dec) | ||
| Polar Frontal Zone, Atlantic Sector | Summer (Dec–Jan) | [40] | |
| Crozet Plateau (Polar Frontal Zone) | Spring–Summer (Nov–Jan) | [37,47] | |
| Amundsen Sea | Summer (Jan–Feb) | [49] | |
| Scotia Sea | Spring (Oct–Dec) Summer (Dec–Feb) | [68] | |
| Amundsen Sea | Early summer (Dec–Jan) | [69] | |
| Ross Sea | Summer (Dec–Feb) | [70] | |
| Subantarctic Zone, Atlantic Sector | Summer (Dec–Feb) | [15] | |
| Polar Frontal Zone (North);Antarctic Zone (South), Atlantic Sector | Summer (Dec–Feb) | [71] | |
| Ross Sea | Early Summer (Dec–Jan) | [72] | |
| East Antarctica | Late Summer (Jan–Mar) | [73] |
3.2. Co-Limitations with Other Environmental Factors
3.2.1. Light
3.2.2. Temperature
3.2.3. Silicates
3.3. Phytoplankton Community and Ecosystem Structure
3.4. Where We Are Today: Artificial Fe Fertilization and Carbon Export
4. Looking to The Future: Could Climate Change Relieve Iron Limitation?
4.1. Iron Supply
4.2. How Will Changes in the Environment and Iron Cycle Affect Phytoplankton Biology?
4.2.1. Warming
4.2.2. Acidification
4.2.3. Mixing and Stratification
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guy, J.; Paul, G. Écosystèmes Pélagiques Marins; Collection d’écologie; Elsevier Masson: Paris, France; New York, NY, USA; Barcelone, Spain, 1986; ISBN 978-2-225-80760-2. [Google Scholar]
- Knox, G.A. Biology of the Southern Ocean, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-11980-4. [Google Scholar]
- Lauritano, C.; Rizzo, C.; Lo Giudice, A.; Saggiomo, M. Physiological and Molecular Responses to Main Environmental Stressors of Microalgae and Bacteria in Polar Marine Environments. Microorganisms 2020, 8, 1957. [Google Scholar] [CrossRef]
- Martin, J.H.; Gordon, R.M.; Fitzwater, S.E. Iron in Antarctic Waters. Nature 1990, 345, 156–158. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Hudson, R.J.M.; Price, N.M. Limitation of Productivity by Trace Metals in the Sea. Limnol. Oceanogr. 1991, 36, 1742–1755. [Google Scholar] [CrossRef]
- Fitzwater, S.E.; Johnson, K.S.; Gordon, R.M.; Coale, K.H.; Smith, W.O. Trace Metal Concentrations in the Ross Sea and Their Relationship with Nutrients and Phytoplankton Growth. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 3159–3179. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Price, N.M. The Biogeochemical Cycles of Trace Metals in the Oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Stoll, H. 30 Years of the Iron Hypothesis of Ice Ages. Nature 2020, 578, 370–371. [Google Scholar] [CrossRef] [Green Version]
- Street, J.H.; Paytan, A. Iron, Phytoplankton Growth, and the Carbon Cycle. In Metal Ions in Biological Systems, Volume 43—Biogeochemical Cycles of Elements; CRC Press: Boca Raton, FL, USA, 2005; pp. 153–193. ISBN 978-0-429-16464-4. [Google Scholar]
- Huang, Y.; Tagliabue, A.; Cassar, N. Data-Driven Modeling of Dissolved Iron in the Global Ocean. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Martin, J.H. Glacial-Interglacial CO2 Change: The Iron Hypothesis. Paleoceanography 1990, 5, 1–13. [Google Scholar] [CrossRef]
- Loscher, B.M.; De Baar, H.J.W.; De Jong, J.T.M.; Veth, C.; Dehairs, F. The Distribution of Fe in the Antarctic Circumpolar Current. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 143–187. [Google Scholar] [CrossRef] [Green Version]
- De Baar, H.J.W.; de Jong, J.T.M.; Bakker, D.C.E.; Löscher, B.M.; Veth, C.; Bathmann, U.; Smetacek, V. Importance of Iron for Plankton Blooms and Carbon Dioxide Drawdown in the Southern Ocean. Nature 1995, 373, 412–415. [Google Scholar] [CrossRef]
- Moore, J.K.; Abbott, M.R. Phytoplankton Chlorophyll Distributions and Primary Production in the Southern Ocean. JGR Ocean. 2000, 105, 28709–28722. [Google Scholar] [CrossRef]
- Ryan-Keogh, T.J.; Thomalla, S.J.; Mtshali, T.N.; van Horsten, N.R.; Little, H.J. Seasonal Development of Iron Limitation in the Sub-Antarctic Zone. Biogeosciences 2018, 15, 4647–4660. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, D.A.; Boyd, W. Marine Phytoplankton and the Changing Ocean Iron Cycle. Nat. Clim. Change 2016, 6, 8. [Google Scholar] [CrossRef]
- Turner, D.R.; Hunter, K.A. The Biogeochemistry of Iron in Seawater; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-0-471-49068-5. [Google Scholar]
- Rivaro, P.; Ardini, F.; Grotti, M.; Aulicino, G.; Cotroneo, Y.; Fusco, G.; Mangoni, O.; Bolinesi, F.; Saggiomo, M.; Celussi, M. Mesoscale Variability Related to Iron Speciation in a Coastal Ross Sea Area (Antarctica) during Summer 2014. Chem. Ecol. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Boyd, P.W.; Arrigo, K.R.; Strzepek, R.; van Dijken, G.L. Mapping Phytoplankton Iron Utilization: Insights into Southern Ocean Supply Mechanisms. JGR Ocean. 2012, 117. [Google Scholar] [CrossRef] [Green Version]
- Death, R.; Wadham, J.L.; Monteiro, F.; Le Brocq, A.M.; Tranter, M.; Ridgwell, A.; Dutkiewicz, S.; Raiswell, R. Antarctic Ice Sheet Fertilises the Southern Ocean. Biogeosciences 2014, 11, 2635–2643. [Google Scholar] [CrossRef] [Green Version]
- Hawkings, J.R.; Wadham, J.L.; Tranter, M.; Raiswell, R.; Benning, L.G.; Statham, P.J.; Tedstone, A.; Nienow, P.; Lee, K.; Telling, J. Ice Sheets as a Significant Source of Highly Reactive Nanoparticulate Iron to the Oceans. Nat. Commun. 2014, 5, 3929. [Google Scholar] [CrossRef] [Green Version]
- Lannuzel, D.; Vancoppenolle, M.; van der Merwe, P.; de Jong, J.; Meiners, K.M.; Grotti, M.; Nishioka, J.; Schoemann, V. Iron in Sea Ice: Review and New Insights. Elem. Sci. Anthr. 2016, 4, 000130. [Google Scholar] [CrossRef] [Green Version]
- Dinniman, M.S.; St-Laurent, P.; Arrigo, K.R.; Hofmann, E.E.; Dijken, G.L. Analysis of Iron Sources in Antarctic Continental Shelf Waters. JGR Ocean. 2020, 125. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Sohst, B.M.; O’Hara, C.; Stammerjohn, S.E.; Loose, B.; Dinniman, M.S.; Buck, N.J.; Resing, J.A.; Ackley, S.F. Seasonal Dynamics of Dissolved Iron on the Antarctic Continental Shelf: Late-Fall Observations From the Terra Nova Bay and Ross Ice Shelf Polynyas. JGR Ocean. 2022, 127, e2022JC018999. [Google Scholar] [CrossRef]
- Hoppema, M.; de Baar, H.J.W.; Fahrbach, E.; Hellmer, H.H.; Klein, B. Substantial Advective Iron Loss Diminishes Phytoplankton Production in the Antarctic Zone. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Watson, A.J.; Bakker, D.C.; Ridgwell, A.J.; Boyd, P.W.; Law, C.S. Effect of Iron Supply on Southern Ocean CO2 Uptake and Implications for Glacial Atmospheric CO2. Nature 2000, 407, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, M.R.; Marra, J.; Smith, W.O.; Goericke, R.; Measures, C.; Vink, S.; Olson, R.J.; Sosik, H.M.; Barber, R.T. Primary Productivity and Its Regulation in the Pacific Sector of the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 533–558. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.R.; Ratnarajah, L.; Holmes, T.M.; Wuttig, K.; Townsend, A.T.; Westwood, K.; Cox, M.; Bell, E.; Nicol, S.; Lannuzel, D. Circumpolar Deep Water and Shelf Sediments Support Late Summer Microbial Iron Remineralization. Glob. Biogeochem. Cycles 2021, 35, e2020GB006921. [Google Scholar] [CrossRef]
- Sedwick, P.N.; Bowie, A.R.; Trull, T.W. Dissolved Iron in the Australian Sector of the Southern Ocean (CLIVAR SR3 Section): Meridional and Seasonal Trends. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 911–925. [Google Scholar] [CrossRef]
- Gerringa, L.J.A.; Alderkamp, A.-C.; Laan, P.; Thuróczy, C.-E.; De Baar, H.J.W.; Mills, M.M.; van Dijken, G.L.; van Haren, H.; Arrigo, K.R. Iron from Melting Glaciers Fuels the Phytoplankton Blooms in Amundsen Sea (Southern Ocean): Iron Biogeochemistry. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 16–31. [Google Scholar] [CrossRef]
- Cullen, J.J. Hypotheses to Explain High-Nutrient Conditions in the Open Sea. Limnol. Oceanogr. 1991, 36, 1578–1599. [Google Scholar] [CrossRef] [Green Version]
- Minas, H.; Minas, M. Net Community Production in High Nutrient-Low Chlorophyll Waters of the Tropical and Antarctic Oceans - Grazing vs Iron Hypothesis. Oceanol. Acta 1992, 15, 145–162. [Google Scholar]
- De Baar, H.J.W.; Buma, A.J.G.; Nolting, R.F.; Cadee, G.C.; Jacques, G.; Treguer, P.J. On Iron Limitation of the Southern Ocean: Experimental Observations in the Weddell and Scotia Seas. Mar. Ecol. Prog. Ser. 1990, 65, 105–122. [Google Scholar] [CrossRef]
- Martin, J.H.; Coale, K.H.; Johnson, K.S.; Fitzwater, S.E.; Gordon, R.M.; Tanner, S.J.; Hunter, C.N.; Elrod, V.A.; Nowicki, J.L.; Coley, T.L.; et al. Testing the Iron Hypothesis in Ecosystems of the Equatorial Pacific Ocean. Nature 1994, 371, 123–129. [Google Scholar] [CrossRef]
- Coale, K.H.; Johnson, K.S.; Fitzwater, S.E.; Gordon, R.M.; Tanner, S.; Chavez, F.P.; Ferioli, L.; Sakamoto, C.; Rogers, P.; Millero, F.; et al. A Massive Phytoplankton Bloom Induced by an Ecosystem-Scale Iron Fertilization Experiment in the Equatorial Pacific Ocean. Nature 1996, 383, 495–501. [Google Scholar] [CrossRef]
- De Baar, H.J.W.; de Jong, J.T.M.; Nolting, R.F.; Timmermans, K.R.; van Leeuwe, M.A.; Bathmann, U.; Rutgers van der Loeff, M.; Sildam, J. Low Dissolved Fe and the Absence of Diatom Blooms in Remote Pacific Waters of the Southern Ocean. Mar. Chem. 1999, 66, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Seeyave, S.; Hickman, A.E.; Allen, J.T.; Lucas, M.I.; Planquette, H.; Pollard, R.T.; Poulton, A.J. Iron–Light Interactions during the CROZet Natural Iron Bloom and EXport Experiment (CROZEX) I: Phytoplankton Growth and Photophysiology. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2045–2065. [Google Scholar] [CrossRef]
- Coale, K.H.; Wang, X.; Tanner, S.J.; Johnson, K.S. Phytoplankton Growth and Biological Response to Iron and Zinc Addition in the Ross Sea and Antarctic Circumpolar Current along 170° W. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 635–653. [Google Scholar] [CrossRef]
- Timmermans, K.; van Leeuwe, M.; de Jong, J.; McKay, R.; Nolting, R.; Witte, H.; van Ooyen, J.; Swagerman, M.; Kloosterhuis, H.; de Baar, H. Iron Stress in the Pacific Region of the Southern Ocean:Evidence from Enrichment Bioassays. Mar. Ecol. Prog. Ser. 1998, 166, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, M.; Croot, P.L.; Bertilsson, S.; Abrahamsson, K.; Karlson, B.; David, R.; Fransson, A.; Sakshaug, E. Iron Enrichment and Photoreduction of Iron under UV and PAR in the Presence of Hydroxycarboxylic Acid: Implications for Phytoplankton Growth in the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 2841–2856. [Google Scholar] [CrossRef]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.W.; Follows, M.; et al. Mesoscale Iron Enrichment Experiments 1993-2005: Synthesis and Future Directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [Green Version]
- De Baar, H.J.W.; Boyd, P.; Coale, K.; Landry, M.; Tsuda, A.; Assmy, P.; Bakker, D.; Bozec, Y.; Barber, R.; Brzezinski, M.; et al. Synthesis of Iron Fertilization Experiments: From the Iron Age in the Age of Enlightenment. JGR Atmos. 2005. [Google Scholar] [CrossRef]
- Yoon, J.-E.; Yoo, K.-C.; Macdonald, A.M.; Yoon, H.-I.; Park, K.-T.; Yang, E.J.; Kim, H.-C.; Lee, J.I.; Lee, M.K.; Jung, J.; et al. Ocean Iron Fertilization Experiments – Past, Present, and Future Looking to a Future Korean Iron Fertilization Experiment in the Southern Ocean (KIFES) Project. Biogeosciences 2018, 15, 5847–5889. [Google Scholar] [CrossRef] [Green Version]
- Blain, S.; Tréguer, P.; Belviso, S.; Bucciarelli, E.; Denis, M.; Desabre, S.; Fiala, M.; Martin-Jezequel, V.; Fèvre, J.; Mayzaud, P.; et al. Biogeochemical Study of an Island Mass Effect in the Context of the Iron Hypothesis in the Southern Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 163–187. [Google Scholar] [CrossRef]
- Blain, S.; Quéguiner, B.; Armand, L.; Belviso, S.; Bombled, B.; Bopp, L.; Bowie, A.; Brunet, C.; Brussaard, C.; Carlotti, F.; et al. Effect of Natural Iron Fertilization on Carbon Sequestration in the Southern Ocean. Nature 2007, 446, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Blain, S.; Sarthou, G.; Laan, P. Distribution of Dissolved Iron during the Natural Iron-Fertilization Experiment KEOPS (Kerguelen Plateau, Southern Ocean). Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 594–605. [Google Scholar] [CrossRef]
- Moore, C.M.; Hickman, A.E.; Poulton, A.J.; Seeyave, S.; Lucas, M.I. Iron-Light Interactions during the CROZet Natural Iron Bloom and EXport Experiment (CROZEX): II-Taxonomic Responses and Elemental Stoichiometry. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2066–2084. [Google Scholar] [CrossRef]
- Cassar, N.; Bender, M.L.; Barnett, B.A.; Fan, S.; Moxim, W.J.; Levy, H.; Tilbrook, B. The Southern Ocean Biological Response to Aeolian Iron Deposition. Science 2007, 317, 1067–1070. [Google Scholar] [CrossRef]
- Mills, M.M.; Alderkamp, A.-C.; Thuróczy, C.-E.; van Dijken, G.L.; Laan, P.; de Baar, H.J.W.; Arrigo, K.R. Phytoplankton Biomass and Pigment Responses to Fe Amendments in the Pine Island and Amundsen Polynyas. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 61–76. [Google Scholar] [CrossRef]
- Arrigo, K.R.; DiTullio, G.R.; Dunbar, R.B.; Robinson, D.H.; VanWoert, M.; Worthen, D.L.; Lizotte, M.P. Phytoplankton Taxonomic Variability in Nutrient Utilization and Primary Production in the Ross Sea. J. Geophys. Res. Ocean. 2000, 105, 8827–8846. [Google Scholar] [CrossRef]
- Maucher, J.M.; DiTullio, G.R. Flavodoxin as a Diagnostic Indicator of Chronic Iron Limitation in the Ross Sea and New Zealand Sector of the Southern Ocean. Antarct. Res. Ser. 2003, 78, 11. [Google Scholar]
- Boyd, P.W.; Watson, A.J.; Law, C.S.; Abraham, E.R.; Trull, T.; Murdoch, R.; Bakker, D.C.E.; Bowie, A.R.; Buesseler, K.O.; Chang, H.; et al. A Mesoscale Phytoplankton Bloom in the Polar Southern Ocean Stimulated by Iron Fertilization. Nature 2000, 407, 695–702. [Google Scholar] [CrossRef]
- Boyd, P.W.; Law, C.S. The Southern Ocean Iron RElease Experiment (SOIREE)—Introduction and Summary. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 2425–2438. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.T.; Boyd, P.W.; LaRoche, J.; Strzepek, R.; Waite, A.; Bowie, A.R.; Croot, P.L.; Frew, R.D.; Price, N.M. Iron Uptake and Physiological Response of Phytoplankton during a Mesoscale Southern Ocean Iron Enrichment. Limnol. Oceanogr. 2001, 46, 1802–1808. [Google Scholar] [CrossRef] [Green Version]
- Gervais, F.; Riebesell, U.; Gorbunov, M.Y. Changes in Primary Productivity and Chlorophyll a in Response to Iron Fertilization in the Southern Polar Frontal Zone. Limnol. Oceanogr. 2002, 47, 1324–1335. [Google Scholar] [CrossRef] [Green Version]
- Coale, K.H.; Johnson, K.S.; Chavez, F.P.; Buesseler, K.O.; Barber, R.T.; Brzezinski, M.A.; Cochlan, W.P.; Millero, F.J.; Falkowski, P.G.; Bauer, J.E.; et al. Southern Ocean Iron Enrichment Experiment: Carbon Cycling in High- and Low-Si Waters. Science 2004, 304, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buesseler, K.O.; Andrews, J.E.; Pike, S.M.; Charette, M.A. The Effects of Iron Fertilization on Carbon Sequestration in the Southern Ocean. Science 2004, 304, 414–417. [Google Scholar] [CrossRef]
- Hoffmann, L.J.; Peeken, I.; Lochte, K.; Assmy, P.; Veldhuis, M. Different Reactions of Southern Ocean Phytoplankton Size Classes to Iron Fertilization. Limnol. Oceanogr. 2006, 51, 1217–1229. [Google Scholar] [CrossRef] [Green Version]
- Harvey, M.J.; Law, C.S.; Smith, M.J.; Hall, J.A.; Abraham, E.R.; Stevens, C.L.; Hadfield, M.G.; Ho, D.T.; Ward, B.; Archer, S.D.; et al. The SOLAS Air–Sea Gas Exchange Experiment (SAGE) 2004. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.; van der Loeff, M.R.; Cassar, N.; Vandromme, P.; d’Ovidio, F.; Stemmann, L.; Rengarajan, R.; Soares, M.; González, H.E.; Ebersbach, F.; et al. Iron Fertilization Enhanced Net Community Production but Not Downward Particle Flux during the Southern Ocean Iron Fertilization Experiment LOHAFEX. Glob. Biogeochem. Cycles 2013, 27, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Smetacek, V.; Naqvi, S.W.A. The Expedition of the Research Vessel “Polarstern” to the Antarctic in 2009 (ANT-XXV/3 - LOHAFEX); Berichte zur Polar- und Meeresforschung (Reports on Polar and Marine Research); Alfred Wegener Institute for Polar and Marine Research: Bremerhaven, Germany, 2010; Volume 613. [Google Scholar]
- Pollard, R.T.; Salter, I.; Sanders, R.J.; Lucas, M.I.; Moore, C.M.; Mills, R.A.; Statham, P.J.; Allen, J.T.; Baker, A.R.; Bakker, D.C.E.; et al. Southern Ocean Deep-Water Carbon Export Enhanced by Natural Iron Fertilization. Nature 2009, 457, 577–580. [Google Scholar] [CrossRef]
- Christaki, U.; Lefèvre, D.; Georges, C.; Colombet, J.; Catala, P.; Courties, C.; Sime-Ngando, T.; Blain, S.; Obernosterer, I. Microbial Food Web Dynamics during Spring Phytoplankton Blooms in the Naturally Iron-Fertilized Kerguelen Area (Southern Ocean). Biogeosciences 2014, 11, 6739–6753. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.; LaRoche, J.; Gall, M.; Frew, R.; McKay, R.M.L. Role of Iron, Light, and Silicate in Controlling Algal Biomass in Subantarctic Waters SE of New Zealand. JGR Ocean. 1999, 104, 13395–13408. [Google Scholar] [CrossRef] [Green Version]
- Franck, V.M.; Brzezinski, M.A.; Coale, K.H.; Nelson, D.M. Iron and Silicic Acid Concentrations Regulate Si Uptake North and South of the Polar Frontal Zone in the Pacific Sector of the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 3315–3338. [Google Scholar] [CrossRef]
- Boyd, P.W.; Crossley, A.C.; DiTullio, G.R.; Griffiths, F.B.; Hutchins, D.A.; Queguiner, B.; Sedwick, P.N.; Trull, T.W. Control of Phytoplankton Growth by Iron Supply and Irradiance in the Subantarctic Southern Ocean: Experimental Results from the SAZ Project. JGR Ocean. 2001, 106, 31573–31583. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, D.A.; Sedwick, P.N.; DiTullio, G.R.; Boyd, P.W.; Quéguiner, B.; Griffiths, F.B.; Crossley, C. Control of Phytoplankton Growth by Iron and Silicic Acid Availability in the Subantarctic Southern Ocean: Experimental Results from the SAZ Project. J. Geophys. Res. Ocean. 2001, 106, 31559–31572. [Google Scholar] [CrossRef] [Green Version]
- Hinz, D.J.; Nielsdóttir, M.C.; Korb, R.E.; Whitehouse, M.J.; Poulton, A.J.; Moore, C.M.; Achterberg, E.P.; Bibby, T.S. Responses of Microplankton Community Structure to Iron Addition in the Scotia Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 59–60, 36–46. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; van Dijken, G.L.; Lowry, K.E.; Connelly, T.L.; Lagerström, M.; Sherrell, R.M.; Haskins, C.; Rogalsky, E.; Schofield, O.; Stammerjohn, S.E.; et al. Fe Availability Drives Phytoplankton Photosynthesis Rates during Spring Bloom in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anthr. 2015, 3, 000043. [Google Scholar] [CrossRef] [Green Version]
- Ryan-Keogh, T.J.; DeLizo, L.M.; Smith, W.O.; Sedwick, P.N.; McGillicuddy, D.J.; Moore, C.M.; Bibby, T.S. Temporal Progression of Photosynthetic-Strategy in Phytoplankton in the Ross Sea, Antarctica. J. Mar. Syst. 2017, 166, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, J.J.; Philibert, R.; Van Horsten, N.; Mtshali, T.; Roychoudhury, A.N.; Thomalla, S.; Fietz, S. Phytoplankton Response in Growth, Photophysiology and Community Structure to Iron and Light in the Polar Frontal Zone and Antarctic Waters. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 141, 118–129. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; van Dijken, G.L.; Lowry, K.E.; Lewis, K.M.; Joy-Warren, H.L.; van de Poll, W.; Laan, P.; Gerringa, L.; Delmont, T.O.; Jenkins, B.D.; et al. Effects of Iron and Light Availability on Phytoplankton Photosynthetic Properties in the Ross Sea. Mar. Ecol. Prog. Ser. 2019, 621, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Vives, C.R.; Schallenberg, C.; Strutton, P.G.; Westwood, K.J. Iron and Light Limitation of Phytoplankton Growth off East Antarctica. J. Mar. Syst. 2022, 234, 103774. [Google Scholar] [CrossRef]
- Moore, J.K.; Doney, S.C.; Glover, D.M.; Fung, I.Y. Iron Cycling and Nutrient-Limitation Patterns in Surface Waters of the World Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 463–507. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.W.; Strzepek, R.; Fu, F.; Hutchins, D.A. Environmental Control of Open-Ocean Phytoplankton Groups: Now and in the Future. Limnol. Oceanogr. 2010, 55, 1353–1376. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Interrelated Influence of Iron, Light and Cell Size on Marine Phytoplankton Growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Hunter, K.A.; Frew, R.D.; Harrison, P.J.; Boyd, P.W. Iron-Light Interactions Differ in Southern Ocean Phytoplankton. Limnol. Oceanogr. 2012, 57, 1182–1200. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.M.; Lin, Y.; Davies, S.; Monbureau, E.; Cassar, N.; Marchetti, A. Examination of Gene Repertoires and Physiological Responses to Iron and Light Limitation in Southern Ocean Diatoms. Polar Biol. 2018, 41, 679–696. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Boyd, P.W.; Sunda, W.G. Photosynthetic Adaptation to Low Iron, Light, and Temperature in Southern Ocean Phytoplankton. Proc. Natl. Acad. Sci. USA 2019, 116, 4388–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coale, T.H.; Bertrand, E.M.; Lampe, R.H.; Allen, A.E. Molecular Mechanisms Underlying Micronutrient Utilization in Marine Diatoms. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 567–604. ISBN 978-3-030-92499-7. [Google Scholar]
- Rose, J.M.; Feng, Y.; DiTullio, G.R.; Dunbar, R.B.; Hare, C.E.; Lee, P.A.; Lohan, M.; Long, M.; Smith, W.O., Jr.; Sohst, B.; et al. Synergistic Effects of Iron and Temperature on Antarctic Phytoplankton and Microzooplankton Assemblages. Biogeosciences 2009, 6, 3131–3147. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xu, K.; Fu, F.; Spackeen, J.; Bronk, D.; Hutchins, D. A Comparative Study of Iron and Temperature Interactive Effects on Diatoms and Phaeocystis Antarctica from the Ross Sea, Antarctica. Mar. Ecol. Prog. Ser. 2016, 550, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Jabre, L.J.; Allen, A.E.; McCain, J.S.P.; McCrow, J.P.; Tenenbaum, N.; Spackeen, J.L.; Sipler, R.E.; Green, B.R.; Bronk, D.A.; Hutchins, D.A.; et al. Molecular Underpinnings and Biogeochemical Consequences of Enhanced Diatom Growth in a Warming Southern Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2107238118. [Google Scholar] [CrossRef]
- Booth, B.C.; Lewin, J.; Postel, J.R. Temporal Variation in the Structure of Autotrophic and Heterotrophic Communities in the Subarctic Pacific. Prog. Oceanogr. 1993, 32, 57–99. [Google Scholar] [CrossRef]
- Mangoni, O.; Saggiomo, V.; Bolinesi, F.; Margiotta, F.; Budillon, G.; Cotroneo, Y.; Misic, C.; Rivaro, P.; Saggiomo, M. Phytoplankton Blooms during Austral Summer in the Ross Sea, Antarctica: Driving Factors and Trophic Implications. PLoS ONE 2017, 12, e0176033. [Google Scholar] [CrossRef] [Green Version]
- Kohut, J.T.; Kustka, A.B.; Hiscock, M.R.; Lam, P.J.; Measures, C.; Milligan, A.; White, A.; Carvalho, F.; Hatta, M.; Jones, B.M.; et al. Mesoscale Variability of the Summer Bloom over the Northern Ross Sea Shelf: A Tale of Two Banks. J. Mar. Syst. 2017, 166, 50–60. [Google Scholar] [CrossRef]
- Saggiomo, V.; Catalano, G.; Mangoni, O.; Budillon, G.; Carrada, G.C. Primary Production Processes in Ice-Free Waters of the Ross Sea (Antarctica) during the Austral Summer 1996. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1787–1801. [Google Scholar] [CrossRef]
- Hatta, M.; Measures, C.I.; Lam, P.J.; Ohnemus, D.C.; Auro, M.E.; Grand, M.M.; Selph, K.E. The Relative Roles of Modified Circumpolar Deep Water and Benthic Sources in Supplying Iron to the Recurrent Phytoplankton Blooms above Pennell and Mawson Banks, Ross Sea, Antarctica. J. Mar. Syst. 2017, 166, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.S.; Giulivi, C.F. Large Multidecadal Salinity Trends near the Pacific–Antarctic Continental Margin. J. Clim. 2010, 23, 4508–4524. [Google Scholar] [CrossRef]
- Budillon, G.; Castagno, P.; Aliani, S.; Spezie, G.; Padman, L. Thermohaline Variability and Antarctic Bottom Water Formation at the Ross Sea Shelf Break. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 1002–1018. [Google Scholar] [CrossRef]
- Bolinesi, F.; Saggiomo, M.; Ardini, F.; Castagno, P.; Cordone, A.; Fusco, G.; Rivaro, P.; Saggiomo, V.; Mangoni, O. Spatial-Related Community Structure and Dynamics in Phytoplankton of the Ross Sea, Antarctica. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Saggiomo, M.; Escalera, L.; Saggiomo, V.; Bolinesi, F.; Mangoni, O. Phytoplankton Blooms Below the Antarctic Landfast Ice During the Melt Season Between Late Spring and Early Summer. J. Phycol. 2021, 57, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Rivaro, P.; Ianni, C.; Langone, L.; Ori, C.; Aulicino, G.; Cotroneo, Y.; Saggiomo, M.; Mangoni, O. Physical and Biological Forcing of Mesoscale Variability in the Carbonate System of the Ross Sea (Antarctica) during Summer 2014. J. Mar. Syst. 2017, 166, 144–158. [Google Scholar] [CrossRef]
- Bender, S.J.; Moran, D.M.; McIlvin, M.R.; Zheng, H.; McCrow, J.P.; Badger, J.; DiTullio, G.R.; Allen, A.E.; Saito, M.A. Colony Formation in Phaeocystis Antarctica: Connecting Molecular Mechanisms with Iron Biogeochemistry. Biogeosciences 2018, 15, 4923–4942. [Google Scholar] [CrossRef] [Green Version]
- Martínez-García, A.; Sigman, D.M.; Ren, H.; Anderson, R.F.; Straub, M.; Hodell, D.A.; Jaccard, S.L.; Eglinton, T.I.; Haug, G.H. Iron Fertilization of the Subantarctic Ocean During the Last Ice Age. Science 2014, 343, 1347–1350. [Google Scholar] [CrossRef]
- Smetacek, V.; Naqvi, S.W.A. The next Generation of Iron Fertilization Experiments in the Southern Ocean. Phil. Trans. R. Soc. A. 2008, 366, 3947–3967. [Google Scholar] [CrossRef]
- Denman, K.L. Climate Change, Ocean Processes and Ocean Iron Fertilization. Mar. Ecol. Prog. Ser. 2008, 364, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Smetacek, V.; Klaas, C.; Strass, V.H.; Assmy, P.; Montresor, M.; Cisewski, B.; Savoye, N.; Webb, A.; d’Ovidio, F.; Arrieta, J.M.; et al. Deep Carbon Export from a Southern Ocean Iron-Fertilized Diatom Bloom. Nature 2012, 487, 313–319. [Google Scholar] [CrossRef]
- Tripathy, S.C.; Jena, B. Iron-Stimulated Phytoplankton Blooms in the Southern Ocean: A Brief Review. Remote Sens. Earth Syst. Sci. 2019, 2, 64–77. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- IPCC. Climate Change 2007: Mitigation. In Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Lambert, F.; Tagliabue, A.; Shaffer, G.; Lamy, F.; Winckler, G.; Farias, L.; Gallardo, L.; De Pol-Holz, R. Dust Fluxes and Iron Fertilization in Holocene and Last Glacial Maximum Climates. Geophys. Res. Lett. 2015, 42, 6014–6023. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.W.; Claustre, H.; Levy, M.; Siegel, D.A.; Weber, T. Multi-Faceted Particle Pumps Drive Carbon Sequestration in the Ocean. Nature 2019, 568, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Bowie, A.R.; Boyd, P.W.; Buck, K.N.; Johnson, K.S.; Saito, M.A. The Integral Role of Iron in Ocean Biogeochemistry. Nature 2017, 543, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Oschlies, A.; Koeve, W.; Rickels, W.; Rehdanz, K. Side Effects and Accounting Aspects of Hypothetical Large-Scale Southern Ocean Iron Fertilization. Biogeosciences 2010, 7, 4017–4035. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, J.A.; Capone, D.G. Possible Biogeochemical Consequences of Ocean Fertilization. Limnol. Oceanogr. 1991, 36, 1951–1959. [Google Scholar] [CrossRef]
- Silver, M.W.; Bargu, S.; Coale, S.L.; Benitez-Nelson, C.R.; Garcia, A.C.; Roberts, K.J.; Sekula-Wood, E.; Bruland, K.W.; Coale, K.H. Toxic Diatoms and Domoic Acid in Natural and Iron Enriched Waters of the Oceanic Pacific. Proc. Natl. Acad. Sci. USA 2010, 107, 20762–20767. [Google Scholar] [CrossRef] [Green Version]
- Trick, C.G.; Bill, B.D.; Cochlan, W.P.; Wells, M.L.; Trainer, V.L.; Pickell, L.D. Iron Enrichment Stimulates Toxic Diatom Production in High-Nitrate, Low-Chlorophyll Areas. Proc. Natl. Acad. Sci. USA 2010, 107, 5887–5892. [Google Scholar] [CrossRef] [Green Version]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P.; et al. Climate Change and Southern Ocean Ecosystems I: How Changes in Physical Habitats Directly Affect Marine Biota. Glob. Change Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef] [Green Version]
- Meredith, M.; Sommerkorn, M.; Cassotta, S.; Derksen, C.; Ekaykin, A.; Hollowed, A.; Kofinas, G.; Mackintosh, A.; Melbourne-Thomas, J.; Muelbert, M.M.C.; et al. Polar Regions. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- Shadwick, E.H.; Rintoul, S.R.; Tilbrook, B.; Williams, G.D.; Young, N.; Fraser, A.D.; Marchant, H.; Smith, J.; Tamura, T. Glacier Tongue Calving Reduced Dense Water Formation and Enhanced Carbon Uptake. Geophys. Res. Lett. 2013, 40, 904–909. [Google Scholar] [CrossRef]
- Cape, M.R.; Vernet, M.; Kahru, M.; Spreen, G. Polynya Dynamics Drive Primary Production in the Larsen A and B Embayments Following Ice Shelf Collapse. J. Geophys. Res. Ocean. 2014, 119, 572–594. [Google Scholar] [CrossRef]
- Monien, P.; Lettmann, K.A.; Monien, D.; Asendorf, S.; Wölfl, A.-C.; Lim, C.H.; Thal, J.; Schnetger, B.; Brumsack, H.-J. Redox Conditions and Trace Metal Cycling in Coastal Sediments from the Maritime Antarctic. Geochim. Cosmochim. Acta 2014, 141, 26–44. [Google Scholar] [CrossRef]
- Duprat, L.P.A.M.; Bigg, G.R.; Wilton, D.J. Enhanced Southern Ocean Marine Productivity Due to Fertilization by Giant Icebergs. Nat. Geosci. 2016, 9, 219–221. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Continued Increases in Arctic Ocean Primary Production. Prog. Oceanogr. 2015, 136, 60–70. [Google Scholar] [CrossRef]
- Duprat, L.; Kanna, N.; Janssens, J.; Roukaerts, A.; Deman, F.; Townsend, A.T.; Meiners, K.M.; van der Merwe, P.; Lannuzel, D. Enhanced Iron Flux to Antarctic Sea Ice via Dust Deposition From Ice-Free Coastal Areas. J. Geophys. Res. Ocean. 2019, 124, 8538–8557. [Google Scholar] [CrossRef]
- Van der Merwe, P.; Wuttig, K.; Holmes, T.; Trull, T.W.; Chase, Z.; Townsend, A.T.; Goemann, K.; Bowie, A.R. High Lability Fe Particles Sourced From Glacial Erosion Can Meet Previously Unaccounted Biological Demand: Heard Island, Southern Ocean. Front. Mar. Sci. 2019, 6, 332. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Fu, F.-X.; Hutchins, D.A. Comparative Responses of Two Dominant Antarctic Phytoplankton Taxa to Interactions between Ocean Acidification, Warming, Irradiance, and Iron Availability. Limnol. Oceanogr. 2014, 59, 1919–1931. [Google Scholar] [CrossRef]
- Boyd, P.W.; Dillingham, P.W.; McGraw, C.M.; Armstrong, E.A.; Cornwall, C.E.; Feng, Y.-Y.; Hurd, C.L.; Gault-Ringold, M.; Roleda, M.Y.; Timmins-Schiffman, E.; et al. Physiological Responses of a Southern Ocean Diatom to Complex Future Ocean Conditions. Nat. Clim. Change 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Feng, Y.; Hare, C.E.; Rose, J.M.; Handy, S.M.; DiTullio, G.R.; Lee, P.A.; Smith, W.O.; Peloquin, J.; Tozzi, S.; Sun, J.; et al. Interactive Effects of Iron, Irradiance and CO2 on Ross Sea Phytoplankton. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 368–383. [Google Scholar] [CrossRef]
- Hoppe, C.J.M.; Hassler, C.S.; Payne, C.D.; Tortell, P.D.; Rost, B.; Trimborn, S. Iron Limitation Modulates Ocean Acidification Effects on Southern Ocean Phytoplankton Communities. PLoS ONE 2013, 8, e79890. [Google Scholar] [CrossRef]
- Deppeler, S.; Petrou, K.; Schulz, K.G.; Westwood, K.; Pearce, I.; McKinlay, J.; Davidson, A. Ocean Acidification of a Coastal Antarctic Marine Microbial Community Reveals a Critical Threshold for CO2 Tolerance in Phytoplankton Productivity. Biogeosciences 2018, 15, 209–231. [Google Scholar] [CrossRef] [Green Version]
- Schofield, O.; Brown, M.; Kohut, J.; Nardelli, S.; Saba, G.; Waite, N.; Ducklow, H. Changes in the Upper Ocean Mixed Layer and Phytoplankton Productivity along the West Antarctic Peninsula. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170173. [Google Scholar] [CrossRef]
- Panassa, E.; Völker, C.; Wolf-Gladrow, D.; Hauck, J. Drivers of Interannual Variability of Summer Mixed Layer Depth in the Southern Ocean Between 2002 and 2011. J. Geophys. Res. Ocean. 2018, 123, 5077–5090. [Google Scholar] [CrossRef]
- Leung, S.; Cabré, A.; Marinov, I. A Latitudinally Banded Phytoplankton Response to 21st Century Climate Change in the Southern Ocean across the CMIP5 Model Suite. Biogeosciences 2015, 12, 5715–5734. [Google Scholar] [CrossRef] [Green Version]
- King, A.L.; Sañudo-Wilhelmy, S.A.; Boyd, P.W.; Twining, B.S.; Wilhelm, S.W.; Breene, C.; Ellwood, M.J.; Hutchins, D.A. A Comparison of Biogenic Iron Quotas during a Diatom Spring Bloom Using Multiple Approaches. Biogeosciences 2012, 9, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Luxem, K.E.; Ellwood, M.J.; Strzepek, R.F. Intraspecific Variability in Phaeocystis Antarctica’s Response to Iron and Light Stress. PLoS ONE 2017, 12, e0179751. [Google Scholar] [CrossRef] [Green Version]
- Pinkerton, M.H.; Boyd, P.W.; Deppeler, S.; Hayward, A.; Höfer, J.; Moreau, S. Evidence for the Impact of Climate Change on Primary Producers in the Southern Ocean. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Chappell, P.D.; Whitney, L.P.; Wallace, J.R.; Darer, A.I.; Jean-Charles, S.; Jenkins, B.D. Genetic Indicators of Iron Limitation in Wild Populations of Thalassiosira Oceanica from the Northeast Pacific Ocean. ISME J. 2015, 9, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Whitney, L.; Lins, J.; Hughes, M.; Wells, M.; Chappell, P.; Jenkins, B. Characterization of Putative Iron Responsive Genes as Species-Specific Indicators of Iron Stress in Thalassiosiroid Diatoms. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdner, D.L.; Price, N.M.; Doucette, G.J.; Peleato, M.L.; Anderson, D.M. Characterization of Ferredoxin and Flavodoxin as Markers of Iron Limitation in Marine Phytoplankton. Mar. Ecol. Prog. Ser. 1999, 184, 43–53. [Google Scholar] [CrossRef]
- Marchetti, A.; Moreno, C.M.; Cohen, N.R.; Oleinikov, I.; de Long, K.; Twining, B.S.; Armbrust, E.V.; Lampe, R.H. Development of a Molecular-Based Index for Assessing Iron Status in Bloom-Forming Pennate Diatoms. J. Phycol. 2017, 53, 820–832. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzani, E.; Lauritano, C.; Saggiomo, M. Southern Ocean Iron Limitation of Primary Production between Past Knowledge and Future Projections. J. Mar. Sci. Eng. 2023, 11, 272. https://doi.org/10.3390/jmse11020272
Bazzani E, Lauritano C, Saggiomo M. Southern Ocean Iron Limitation of Primary Production between Past Knowledge and Future Projections. Journal of Marine Science and Engineering. 2023; 11(2):272. https://doi.org/10.3390/jmse11020272
Chicago/Turabian StyleBazzani, Emma, Chiara Lauritano, and Maria Saggiomo. 2023. "Southern Ocean Iron Limitation of Primary Production between Past Knowledge and Future Projections" Journal of Marine Science and Engineering 11, no. 2: 272. https://doi.org/10.3390/jmse11020272
APA StyleBazzani, E., Lauritano, C., & Saggiomo, M. (2023). Southern Ocean Iron Limitation of Primary Production between Past Knowledge and Future Projections. Journal of Marine Science and Engineering, 11(2), 272. https://doi.org/10.3390/jmse11020272







