Can a 16th Century Shipwreck Be Considered a Mercury Source in the 21st Century?—A Case Study in the Azores Archipelago (Portugal)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Total Mercury Quantification
2.3. Assessment of Hg Pollution
2.3.1. Geoaccumulation Index (Igeo)
2.3.2. Contamination Factor (CF)
2.3.3. Potential Ecological Risk Index (PERI)
2.4. Statistical Analysis
3. Results and Discussion
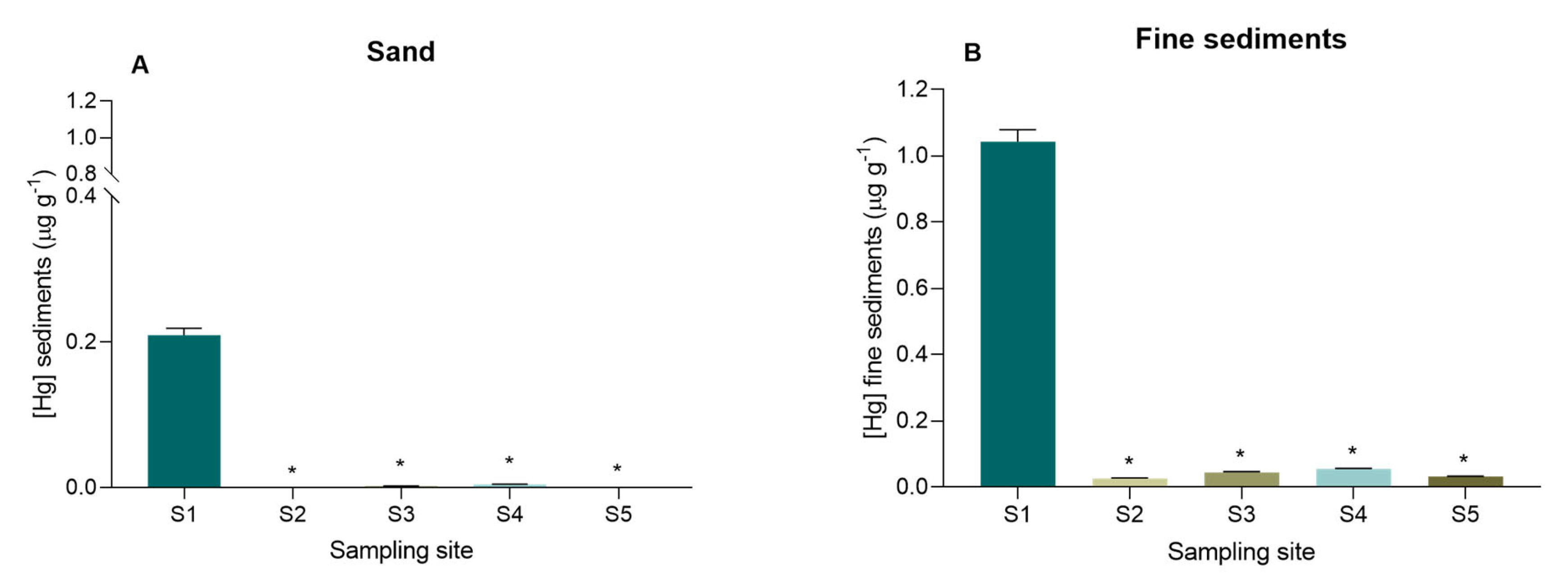
3.1. Mercury in Surface Sediments
3.2. Assessment of Mercury Pollution
3.3. Mercury Accumulation in Intertidal Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engstrom, D.R.; Fitzgerald, W.F.; Cooke, C.A.; Lamborg, C.H.; Drevnick, P.E.; Swain, E.B.; Balogh, S.J.; Balcom, P.H. Atmospheric Hg Emissions from Preindustrial Gold and Silver Extraction in the Americas: A Reevaluation from Lake-Sediment Archives. Environ. Sci. Technol. 2014, 48, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D. Global Mercury Emissions from Gold and Silver Mining. Water Air Soil Pollut 1997, 97, 209–221. [Google Scholar] [CrossRef]
- Gibb, H.; O’Leary, K.G. Mercury Exposure and Health Impacts among Individuals in the Artisanal and Small-Scale Gold Mining Community: A Comprehensive Review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.M.; Maxson, P.A.; Hylander, L.D. Origin and consumption of mercury in small-scale gold mining. J. Clean. Prod. 2006, 14, 436–447. [Google Scholar] [CrossRef]
- Malm, O. Gold Mining as a Source of Mercury Exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78. [Google Scholar] [CrossRef]
- Leshikar-Denton, M.E. Problems and Progress in the Caribbean. In International Handbook of Underwater Archaeology; Ruppé, C.V., Barstad, J.F., Eds.; Springer: Boston, MA, USA, 2002; pp. 279–298. [Google Scholar]
- Nriagu, J.O. Mercury pollution from the past mining of gold and silver in the Americas. Sci. Total Environ. 1994, 149, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Rogowska, J.; Kudłak, B.; Tsakovski, S.; Gałuszka, A.; Bajger-Nowak, G.; Simeonov, V.; Konieczka, P.; Wolska, L.; Namieśnik, J. Surface sediments pollution due to shipwreck s/s “Stuttgart”: A multidisciplinary approach. Stoch. Environ. Res. Risk Assess. 2015, 29, 1797–1807. [Google Scholar] [CrossRef]
- Smith, R.C. Discovery, Development, and Interpretation of Florida’s Earliest Shipwreck: A Partnership in Research and Historic Preservation. In Underwater Archaeology; The Society for Historical Archaeology: Uniontown, PA, USA, 1998; pp. 115–121. [Google Scholar]
- Monteiro, A. Underwater Archaeological Trails and Preserves in Portugal; Instituto de Arqueologia e Paleociências, Faculdade de Ciências Socias e Humanas, Universidade Nova de Lisboa: Lisbon, Protugal, 2013. [Google Scholar]
- Garcia, C.; Monteiro, P. The excavation and dismantling of Angra D, a probable Iberian seagoing ship, Angra bay, Terceira Island, Azores, Portugal. Preliminary assessment. In Trabalhos de Arqueologia, Proceedings of the International Symposium on Archaeology of Medieval and Modern Ships of Iberian-Atlantic Tradition, Lisbon, Portugal, 7–9 September 1998; Instituto Português de Arqueologia: Lisbon, Portugal, 2001; pp. 431–447. [Google Scholar]
- Leermakers, M.; Baeyens, W.; Quevauviller, P.; Horvat, M. Mercury in environmental samples: Speciation, artifacts and validation. TrAC Trends Anal. Chem. 2005, 24, 383–393. [Google Scholar] [CrossRef]
- Nyström, J. The OSPAR List of Chemicals for Priority Action: Suggestions for Future Actions; Swedish Environmental Protection Agency: Stockholm, Sweden, 2018. [Google Scholar]
- Ma, M.; Du, H.; Wang, D. Mercury methylation by anaerobic microorganisms: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1893–1936. [Google Scholar] [CrossRef]
- Gonzalez-Raymat, H.; Liu, G.; Liriano, C.; Li, Y.; Yin, Y.; Shi, J.; Jiang, G.; Cai, Y. Elemental mercury: Its unique properties affect its behavior and fate in the environment. Environ. Pollut. 2017, 229, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003, 56, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Amyot, M.; Morel, F.M.M.; Ariya, P.A. Dark Oxidation of Dissolved and Liquid Elemental Mercury in Aquatic Environments. Environ. Sci. Technol. 2005, 39, 110–114. [Google Scholar] [CrossRef]
- Wang, K.; Liu, G.; Cai, Y. Possible pathways for mercury methylation in oxic marine waters. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3997–4015. [Google Scholar] [CrossRef]
- Guzzi, G.; La Porta, C.A.M. Molecular mechanisms triggered by mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef]
- Kwasigroch, U.; Bełdowska, M.; Jędruch, A.; Łukawska-Matuszewska, K. Distribution and bioavailability of mercury in the surface sediments of the Baltic Sea. Environ. Sci. Pollut. Res. 2021, 28, 35690–35708. [Google Scholar] [CrossRef]
- Weis, J.S. Physiological, Developmental and Behavioral Effects of Marine Pollution; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Tsui, M.T.-K.; Blum, J.D.; Finlay, J.C.; Balogh, S.J.; Nollet, Y.H.; Palen, W.J.; Power, M.E. Variation in Terrestrial and Aquatic Sources of Methylmercury in Stream Predators as Revealed by Stable Mercury Isotopes. Environ. Sci. Technol. 2014, 48, 10128–10135. [Google Scholar] [CrossRef] [Green Version]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Fitzgerald, W.F.; Lamborg, C.H.; Hammerschmidt, C.R. Marine Biogeochemical Cycling of Mercury. Chem. Rev. 2007, 107, 641–662. [Google Scholar] [CrossRef]
- Vieira, H.C.; Bordalo, M.D.; Figueroa, A.G.; Soares, A.M.V.M.; Morgado, F.; Abreu, S.N.; Rendón-von Osten, J. Mercury distribution and enrichment in coastal sediments from different geographical areas in the North Atlantic Ocean. Mar. Pollut. Bull. 2021, 165, 112153. [Google Scholar] [CrossRef]
- Chakraborty, P.; Sarkar, A.; Vudamala, K.; Naik, R.; Nath, B.N. Organic matter—A key factor in controlling mercury distribution in estuarine sediment. Mar. Chem. 2015, 173, 302–309. [Google Scholar] [CrossRef]
- Cukrov, N.; Doumandji, N.; Garnier, C.; Tucaković, I.; Dang, D.H.; Omanović, D.; Cukrov, N. Anthropogenic mercury contamination in sediments of Krka River estuary (Croatia). Environ. Sci. Pollut. Res. 2020, 27, 7628–7638. [Google Scholar] [CrossRef]
- Landquist, H.; Hassellöv, I.-M.; Rosén, L.; Lindgren, J.; Dahllöf, I. Evaluating the needs of risk assessment methods of potentially polluting shipwrecks. J. Environ. Manag. 2013, 119, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.E.; Bolam, S.G.; Brant, J.L.; Brash, R.; Goodsir, F.; Hynes, C.; McGenity, T.J.; McIlwaine, P.S.O.; McKew, B.A. Evaluation of Polycyclic Aromatic Hydrocarbon Pollution From the HMS Royal Oak Shipwreck and Effects on Sediment Microbial Community Structure. Front. Mar. Sci. 2021, 8, 650139. [Google Scholar] [CrossRef]
- Schiel, D.R.; Ross, P.M.; Battershill, C.N. Environmental effects of the MV Rena shipwreck: Cross-disciplinary investigations of oil and debris impacts on a coastal ecosystem. N. Z. J. Mar. Freshw. Res. 2016, 50, 1–9. [Google Scholar] [CrossRef]
- Faksness, L.-G.; Daling, P.; Altin, D.; Dolva, H.; Fosbæk, B.; Bergstrøm, R. Relative bioavailability and toxicity of fuel oils leaking from World War II shipwrecks. Mar. Pollut. Bull. 2015, 94, 123–130. [Google Scholar] [CrossRef]
- Ventikos, N.P.; Louzis, K.; Koimtzoglou, A. The Shipwrecks in Greece are Going Fuzzy: A Study for the Potential of Oil Pollution from Shipwrecks in Greek Waters. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 462–491. [Google Scholar] [CrossRef]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Müller, G. Schadstoffe in sedimenten-sedimente als schadstoffe. Mitt Österr. Geol. Ges. 1986, 79, 107–126. [Google Scholar]
- ICES. Report of the ICES Advisory Committee on the Marine Environment; ICES Advice; ICES: Copenhagen, Denmark, 2004; Volume 1, Number 1, 283p. [Google Scholar]
- Williams, J.A.; Antoine, J. Evaluation of the elemental pollution status of Jamaican surface sediments using enrichment factor, geoaccumulation index, ecological risk and potential ecological risk index. Mar. Pollut. Bull. 2020, 157, 111288. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Ben Mna, H.; Helali, M.A.; Oueslati, W.; Amri, S.; Aleya, L. Spatial distribution, contamination assessment and potential ecological risk of some trace metals in the surface sediments of the Gulf of Tunis, North Tunisia. Mar. Pollut. Bull. 2021, 170, 112608. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Setia, R. A meta-analysis of potential ecological risk evaluation of heavy metals in sediments and soils. Gondwana Res. 2022, 103, 487–501. [Google Scholar] [CrossRef]
- Haris, H.; Aris, A.Z.; Mokhtar, M.b. Mercury and methylmercury distribution in the intertidal surface sediment of a heavily anthrophogenically impacted saltwater-mangrove-sediment interplay zone. Chemosphere 2017, 166, 323–333. [Google Scholar] [CrossRef]
- Haris, H.; Aris, A.Z.; Mokhtar, M.B.; Looi, L.J. The accumulation of metals and methylmercury in Nerita lineata and the relation to intertidal surface sediment concentrations. Chemosphere 2020, 245, 125590. [Google Scholar] [CrossRef]
- Morgado, F.; Santos, R.M.A.L.; Sampaio, D.; de Lacerda, L.D.; Soares, A.M.V.M.; Vieira, H.C.; Abreu, S. Chronological Trends and Mercury Bioaccumulation in an Aquatic Semiarid Ecosystem under a Global Climate Change Scenario in the Northeastern Coast of Brazil. Animals 2021, 11, 2402. [Google Scholar] [CrossRef]
- Bełdowski, J.; Pempkowiak, J. Mercury transformations in marine coastal sediments as derived from mercury concentration and speciation changes along source/sink transport pathway (Southern Baltic). Estuar. Coast. Shelf Sci. 2007, 72, 370–378. [Google Scholar] [CrossRef]
- Bravo, A.G.; Bouchet, S.; Amouroux, D.; Poté, J.; Dominik, J. Distribution of mercury and organic matter in particle-size classes in sediments contaminated by a waste water treatment plant: Vidy Bay, Lake Geneva, Switzerland. J. Environ. Monit. 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Fang, T.-H.; Chen, R.-Y. Mercury contamination and accumulation in sediments of the East China Sea. J. Environ. Sci. 2010, 22, 1164–1170. [Google Scholar] [CrossRef]
- Ndungu, K.; Beylich, B.A.; Staalstrøm, A.; Øxnevad, S.; Berge, J.A.; Braaten, H.F.V.; Schaanning, M.; Bergstrøm, R. Petroleum oil and mercury pollution from shipwrecks in Norwegian coastal waters. Sci. Total Environ. 2017, 593–594, 624–633. [Google Scholar] [CrossRef]
- Sbriz, L.; Aquino, M.R.; Fowler, S.W.; Sericano, J.L. Levels of chlorinated hydrocarbons and trace metals in bivalves and nearshore sediments from the Dominican Republic. Mar. Pollut. Bull. 1998, 36, 971–979. [Google Scholar] [CrossRef]
- Oliveri, E.; Salvagio Manta, D.; Bonsignore, M.; Cappello, S.; Tranchida, G.; Bagnato, E.; Sabatino, N.; Santisi, S.; Sprovieri, M. Mobility of mercury in contaminated marine sediments: Biogeochemical pathways. Mar. Chem. 2016, 186, 1–10. [Google Scholar] [CrossRef]
- Bengtsson, G.; Picado, F. Mercury sorption to sediments: Dependence on grain size, dissolved organic carbon, and suspended bacteria. Chemosphere 2008, 73, 526–531. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, R.; Zhang, C.; Yin, D.; Yang, S.; Huang, X. Geochemical controls on the distribution of mercury and methylmercury in sediments of the coastal East China Sea. Sci. Total Environ. 2019, 667, 133–141. [Google Scholar] [CrossRef]
- Soana, E.; Naldi, M.; Bartoli, M. Effects of increasing organic matter loads on pore water features of vegetated (Vallisneria spiralis L.) and plant-free sediments. Ecol. Eng. 2012, 47, 141–145. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, B.-O.; Kim, B.; Noh, J.; Hwang, K.; Ryu, J.; Park, J.; Hong, S.; Khim, J.S. Natural and anthropogenic signatures on sedimentary organic matters across varying intertidal habitats in the Korean waters. Environ. Int. 2019, 133, 105166. [Google Scholar] [CrossRef]
- González-Macías, C.; Schifter, I.; Lluch-Cota, D.B.; Méndez-Rodríguez, L.; Hernández-Vázquez, S. Distribution, Enrichment and Accumulation of Heavy Metals in Coastal Sediments of Salina Cruz Bay, México. Environ. Monit. Assess. 2006, 118, 211–230. [Google Scholar] [CrossRef]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs); Office of Response and Restoriation Division, National Oceanic and Atmospheric Administration: Seattle, WA, USA, 2008. [Google Scholar]
- Bignert, A.; Cossa, D.; Emmerson, R.; Fryer, R.; Füll, C.; Fumega, J.; Laane, R.; Calls, H.; McHugh, B.; Miller, B. Final Report. In Proceedings of the OSPAR/ICES Workshop on the Evaluation and Update of Background Reference Concentrations (B/RCs) and Ecotoxicological Assessment Criteria (EACs) and How These Assessment Tools Should Be Used in Assessing Contaminants in Water, Sediment, and Biota, The Hague, The Netherlands, 9–13 February 2004. [Google Scholar]
- CCME. Canadian sediment quality guidelines for the protection of aquatic life: Mercury. In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Vieira, H.C.; Morgado, F.; Soares, A.M.V.M.; Abreu, S.N. Real and Potential Mercury Accumulation in Human Scalp of Adolescents: A Case Study. Biol. Trace Elem. Res. 2015, 163, 19–27. [Google Scholar] [CrossRef]
- Vieira, H.C.; Morgado, F.; Soares, A.M.V.M.; Abreu, S.N. Mercury in Scalp Hair Near the Mid-Atlantic Ridge (MAR) in Relation to High Fish Consumption. Biol. Trace Elem. Res. 2013, 156, 29–35. [Google Scholar] [CrossRef]
- Quartau, R. The Insular Shelf of Faial: Morphological and Sedimentary Evolution; Universidade de Aveiro: Aveiro, Portugal, 2007. [Google Scholar]
- Cabral-Oliveira, J.; Coelho, H.; Pratas, J.; Mendes, S.; Pardal, M.A. Arsenic accumulation in intertidal macroalgae exposed to sewage discharges. J. Appl. Phycol. 2016, 28, 3697–3703. [Google Scholar] [CrossRef]
- Areco, M.M.; Salomone, V.N.; Afonso, M.d.S. Ulva lactuca: A bioindicator for anthropogenic contamination and its environmental remediation capacity. Mar. Environ. Res. 2021, 171, 105468. [Google Scholar] [CrossRef] [PubMed]
- Kamala-Kannan, S.; Prabhu Dass Batvari, B.; Lee, K.J.; Kannan, N.; Krishnamoorthy, R.; Shanthi, K.; Jayaprakash, M. Assessment of heavy metals (Cd, Cr and Pb) in water, sediment and seaweed (Ulva lactuca) in the Pulicat Lake, South East India. Chemosphere 2008, 71, 1233–1240. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Herranz, I.; González-Lorenzo, G.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Paz, S.; Gutiérrez, Á.J. Limpets as bioindicators of element pollution in the coasts of Tenerife (Canary Islands). Environ. Sci. Pollut. Res. 2021, 28, 42999–43006. [Google Scholar] [CrossRef]
- Álvaro, N.V.; Neto, A.I.; Couto, R.P.; Azevedo, J.M.N.; Rodrigues, A.S. Crabs tell the difference—Relating trace metal content with land use and landscape attributes. Chemosphere 2016, 144, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Caliani, I.; Cannicci, S.; Pretti, C.; Baratti, M.; Contini, G.; Vitale, M.; Casini, S.; Fossi, M.C.; Iannucci, A.; Fratini, S. A multidisciplinary integrated approach using Pachygrapsus marmoratus to assess the impact of port activities on mediterranean marine protected areas. Chemosphere 2023, 312, 137129. [Google Scholar] [CrossRef]
- Lozano, G.; Herraiz, E.; Hardisson, A.; Gutiérrez, A.J.; González-Weller, D.; Rubio, C. Heavy and trace metal concentrations in three rockpool shrimp species (Palaemon elegans, Palaemon adspersus and Palaemon serratus) from Tenerife (Canary Islands). Environ. Monit. Assess. 2010, 168, 451–460. [Google Scholar] [CrossRef]
- Shiber, J.; Washburn, E. Lead, mercury, and certain nutrient elements in ulva lactuca (linnaeus) from Ras Beirut, Lebanon. Hydrobiologia 1978, 61, 187–192. [Google Scholar] [CrossRef]
- Green-Ruiz, C.; Ruelas-Inzunza, J.; Páez-Osuna, F. Mercury in surface sediments and benthic organisms from Guaymas Bay, east coast of the Gulf of California. Environ. Geochem. Health 2005, 27, 321. [Google Scholar] [CrossRef]
- Cunha, L.; Amaral, A.; Medeiros, V.; Martins, G.M.; Wallenstein, F.F.M.M.; Couto, R.P.; Neto, A.I.; Rodrigues, A. Bioavailable metals and cellular effects in the digestive gland of marine limpets living close to shallow water hydrothermal vents. Chemosphere 2008, 71, 1356–1362. [Google Scholar] [CrossRef]
- Rua-Ibarz, A.; Bolea-Fernandez, E.; Maage, A.; Frantzen, S.; Valdersnes, S.; Vanhaecke, F. Assessment of Hg Pollution Released from a WWII Submarine Wreck (U-864) by Hg Isotopic Analysis of Sediments and Cancer pagurus Tissues. Environ. Sci. Technol. 2016, 50, 10361–10369. [Google Scholar] [CrossRef] [PubMed]
- Cairrão, E.; Pereira, M.J.; Pastorinho, M.R.; Morgado, F.; Soares, A.M.V.M.; Guilhermino, L. Fucus spp. as a Mercury Contamination Bioindicator in Costal Areas (Northwestern Portugal). Bull. Environ. Contam. Toxicol. 2007, 79, 388–395. [Google Scholar] [CrossRef] [PubMed]




| Guideline | PEL | TEL |
|---|---|---|
| NOOA SQuiRTs 1 | 0.13 | 0.7 |
| EU-WFD 2 | 0.05–0.5 | ▬ |
| Canadian SQG 3 | ▬ | 0.7 |
| Sampling Sites | Assessment of Mercury Pollution | ||
|---|---|---|---|
| Igeo (Obtained Value) | CF (Obtained Value) | PERI (Obtained Value) | |
| S1 | Strongly polluted (4) | Very high contamination (21) | High-risk condition (834) |
| S2 | Practically unpolluted (−1) | Low contamination (0.5) | Low risk (21) |
| S3 | Practically unpolluted (0) | Low contamination (0.9) | Low risk (34) |
| S4 | Practically unpolluted (0) | Low contamination (1) | Low risk (45) |
| S5 | Practically unpolluted (−1) | Low contamination (0.6) | Low risk (26) |
| Sampling Sites | Gravel | Sand | Fine Sediments (Silt) | |||
|---|---|---|---|---|---|---|
| Weight (g) | % | Weight (g) | % | Weight (g) | % | |
| S1 | 0 | 0 | 320.38 | 99.95 | 0.15 | 0.05 |
| S2 | 0 | 0 | 426.89 | 99.94 | 0.24 | 0.06 |
| S3 | 0 | 0 | 297.75 | 99.58 | 1.26 | 0.42 |
| S4 | 0 | 0 | 499.85 | 99.97 | 0.17 | 0.03 |
| S5 | 0 | 0 | 313.78 | 99.96 | 0.13 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, H.C.; Bordalo, M.D.; Osten, J.R.-v.; Soares, A.M.V.M.; Abreu, S.N.; Morgado, F. Can a 16th Century Shipwreck Be Considered a Mercury Source in the 21st Century?—A Case Study in the Azores Archipelago (Portugal). J. Mar. Sci. Eng. 2023, 11, 276. https://doi.org/10.3390/jmse11020276
Vieira HC, Bordalo MD, Osten JR-v, Soares AMVM, Abreu SN, Morgado F. Can a 16th Century Shipwreck Be Considered a Mercury Source in the 21st Century?—A Case Study in the Azores Archipelago (Portugal). Journal of Marine Science and Engineering. 2023; 11(2):276. https://doi.org/10.3390/jmse11020276
Chicago/Turabian StyleVieira, Hugo C., Maria D. Bordalo, Jaime Rendón-von Osten, Amadeu M. V. M. Soares, Sizenando N. Abreu, and Fernando Morgado. 2023. "Can a 16th Century Shipwreck Be Considered a Mercury Source in the 21st Century?—A Case Study in the Azores Archipelago (Portugal)" Journal of Marine Science and Engineering 11, no. 2: 276. https://doi.org/10.3390/jmse11020276







