The Impact of IMTA on the Spatial and Temporal Distribution of the Surface Planktonic Bacteria Community in the Surrounding Sea Area of Xiasanhengshan Island of the East China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Profile and Study Site
2.2. Sample Collection and Processing
2.3. Determination of Environmental Physical and Chemical Properties
2.4. Sequencing Experiment Process
2.5. Data Processing and Bioinformatics Analysis
3. Results and Analysis
3.1. Fish and Shellfish Culture and Yield in the IMTA System and Macroalgae Distribution of Xiasanhengshan Island
3.2. Characteristic Analysis of Environmental Factors
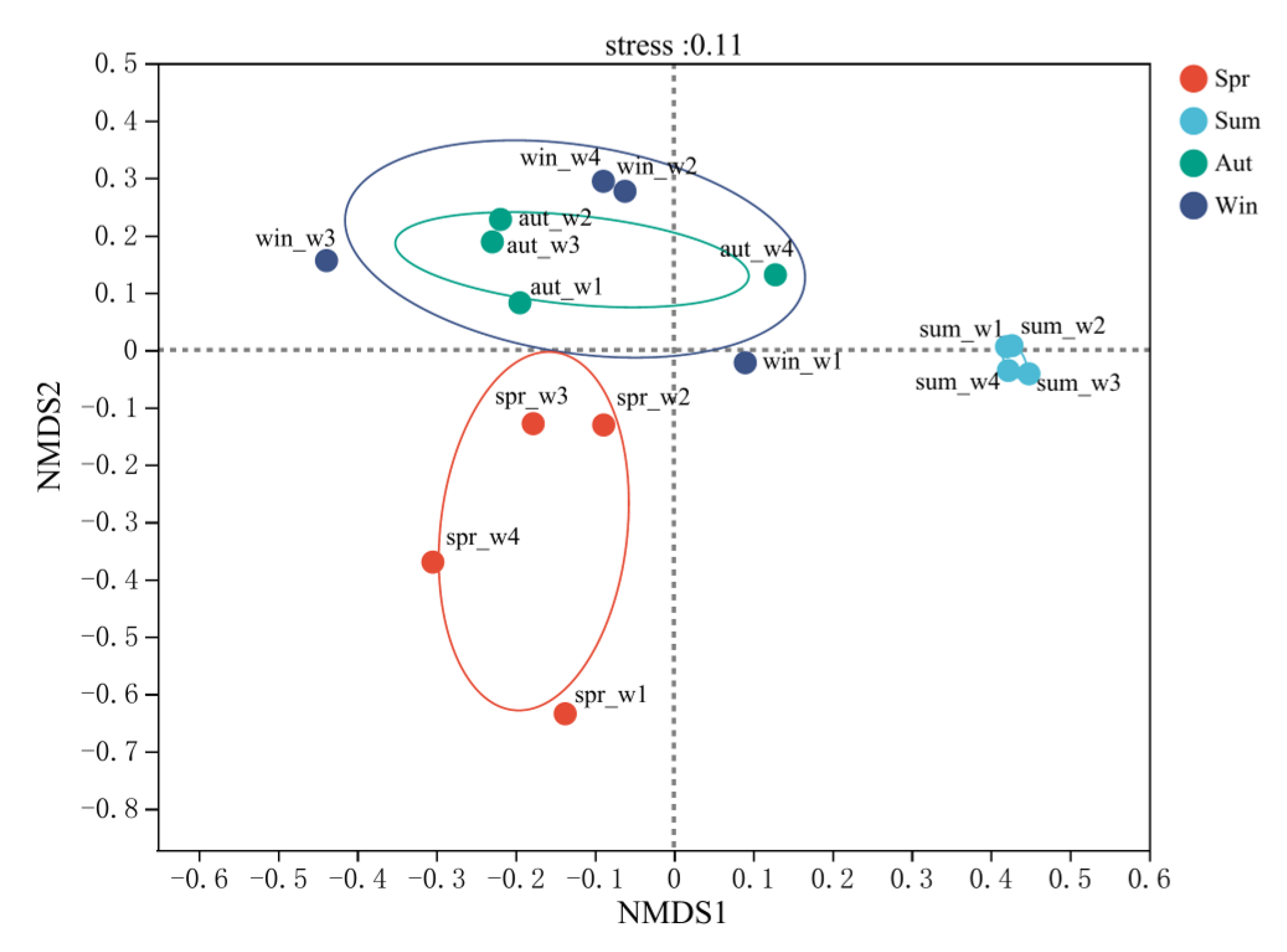
3.3. Diversity Assessment of Planktonic Bacteria
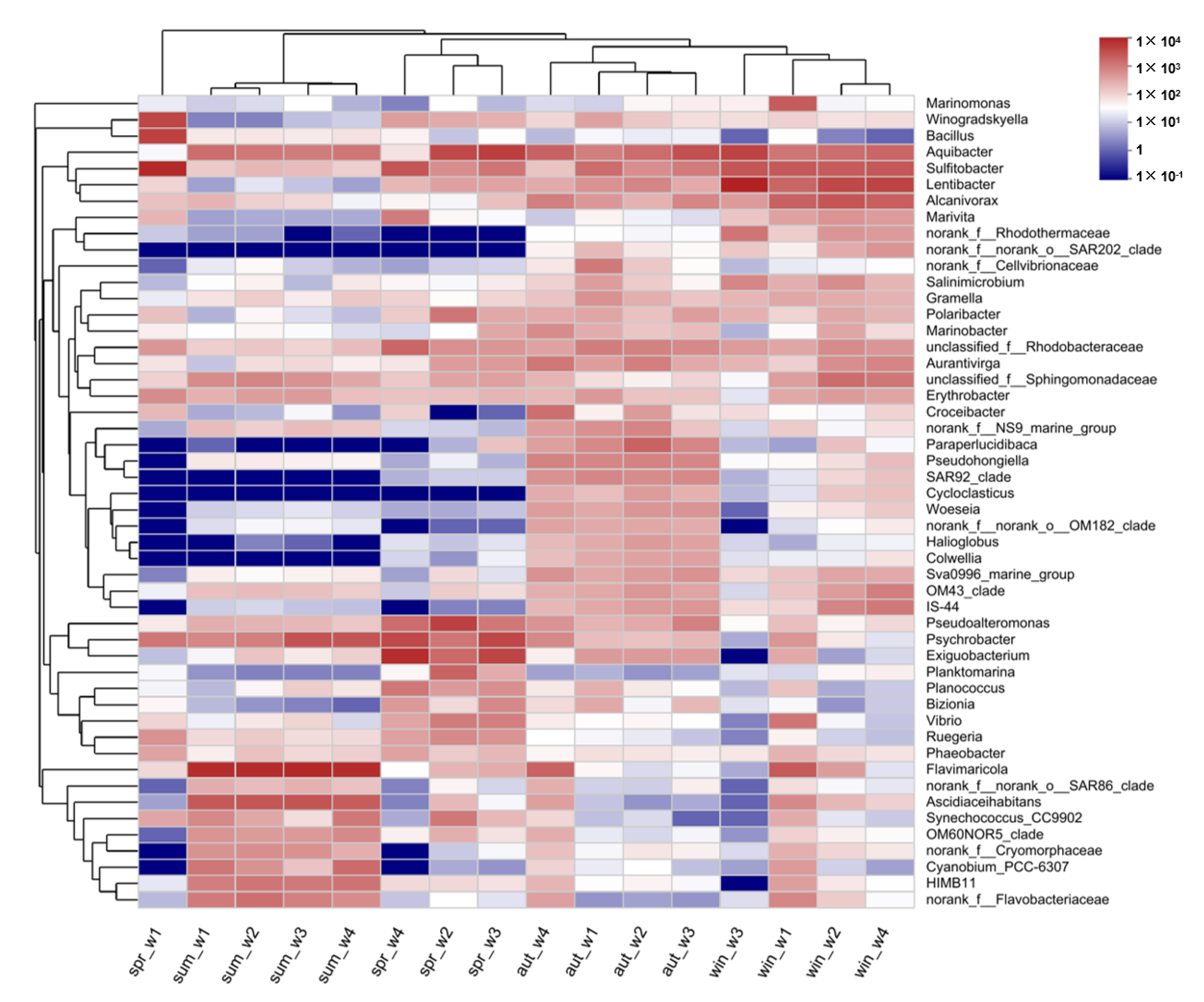
3.4. Species Composition of Planktonic Bacteria Community
3.5. Analysis of the Species Difference of Planktonic Bacteria in Fish Culture Areas, Shellfish Culture Areas, and Macroalgae Distribution Areas in Four Seasons
3.6. Environmental Factors and Their Correlation with Microorganisms
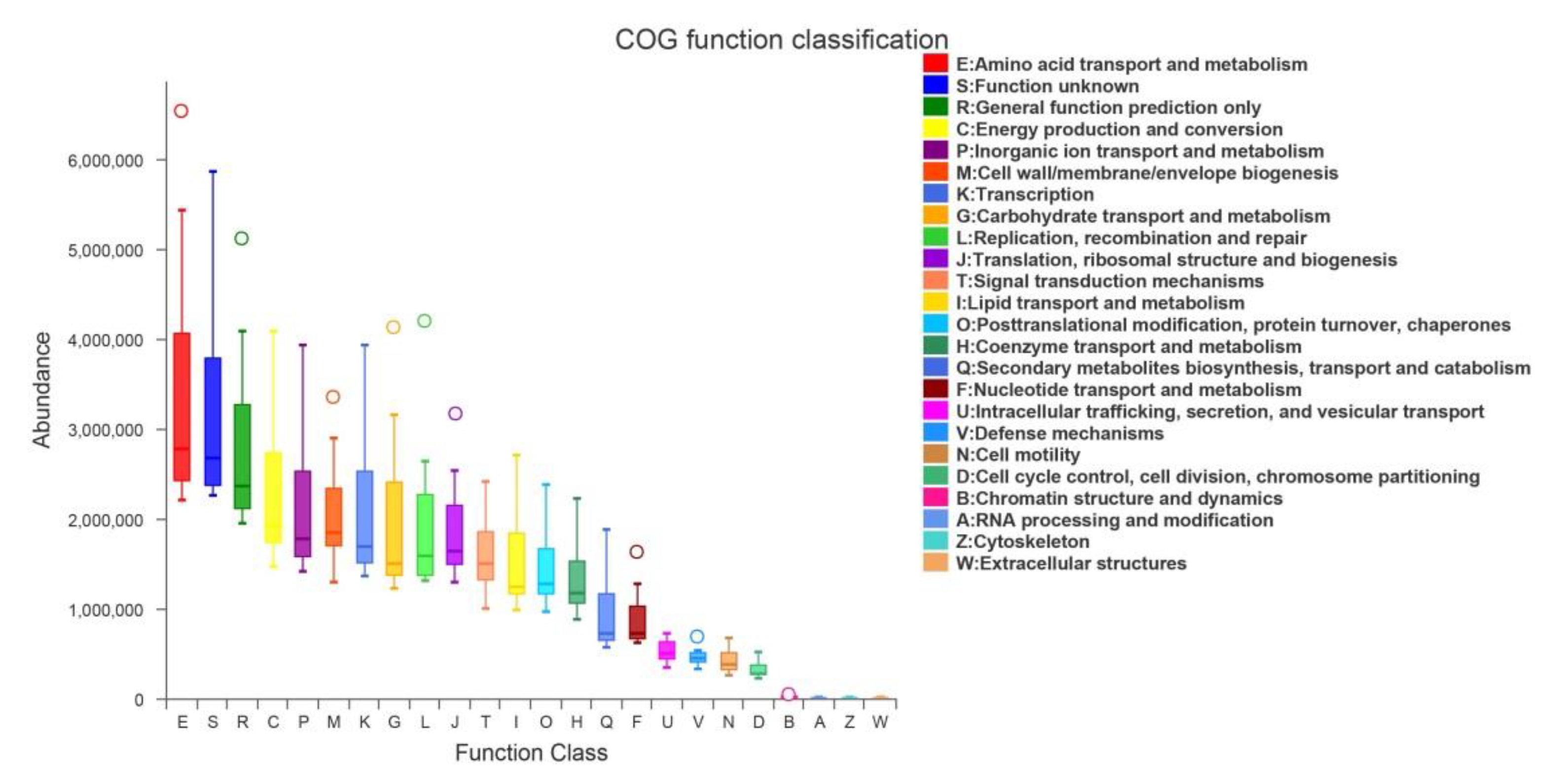
3.7. Function Statistics of the Microbial Community
4. Discussion
4.1. Planktonic Bacterial Community Analysis of α-Diversity and Stability
4.2. Community Composition and Community Function Analysis of Planktonic Bacteria
4.3. Correlation Analysis between Planktonic Bacteria and Environmental Factors
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, K.T. Marine Biodiversity and Fishery Sustainability. Asia Pac. J. Clin. Nutr. 2009, 18, 527–531. [Google Scholar] [PubMed]
- Ding, D.W.; He, P.M.; Ye, S.F.; Chen, B.; Huang, L.F.; Yang, J.L.; He, W.H. Coastal Marine Ecology in China: Research and Management; Science Press: Beijing, China, 2022; ISBN 9787030705594. [Google Scholar]
- Campbell, B.; Pauly, D. Mariculture: A Global Analysis of Production Trends Since 1950. Mar. Policy 2013, 39, 94–100. [Google Scholar] [CrossRef]
- Wu, R.S.S. The Environmental Impact of Marine Fish Culture: Towards a Sustainable Future. Mar. Pollut. Bull. 1995, 31, 159–166. [Google Scholar] [CrossRef]
- He, P.M.; Xu, S.N.; Zhang, H.Y.; Wen, S.S.; Dai, Y.J.; Lin, S.J.; Yarish, C. Bioremediation Efficiency in the Removal of Dissolved Inorganic Nutrients by the Red Seaweed, Porphyra yezoensis, Cultivated in the Open Sea. Water Res. 2008, 42, 1281–1289. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating Seaweeds into Marine Aquaculture Systems: A Key toward Sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- He, P.M.; Duan, Y.L.; Liu, Q.; Liu, J.L.; Liu, W.; Zhang, J.H.; Fang, J.G.; Jiang, Z.J.; Wu, H.L.; Li, X.S.; et al. Strategy of Macroalgae Eco-Remediation with Cases in Nearshore China. J. Appl. Oceanogr. 2021, 40, 557–563. [Google Scholar]
- Tang, Q.S.; Fang, J.G.; Zhang, J.H.; Jiang, Z.J.; Liu, H.M. Impacts of Multiple Stressors on Coastal Ocean Ecosystems and Integrated Multi-Trophic Aquaculture. Prog. Fish. Sci. 2013, 34, 1–11. [Google Scholar]
- Wei, Z.L.; Huo, Y.Z.; Liu, Q.; Yang, F.F.; Long, L.J.; Bi, H.S.; Fan, C.L.; He, P.M. A Field Scale Evaluation of Gracilaria Lemaneiformis Co-Cultured with Crassostrea gigas as a Nutrient Bioextraction Strategy in Yantian Bay, China. Algal Res. 2019, 38, 101407. [Google Scholar] [CrossRef]
- Bao, Y.L.; Huo, Y.Z.; Duan, Y.L.; He, P.M.; Wu, M.Q.; Yang, N.; Sun, B. Growth and Nutrient Uptake of Myriophyllum spicatum under Different Nutrient Conditions and Its Potential Ecosystem Services in an Enclosed Sea Area in the East China Sea. Mar. Pollut. Bull. 2020, 151, 110801. [Google Scholar] [CrossRef]
- Edwards, P.; Pullin, R.S.V.; Gartner, J.A. Research and Education for the Development of Integrated Crop-Livestock-Fish Farming Systems in the Tropics. Agric. Syst. 1988, 32, 293–294. [Google Scholar]
- Paerl, H.W.; Dyble, J.; Twomey, L.; Pinckney, J.L.; Nelson, J.; Kerkhof, L. Characterizing Man-Made and Natural Modifications of Microbial Diversity and Activity in Coastal Ecosystems. Antonie Leeuwenhoek 2002, 81, 487–507. [Google Scholar] [CrossRef]
- Bao, Y.L.; He, W.H.; Zhao, S.; Liu, Q.; Li, K.; He, P.M.; Li, K.J. Planktonic and Sediment Bacterial Communities in an Integrated Mariculture System. Lett. Appl. Microbiol. 2021, 72, 341–350. [Google Scholar] [CrossRef]
- An, X.L.; Zhou, Q.X.; Xing, G.M. A Review: Application of Marine Microorganisms in Marine-Pollution Treatment. Fish. Sci. 2006, 25, 97–100. [Google Scholar]
- Salazar, G.; Sunagawa, S. Marine Microbial Diversity. Curr. Biol. 2017, 27, R489–R494. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.M.; Wang, X.; Xiong, J.B.; Zhu, J.L.; Wang, Y.N.; Zhao, Q.F.; Chen, H.P.; Guo, A.N.; Wu, J.F.; Dai, H.P. Bacterioplankton Assemblages as Biological Indicators of Shrimp Health Status. Ecol. Indic. 2014, 38, 218–224. [Google Scholar] [CrossRef]
- Humayoun, S.B.; Bano, N.; Hollibaugh, J.T. Depth Distribution of Microbial Diversity in Mono Lake, a meromictic soda lake in California. Appl. Environ. Microbiol. 2003, 69, 1030–1042. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.Y.; Yu, Y.H.; Feng, W.S.; Yu, Z.G.; Chen, H.T. Plankton Community Composition in the Three Gorges Reservoir Region revealed by PCR-DGGE and its relationships with environmental factors. J. Environ. Sci. 2008, 20, 732–738. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Ma, Y.; Wei, C.L.; Jiao, N.Z.; Tang, K.X.; Wu, Z.H.; Jian, J.C. Bacterial Diversity in Various Coastal Mariculture Ponds in Southeast China and in Diseased Eels as Revealed by Culture and Culture-Independent Molecular Techniques. Aquacult. Res. 2010, 41, e172–e186. [Google Scholar] [CrossRef]
- Chen, M. Chemical Oceanography; Science Press: Beijing, China, 2009; ISBN 9787502775346. [Google Scholar]
- Hu, C.J.; Xiong, J.B.; Chen, H.P.; Wang, K.; Ye, R.; Cui, Y.P.; Zhu, J.L.; Zhang, D.M. Distribution of Bacterioplankton Communities in Cage Culture and Noncultured Areas of Xiangshan Bay, Ningbo, China. Acta Ecol. Sin. 2015, 35, 8053–8061. [Google Scholar]
- Xu, Z.H.; Guo, Z.R.; Xu, X.; Huang, D.J.; Sun, X.X.; Jiang, L.M.; Yang, J.F. The Impact of Nutrient Enrichment on the Phytoplankton and Bacterioplankton Community during a Mesocosm Experiment in Nan’ao of Daya Bay. Mar. Biol. Res. 2014, 10, 374–382. [Google Scholar] [CrossRef]
- Mezhoud, N.; Temimi, M.; Zhao, J.; Al Shehhi, M.R.; Ghedira, H. Analysis of the Spatio-Temporal Variability of Seawater Quality in the Southeastern Arabian Gulf. Mar. Pollut. Bull. 2016, 106, 127–138. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, M.M.; Zhang, Q.; Bao, Y.L.; Yang, N.; Huo, Y.Z.; He, P.M. Epiphytic Bacterial Community Composition on the Surface of the Submerged Macrophyte in a Low-Salinity Sea Area of Hangzhou Bay. Oceanol. Hydrobiol. Stud. 2019, 48, 43–55. [Google Scholar] [CrossRef]
- Lu, J.W.; Zhang, X.Q.; Du, L.L.; Yang, Z.J.; Wu, M.; Lu, L.P. Bacterial Isolation and Diversity Analysis of Four Seawater Sampling Sites of the East China Sea and the South China Sea. J. Zhejiang Univ. 2012, 39, 443–449. [Google Scholar]
- Liu, Q. Community Structure of Picoplankton in the Coastal Waters of Zhoushan and Correlation with Environmental Parameters. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2018. [Google Scholar]
- Bao, Y.L. The Construction of the Island Reef Fish-shell IMTA Model and Its Impact on Theecological Environment of the Nearby Sea. J. Shanghai Oc. Univ. 2020, 598, 97–108. [Google Scholar]
- Wu, W.J. Enrichment Culture of Bacteria from Coastal Sediment off Weihai and Polyphasic Taxonomy Analysis of Three Novel Bacteroidetes Strains. Master’s Thesis, Shandong University, Jinan, China, 2016. [Google Scholar]
- Cottrell, M.T.; Kirchman, D.L. Natural Assemblages of Marine Proteobacteria and Members of the Cytophaga-Flavobacter Cluster Consuming Low-and High-Molecular-Weight Dissolved Organic Matter. Appl. Environ. Microbiol. 2000, 66, 1692–1697. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, M.T.; Kirchman, D.L. Community Composition of Marine Bacterioplankton Determined by 16S Rrna Gene Clone Libraries and Fluorescence in Situ Hybridization. Appl. Environ. Microbiol. 2000, 66, 5116–5122. [Google Scholar] [CrossRef] [Green Version]
- Rinta-Kanto, J.M.; Sun, S.; Sharma, S.; Kiene, R.P.; Moran, M.A. Bacterial Community Transcription Patterns During a Marine Phytoplankton Bloom. Environ. Microbiol. 2012, 14, 228–239. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.M.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.M.; Kassabgy, M.; Huang, S.; Mann, A.J.; Waldmann, J. Substrate-Controlled Succession of Marine Bacterioplankton Populations Induced by a Phytoplankton Bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.Y.; Zheng, T.L. Bacterial Survival Modes and Community Characteristics in Natural Environment. Chin. J. App. Environ. Biol. 2013, 19, 553–560. [Google Scholar] [CrossRef]
- Ghiglione, J.F.; Murray, A.E. Pronounced Summer to Winter Differencesand Higher Wintertime Richness in Coastal Antarctic Marine Bacterioplankton. Environ. Microbiol. 2012, 14, 617–629. [Google Scholar] [CrossRef]
- Taniguchi, A.; Tada, Y.; Hamasaki, K. Seasonal Variations in the Community Structure of Actively Growing Bacteria in Neritic Waters of Hiroshima bay, Western Japan. Jap. Soc. Microb. Eco. 2011, 26, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Shao, K.Q.; Yao, X.; Wu, Z.S.; Jiang, X.Y.; Hu, Y.; Tang, X.M.; Xu, Q.J.; Gao, G. The Bacterial Community Composition and Its Environmental Drivers in the Rivers around Eutrophic Chaohu Lake, China. BMC Microbiol. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrueck, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining Seasonal Marine Microbial Community Dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.J.; Yu, H.L.; Sheng, H.X.; Tang, Y.L.; Liang, Z.L. Comparative Analysis of Microbial Communities Between Water and Sediment in Laoshan Bay Marine Ranching with Varied Aquaculture Activities. Mar. Pollut. Bull. 2021, 173, 112990. [Google Scholar] [CrossRef]
- Li, W.K.W. Annual Average Abundance of Heterotrophic Bacteria and Synechococcus in Surface Ocean Waters. Limnol. Oceanogr. 1998, 43, 1746–1753. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Liu, J.J.; Yao, Q.; Jin, J.; Liu, X.B.; Wang, G.H. Comparative Analysis of Bacterial Community Compositions Between Sediment and Water in Different Types of Wetlands of Northeast China. J. Soils. Sediments. 2019, 19, 3083–3097. [Google Scholar] [CrossRef]
- Wu, S.K.; Xie, P.; Ni, L.Y. Cyanobacteria Genus and Its Relationship with Nitrogen and Phosphorus Concentration in Lakes along the Middle and Lower Reaches of the Yangtze River. J. Hydrol. 2014, 35, 19–25. [Google Scholar]
- Zhou, W.H.; Li, T.; Cai, C.H.; Huang, L.M.; Wang, H.K.; Xu, J.R.; Dong, J.D.; Zhang, S. Spatial and Temporal Dynamics of Phytoplankton and Bacterioplankton Biomass in Sanya Bay, Northern South China Sea. J. Environ. Sci. 2009, 21, 595–603. [Google Scholar] [CrossRef]
- Hagstrom, A.; Larsson, U.; Horstedt, P.; Normark, S. Frequency of Dividing Cells, a New Approach to the Determination of Bacterial Growth Rates in Aquatic Environments. Appl. Environ. Microbiol. 1979, 37, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, J.A.; Azam, F. Bacterioplankton Secondary Production Estimates for Coastal Waters of British Columbia, Antarctica, and California. Appl. Environ. Microbiol. 1980, 39, 1085–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casotti, R.; Brunet, C.; Aronne, B.; d’Alcala, M.R. Mesoscale Features of Phytoplankton and Planktonic Bacteria in a Coastal Area as Induced by External Water Masses. Mar. Ecol. Prog. Ser. 2000, 195, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Wang, C.S.; Xu, X.W.; Liu, Z.Y. Advances in Studies on Biological Effect of Estuary Hypoxia. Acta Ecol. Sin. 2009, 29, 2595–2602. [Google Scholar]
- Santos, L.; Santos, A.L.; Coelho, F.J.R.C.; Gomes, N.C.M.; Dias, J.M.; Cunha, A.; Almeida, A. Heterotrophic Activities of Neustonic and Planktonic Bacterial Communities in an Estuarine Environment (Ria de Aveiro). J. Plankton. Res. 2014, 36, 230–242. [Google Scholar] [CrossRef] [Green Version]







| Sequencing Area | Primer Name | Primer Sequence | |
|---|---|---|---|
| Bacteria | 338F_806R | 338F | ACCGATAGCAAACAAGTA |
| 806R | TCCTTGGTCCGTGTTTCA |
| Sites | Chl-a | T | pH | S | DO | COD | TN | TP | SiO32− | NH4+ | NO2− | NO3− | DIP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| spr-W1 | 1.112 | 19.88 | 8.47 | 30.01 | 11.54 | 0.594 | 0.380 | 0.008 | 0.775 | 0.000 | 0.011 | 0.129 | 0.003 |
| spr-W2 | 1.124 | 19.73 | 8.53 | 29.99 | 11.25 | 0.812 | 0.385 | 0.015 | 0.690 | 0.001 | 0.011 | 0.212 | 0.003 |
| spr-W3 | 1.285 | 19.62 | 8.54 | 30.00 | 15.56 | 0.505 | 0.390 | 0.022 | 0.889 | 0.006 | 0.009 | 0.181 | 0.009 |
| spr-W4 | 1.378 | 19.78 | 8.25 | 29.99 | 11.41 | 0.601 | 0.390 | 0.019 | 0.818 | 0.008 | 0.008 | 0.236 | 0.009 |
| sum-W1 | 0.411 | 25.00 | 8.03 | 29.01 | 9.36 | 3.701 | 0.490 | 0.024 | 1.591 | 0.014 | 0.005 | 0.661 | 0.005 |
| sum-W2 | 0.511 | 25.01 | 8.01 | 28.95 | 8.65 | 4.087 | 0.866 | 0.022 | 1.329 | 0.019 | 0.006 | 0.655 | 0.009 |
| sum-W3 | 1.087 | 24.76 | 8.02 | 29.01 | 9.00 | 3.120 | 1.015 | 0.024 | 1.587 | 0.011 | 0.007 | 0.761 | 0.012 |
| sum-W4 | 0.358 | 24.72 | 8.11 | 29.02 | 8.62 | 3.897 | 0.562 | 0.026 | 1.396 | 0.016 | 0.006 | 0.393 | 0.007 |
| aut-W1 | 0.519 | 15.72 | 7.90 | 29.05 | 10.75 | 0.642 | 0.813 | 0.010 | 2.024 | 0.010 | 0.002 | 0.592 | 0.010 |
| aut-W2 | 0.333 | 15.62 | 7.89 | 29.23 | 10.20 | 1.212 | 0.279 | 0.016 | 2.024 | 0.006 | 0.003 | 0.591 | 0.009 |
| aut-W3 | 0.294 | 15.72 | 7.93 | 31.12 | 10.53 | 0.451 | 0.402 | 0.014 | 1.981 | 0.020 | 0.003 | 0.665 | 0.006 |
| aut-W4 | 0.209 | 15.71 | 7.96 | 31.05 | 11.14 | 0.798 | 0.258 | 0.007 | 1.677 | 0.020 | 0.002 | 0.397 | 0.012 |
| win-W1 | 0.126 | 9.62 | 8.07 | 35.12 | 15.34 | 0.320 | 0.109 | 0.017 | 0.818 | 0.013 | 0.001 | 0.238 | 0.021 |
| win-W2 | 0.117 | 9.71 | 8.03 | 35.00 | 11.83 | 0.482 | 0.461 | 0.024 | 0.846 | 0.010 | 0.003 | 0.214 | 0.012 |
| win-W3 | 0.145 | 9.87 | 8.04 | 34.99 | 11.88 | 0.258 | 0.913 | 0.026 | 0.701 | 0.006 | 0.003 | 0.239 | 0.017 |
| win-W4 | 0.198 | 10.02 | 8.25 | 35.01 | 11.42 | 0.301 | 0.959 | 0.034 | 0.694 | 0.013 | 0.001 | 0.136 | 0.005 |
| Samples | Shannon | Shannon Even | ACE | Coverage |
|---|---|---|---|---|
| spr_W1 | 2.9036 | 0.5103 | 388.8448 | 0.9973 |
| spr_W2 | 3.8365 | 0.6154 | 675.4457 | 0.995 |
| spr_W3 | 3.6582 | 0.5826 | 704.7729 | 0.9946 |
| spr_W4 | 3.421 | 0.5673 | 558.2343 | 0.9958 |
| sum_W1 | 3.8493 | 0.5798 | 1016.3562 | 0.9924 |
| sum_W2 | 3.8099 | 0.5689 | 1134.5797 | 0.9911 |
| sum_W3 | 3.4472 | 0.5261 | 961.2139 | 0.9926 |
| sum_W4 | 3.468 | 0.5353 | 847.4571 | 0.9935 |
| aut_W1 | 5.2453 | 0.7478 | 1414.3703 | 0.9899 |
| aut_W2 | 5.1113 | 0.7280 | 1426.155 | 0.99 |
| aut_W3 | 5.0432 | 0.7198 | 1385.513 | 0.9905 |
| aut_W4 | 5.1181 | 0.7112 | 1838.2438 | 0.9858 |
| win_W1 | 4.3327 | 0.6479 | 1099.1591 | 0.9916 |
| win_W2 | 4.2882 | 0.6359 | 1155.474 | 0.9911 |
| win_W3 | 2.749 | 0.4401 | 707.5391 | 0.9945 |
| win_W4 | 4.121 | 0.6136 | 1088.6195 | 0.9919 |
| Season | Sample | OTU ID | Contribution Percentage | Taxonomic Status |
|---|---|---|---|---|
| spr | W1 | OTU6 | 12.62% | g__Sulfitobacter |
| OTU191 | 12.30% | g__Aquibacter | ||
| OTU524 | 7.51% | g__Psychrobacter | ||
| spr | W2 | OTU103 | 5.45% | g__Croceitalea |
| OTU139 | 5.45% | f__Hyphomonadaceae | ||
| OTU151 | 3.47% | f__Rhodobacteraceae | ||
| spr | W3 | OTU1493 | 4.26% | g__Oleispira |
| OTU114 | 2.71% | f__Cryomorphaceae | ||
| OTU995 | 2.71% | g__Seonamhaeicola | ||
| sum | W1 | OTU357 | 9.95% | f__Comamonadaceae |
| OTU1351 | 3.32% | g__Nevskia | ||
| OTU326 | 2.37% | f__Methylacidiphilaceae | ||
| sum | W2 | OTU485 | 4.12% | c__Sericytochromatia |
| OTU472 | 3.75% | g__Oleiphilus | ||
| OTU120 | 2.25% | g__Pleurocapsa | ||
| sum | W3 | OTU1967 | 5.71% | s__Salinicoccus_roseus |
| OTU660 | 3.81% | f__Thiotrichaceae | ||
| OTU1776 | 2.86% | s__Micrococcus_luteus | ||
| aut | W1 | OTU892 | 8.87% | f__Legionellaceae |
| OTU947 | 6.28% | f__Arcobacteraceae | ||
| OTU936 | 4.98% | g__Oceanococcus | ||
| aut | W2 | OTU1220 | 15.87% | g__Lachnoclostridium |
| OTU1221 | 11.25% | g__Flavonifractor | ||
| OTU1080 | 10.62% | s__Enterococcus faecalis | ||
| aut | W3 | OTU1491 | 66.41% | o__Flavobacteriales |
| OTU1374 | 1.91% | f__Bacteriovoracaceae | ||
| OTU202 | 1.91% | f__Micavibrionaceae | ||
| win | W1 | OTU118 | 16.19% | g__Exiguobacterium |
| OTU1894 | 8.38% | g__Parvibaculum | ||
| OTU1931 | 6.89% | f__Bdellovibrionaceae | ||
| win | W2 | OTU1229 | 35.14% | o__Parvibaculales |
| OTU2034 | 22.44% | f__Parvibaculaceae | ||
| OTU2079 | 4.71% | g__Alcanivorax | ||
| win | W3 | OTU861 | 25.43% | g__Oceanococcus |
| OTU2118 | 17.37% | f__Criblamydiaceae | ||
| OTU2115 | 9.48% | o__Bradymonadales |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Jiang, R.; Zhang, J.; Li, K.; Zhang, J.; Shao, L.; He, W.; He, P. The Impact of IMTA on the Spatial and Temporal Distribution of the Surface Planktonic Bacteria Community in the Surrounding Sea Area of Xiasanhengshan Island of the East China Sea. J. Mar. Sci. Eng. 2023, 11, 476. https://doi.org/10.3390/jmse11030476
Zhang M, Jiang R, Zhang J, Li K, Zhang J, Shao L, He W, He P. The Impact of IMTA on the Spatial and Temporal Distribution of the Surface Planktonic Bacteria Community in the Surrounding Sea Area of Xiasanhengshan Island of the East China Sea. Journal of Marine Science and Engineering. 2023; 11(3):476. https://doi.org/10.3390/jmse11030476
Chicago/Turabian StyleZhang, Meijing, Ruitong Jiang, Jianlin Zhang, Kejun Li, Jianheng Zhang, Liu Shao, Wenhui He, and Peimin He. 2023. "The Impact of IMTA on the Spatial and Temporal Distribution of the Surface Planktonic Bacteria Community in the Surrounding Sea Area of Xiasanhengshan Island of the East China Sea" Journal of Marine Science and Engineering 11, no. 3: 476. https://doi.org/10.3390/jmse11030476





