Effects of Selenium Yeast Addition on the Growth, Intestinal Health, Immune Status and Body Composition of Juvenile Sea Cucumber Apostichopus japonicus before and after Aestivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Experiment Program
2.3. Sampling Procedures
2.4. Analyzation of Digestive Enzymes and Immune Enzymes
2.5. Analyzation of Body Compositon
2.6. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Digestive Enzyme Activities
3.3. Immune Reponse
3.4. Proximate Compositions
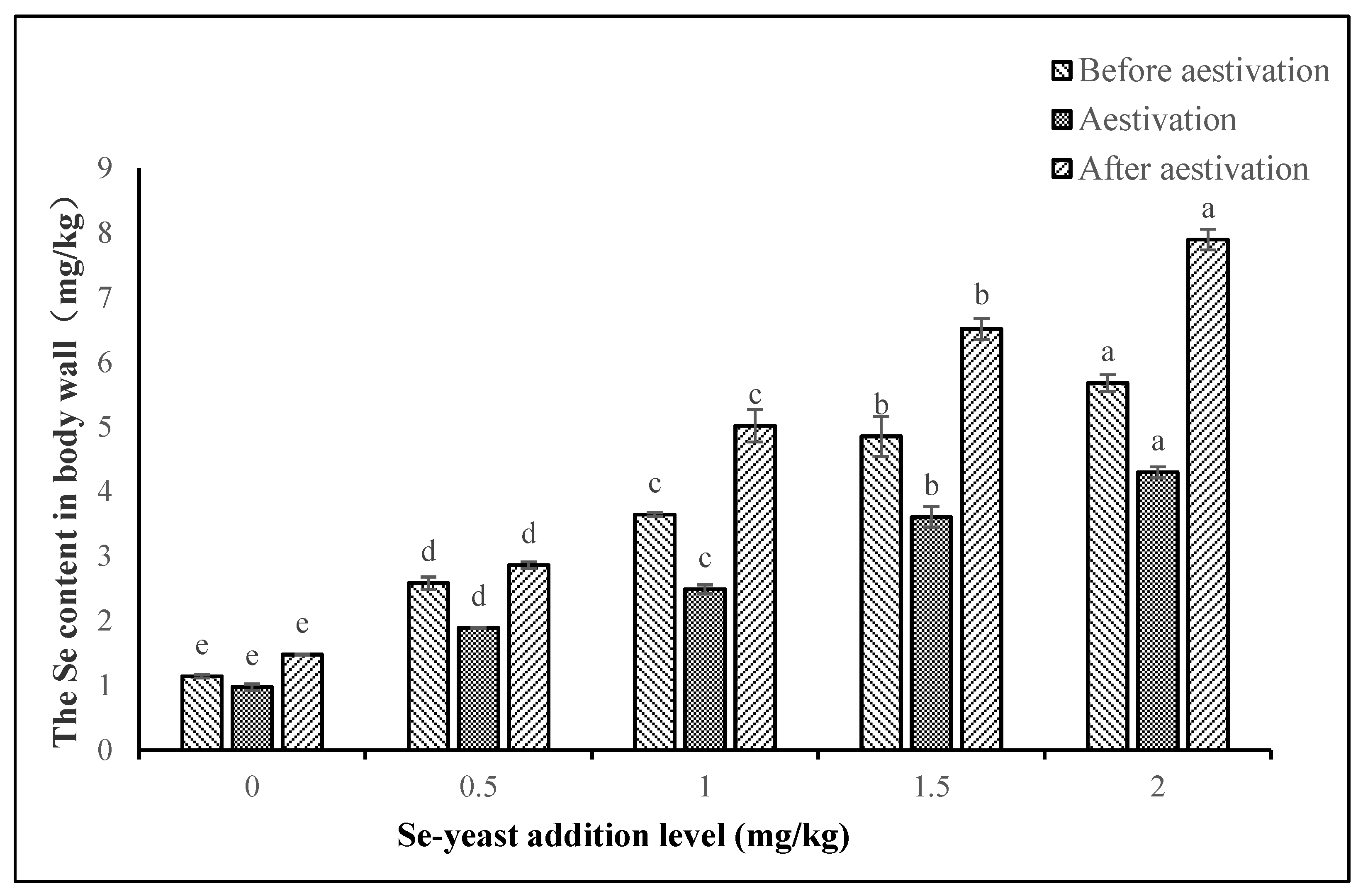
3.5. Selenium Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Xue, X.; Yang, H.; Liao, H.; Han, Y.; Jiang, Z.; Ren, T. Effect of dietary carbohydrate levels on growth performance, nonspecific immune enzymes and acute response to low salinity and high temperature of juvenile sea cucumber Apostichopus japonicus. Aquacult. Nutr. 2020, 26, 683–692. [Google Scholar] [CrossRef]
- Ning, Y.; Wu, X.; Zhou, X.; Ding, J.; Chang, Y.; Yang, Z.; Huang, Z.; Zuo, R. An evaluation on the selenium yeast supplementation in the practical diets of early juvenile sea cucumber (Apostichopus japonicus): Growth performance, digestive enzyme activities, immune and antioxidant capacity, and body composition. Aquacult. Nutr. 2021, 27, 2142–2153. [Google Scholar] [CrossRef]
- Han, Q.; Keesing, J.K.; Liu, D. A Review of Sea Cucumber Aquaculture, Ranching, and Stock Enhancement in China. Rev. Fish. Sci. Aquac. 2016, 24, 326–341. [Google Scholar] [CrossRef]
- Xi, X.; Zhang, L.; Liu, S.; Tao, Z.; Yang, H. Aerated sea mud is beneficial for post-nursery culture of early juvenile sea cucumber Apostichopus japonicus (selenka). Aquacult. Int. 2016, 24, 211–224. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Liu, J.; Storey, K.B. High-throughput sequencing reveals differential expression of miRNAs in intestine from sea cucumber during aestivation. PLoS ONE 2013, 8, e76120. [Google Scholar] [CrossRef]
- Wang, L.; Wu, L.; Liu, Q.; Zhang, D.F.; Yin, J.J.; Xu, Z.; Zhang, X.Z. Improvement of flesh quality in rainbow trout (Oncorhynchus mykiss) fed supranutritional dietary selenium yeast is associated with the inhibited muscle protein degradation. Aquacult. Nutr. 2018, 24, 1351–1360. [Google Scholar] [CrossRef]
- Ewa, J.; Edyta, R.; Jolanta, G.; Edyta, W.; Magdalena, K.; Sara, R.; Wojciech, W. The effect of selenium supplementation on glucose homeostasis and the expression of genes related to glucose metabolism. Nutrients 2016, 8, 772. [Google Scholar]
- Durigon, E.G.; Kunz, D.F.; Peixoto, N.C.; Uczay, J.; Lazzari, R. Diet selenium improves the antioxidant defense system of juveniles Nile tilapia (Oreochromis niloticus L.). Braz. J. Biol. 2018, 79, 527–532. [Google Scholar] [CrossRef]
- Guo, H.; Lin, W.; Hou, J.; Wang, L.; Zhang, D.; Wu, X.; Li, D. The protective roles of dietary selenium yeast and tea polyphenols on growth performance and ammonia tolerance of juvenile wuchang bream (Megalobrama amblycephala). Front. Physiol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Hao, X.; Ling, Q.; Hong, F. Effects of dietary selenium on the pathological changes and oxidative stress in loach (Paramisgurnus dabryanus). Fish Physiol. Biochem. 2014, 40, 1313–1323. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Fernandes, J.B.K.; Ullah, I.; Sarwar, H. An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol. Biochem. 2017, 43, 1689–1705. [Google Scholar] [CrossRef]
- Khalil, H.; Mansour, A.T.; Goda, A.; Omar, E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture 2018, 501, 135–143. [Google Scholar] [CrossRef]
- Payne, R.L.; Southern, L.L. Comparison of inorganic and organic selenium sources for broilers. Poult. Sci. 2005, 84, 898–902. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquacult. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, Z.X.; Zhang, K.; Zhang, Y.M.; Jiang, Y.S.; Liu, C.B.; WU, Y.Q. Effects of dietary selenomethionine levels on growth and some immune indices in juvenile sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 2012, 27, 110–115. [Google Scholar]
- Zhou, W.; Cao, Q.; Wang, Z.F.; Xiao-Jie, H.U.; Zhang, J.Y.; Liu, J.B. Effect of dietary methionine selenium on growth and digestive index of sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 2015, 30, 181–184. [Google Scholar]
- Hu, Y.; Han, Y.; Wang, L.; Bai, Z.; Ren, T. Toxicity effects of different dietary selenium forms on sea cucumber, Apostichopus Japonicus. Aquacult. Rep. 2019, 15, 100209. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, MA, USA, 1995; pp. 16–26. [Google Scholar]
- Zeng, F.; Rabbi, M.H.; Hu, Y.; Li, Z.; Ren, T. Synergistic Effects of Dietary Selenomethionine and Vitamin C on the Immunity, Antioxidant Status, and Intestinal Microbiota in Sea Cucumber (Apostichopus japonicus). Biol. Trace Elem. Res. 2021, 199, 3905–3917. [Google Scholar] [CrossRef]
- Iqbal, S.; Atique, U.; Mughal, M.S.; Khan, N.; Haider, M.S.; Iqbal, K.J.; Rana, M.A. Effect of selenium incorporated in feed on the hematological profile of tilapia (Oreochromis niloticus). J. Aquac. Res. Devel. 2017, 8, 100513. [Google Scholar] [CrossRef]
- Lee, S.; Nambi, R.W.; Won, S.; Katya, K.; Bai, S.C. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158. [Google Scholar] [CrossRef]
- Iqbal, S.; Atique, U.; Mahboob, S.; Haider, M.S.; Iqbal, H.S.; Al-Ghanim, K.A.; Mughal, M.S. Effect of supplemental selenium in fish feed boosts growth and gut enzyme activity in juvenile tilapia (Oreochromis niloticus). J. King Saud Univ.-Sci. 2020, 32, 2610–2616. [Google Scholar] [CrossRef]
- Su, C.F.; Luo, L.; Wen, H.; Chen, X.C.; Sheng, X.S.; Chen, Z. Efects of dietary selenium on growth performance, quality and digestive enzyme activities of grass carp. J. Shanghai Fish. Univ. 2007, 16, 124–129. [Google Scholar]
- Takahashi, L.S.; Biller-Takahashi, J.D.; Mansano, C.; Urbinati, E.C.; Saita, M.V. Long-term organic selenium supplementation overcomes the trade-off between immune and antioxidant systems in pacu (Piaractus mesopotamicus). Fish Shellfish. Immun. 2017, 60, 311–317. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yan, L.; Cao, J.; Xie, L. Subchronic effects of dietary selenium yeast and selenite on growth performance and the immune and antioxidant systems in Nile tilapia Oreochromis niloticus. Fish Shellfish Immun. 2019, 97, 283–293. [Google Scholar] [CrossRef]
- Cheng, X.; Rui, Z.; Wen, Z. Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 2018, 49, 73–84. [Google Scholar]
- Halder, D.; Pahari, S.K. An overview of sea cucumber: Chemistry and pharmacology of its metabolites. Indian Res. J. Pharm. Sci. 2020, 7, 2277–2298. [Google Scholar] [CrossRef]
- Zhao, Z.; Barcus, M.; Kim, J.; Lum, K.L.; Mills, C.; Lei, X.G. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J. Nutr. 2016, 146, 1625–1633. [Google Scholar] [CrossRef]
- Lin, Y.H. Effects of dietary organic and inorganic selenium on the growth, selenium concentration and meat quality of juvenile grouper Epinephelus malabaricus. Aquaculture 2014, 430, 114–119. [Google Scholar] [CrossRef]
| Ingredient | Selenium Yeast Addition Level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Sea mud a | 500 | 500 | 500 | 500 | 500 |
| Sargassum fusiforme powder b | 400 | 400 | 400 | 400 | 400 |
| Sargassum thunbergii powder c | 60 | 60 | 60 | 60 | 60 |
| Shell powder powder | 30 | 30 | 30 | 30 | 30 |
| Selenium-free yeast | 10 | 9.75 | 9.5 | 8.7 | 9 |
| Selenium yeast | - | 0.25 | 0.5 | 0.75 | 1 |
| Proximate composition | |||||
| Crude protein (%) | 9.71 | 9.72 | 9.70 | 9.71 | 9.70 |
| Crude lipid (%) | 1.62 | 1.61 | 1.62 | 1.61 | 1.60 |
| Experimental Stages | Before Aestivation | Aestivation | After Aestivation | |
|---|---|---|---|---|
| Feeding procedures | Feeding | + | - | + |
| Water temperature | 19.0–22.0 °C | 23–25 °C | 22–19 °C | |
| Lasting period | 45 days | 60 days | 30 days | |
| Sampling procedures | Digestive tract | + | - | + |
| Body wall | + | + | + | |
| Coelomic fluid | + | + | + | |
| Sample analysis | Digestive enzyme activities | + | - | + |
| Proximate composition | + | + | + | |
| Immune and antioxidant capacity | + | + | + | |
| Indices | Selenium Yeast Addition Level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Before aestivation | |||||
| IBW (g) | 2.97 ± 0.07 | 3.04 ± 0.02 | 2.86 ± 0.01 | 2.88 ± 0.09 | 3.04 ± 0.03 |
| FBW (g) | 4.67 ± 0.04 e | 5.76 ± 0.04 b | 4.99 ± 0.05 d | 5.34 ± 0.04 c | 5.94 ± 0.05 a |
| BL (cm) | 3.68 ± 0.05 c | 4.35 ± 0.11 a | 3.81 ± 0.06 c | 3.7 ± 0.04 c | 4.10 ± 0.10 b |
| BW (cm) | 1.17 ± 0.05 c | 1.27 ± 0.03 bc | 1.23 ± 0.02 bc | 1.54 ± 0.04 a | 1.34 ± 0.04 b |
| DL (cm) | 11.91 ± 0.33 c | 14.56 ± 0.57 b | 17.65 ± 0.08 a | 12.94 ± 0.72 bc | 14.38 ± 0.65 b |
| DW (g) | 0.24 ± 0.02 b | 0.25 ± 0.01 b | 0.25 ± 0.01 b | 0.29 ± 0.01 b | 0.34 ± 0.02 a |
| WGR (%) | 57.90 ± 1.0 d | 83.3 ± 1.6 b | 74.3 ± 0.6 c | 85.6 ± 0.5 b | 94.3 ± 1.4 a |
| SR (%) | 96.43 ± 1.11 | 98.85 ± 1.15 | 97.41 ± 1.37 | 100.00 | 97.04 ± 1.61 |
| RDL (%) | 3.24 ± 0.13 b | 3.27 ± 0.19 b | 4.58 ± 0.13 a | 3.53 ± 0.20 b | 3.5 ± 0.10 b |
| RDW (%) | 0.05 ± 0.00 b | 0.06 ± 0.00 a | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b |
| ER (%) | 5.93 ± 0.49 b | 8.93 ± 0.47 a | 9.63 ± 0.35 a | 0 c | 0 c |
| VSI (%) | 45.44 ± 0.10 c | 69.79 ± 2.58 a | 52.93 ± 1.80 b | 58.01 ± 0.38 b | 47.11 ± 0.62 c |
| VBI (%) | 32.49 ± 1.25 b | 40.92 ± 0.25 a | 32.28 ± 1.28 b | 33.84 ± 0.42 b | 31.85 ± 0.42 b |
| After aestivation | |||||
| IBW (g) | 1.89 ± 0.24 | 2.09 ± 0.05 | 1.65 ± 0.23 | 1.55 ± 0.04 | 1.77 ± 0.28 |
| FBW (g) | 3.38 ± 0.41 ab | 4.13 ± 0.13 a | 3.40 ± 0.01 ab | 3.07 ± 0.21 b | 3.66 ± 0.15 ab |
| BL (cm) | 3.36 ± 0.11 b | 4.73 ± 0.43 a | 3.81 ± 0.22 ab | 3.72 ± 0.49 ab | 3.79 ± 0.10 ab |
| BW (cm) | 1.49 ± 0.01 | 1.52 ± 0.11 | 1.53 ± 0.09 | 1.55 ± 0.11 | 2.06 ± 0.61 |
| DL (cm) | 10.58 ± 0.75 | 10.43 ± 1.40 | 10.99 ± 0.17 | 11.20 ± 1.92 | 10.01 ± 1.28 |
| DW (g) | 0.22 ± 0.02 | 0.32 ± 0.06 | 0.29 ± 0.02 | 0.35 ± 0.19 | 0.39 ± 0.02 |
| WGR (%) | 89.0 ± 2.3 c | 104.1 ± 6.2 bc | 130.6 ± 1.6 a | 103.2 ± 3.1 bc | 121.5 ± 5.2 ab |
| SR (%) | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 94.66 ± 2.68 b |
| RDL (%) | 3.14 ± 0.12 a | 2.21 ± 0.23 b | 2.9 ± 0.18 a | 2.98 ± 0.17 a | 2.63 ± 0.28 ab |
| RDW (%) | 0.07 ± 0.00 | 0.08 ± 0.02 | 0.10 ± 0.01 | 0.12 ± 0.07 | 0.11 ± 0.01 |
| ER (%) | 8.46 ± 1.00 b | 8.10 ± 0.96 b | 9.09 ± 0.05 ab | 3.30 ± 0 c | 11.25 ± 0.72 a |
| VSI (%) | 39.3 ± 4.14 | 40.26 ± 3.96 | 46.26 ± 11.88 | 43.09 ± 5.85 | 44.81 ± 4.21 |
| Indices | Selenium Yeast Addition Level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Before aestivation | |||||
| Pepsin | 1.53 ± 0.01 c | 1.57 ± 0.02 bc | 1.67 ± 0.02 a | 1.63 ± 0.02 ab | 1.56 ± 0.03 bc |
| Amylase | 1.47 ± 0.02 ab | 1.49 ± 0.00 ab | 1.51 ± 0.02 a | 1.50 ± 0.02 a | 1.42 ± 0.03 b |
| Cellulase | 7.98 ± 0.05 e | 10.16 ± 0.11 d | 12.17 ± 0.13 c | 13.34 ± 0.09 b | 15.09 ± 0.08 a |
| Lipase | 4.44 ± 0.06 e | 5.04 ± 0.02 d | 5.35 ± 0.02 c | 5.52 ± 0.01 b | 9.62 ± 0.06 a |
| After aestivation | |||||
| Pepsin | 1.41 ± 0.19 b | 1.59 ± 0.44 b | 2.50 ± 0.14 a | 2.29 ± 0.32 ab | 2.08 ± 0.09 ab |
| Amylase | 1.33 ± 0.10 | 2.24 ± 0.04 | 1.85 ± 0.35 | 1.76 ± 0.29 | 1.87 ± 0.38 |
| Cellulase | 9.30 ± 2.43 | 11.64 ± 1.76 | 12.13 ± 2.12 | 13.53 ± 3.38 | 13.42 ± 1.78 |
| Lipase | 5.24 ± 1.13 c | 10.22 ± 1.65 abc | 13.10 ± 0.71 a | 11.95 ± 1.62 ab | 6.76 ± 0.72 bc |
| Indices | Selenium Yeast Addition Level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Before aestivation | |||||
| T-NOS | 4.81 ± 0.02 e | 6.19 ± 0.03 d | 8.27 ± 0.07 c | 9.72 ± 0.31 b | 11.19 ± 0.73 a |
| AKP | 0.61 ± 0.01 c | 0.68 ± 0.03 c | 0.89 ± 0.02 b | 0.93 ± 0.06 b | 1.28 ± 0.06 a |
| SOD | 135.09 ± 0.97 c | 142.97 ± 0.42 b | 148.22 ± 0.72 a | 144.21 ± 0.24 ab | 142.18 ± 2.83 b |
| CAT | 0.36 ± 0.01 c | 0.40 ± 0.00 b | 0.43 ± 0.01 a | 0.42 ± 0.01 ab | 0.36 ± 0.01 c |
| GSH-Px | 14.82 ± 0.86 a | 10.58 ± 0.07 bc | 12.66 ± 0.58 ab | 13.17 ± 1.08 a | 9.06 ± 0.14 c |
| MDA | 1.06 ± 0.06 a | 1.06 ± 0.05 a | 0.67 ± 0.01 b | 0.56 ± 0.02 b | 0.43 ± 0.05 c |
| Aestivation | |||||
| T-NOS | 1.59 ± 0.184 a | 1.08 ± 0.028 b | 1.57 ± 0.039 a | 1.14 ± 0.078 b | 1.16 ± 0.028 b |
| AKP | 0.74 ± 0.17 c | 1.04 ± 0.06 bc | 1.45 ± 0.12 a | 1.26 ± 0.08 ab | 0.34 ± 0.02 d |
| SOD | 132.91 ± 0.85 ab | 121.01 ± 9.38 b | 121.80 ± 1.14 b | 143.42 ± 2.21 a | 124.63 ± 4.61 b |
| GSH-Px | 13.05 ± 0.83 b | 16.12 ± 3.47 ab | 17.12 ± 2.58 ab | 21.30 ± 0.33 a | 16.64 ± 1.63 ab |
| CAT | 0.31 ± 0.05 | 0.39 ± 0.02 | 0.36 ± 0.06 | 0.34 ± 0.03 | 0.34 ± 0.02 |
| MDA | 0.32 ± 0.07 c | 0.38 ± 0.17 bc | 0.58 ± 0.10 bc | 0.74 ± 0.14 b | 2.09 ± 0.10 a |
| After aestivation | |||||
| T-NOS | 4.00 ± 0.40 | 4.82 ± 0.75 | 4.11 ± 0.44 | 3.55 ± 0.77 | 4.96 ± 0.37 |
| AKP | 1.08 ± 0.21 a | 0.63 ± 0.13 b | 0.61 ± 0.05 b | 0.54 ± 0.08 b | 0.59 ± 0.15 b |
| SOD | 130.34 ± 0.75 b | 130.37 ± 0.34 b | 139.77 ± 5.80 ab | 135.27 ± 0.77 ab | 141.82 ± 0.19 a |
| GSH-Px | 19.76 ± 0.71 a | 17.23 ± 1.28 ab | 15.13 ± 1.09 bc | 11.59 ± 2.40 cd | 10.38 ± 0.59 d |
| CAT | 0.28 ± 0.03 c | 0.43 ± 0.02 a | 0.43 ± 0.02 a | 0.36 ± 0.00 b | 0.31 ± 0.01 bc |
| MDA | 0.64 ± 0.08 | 0.83 ± 0.23 | 0.79 ± 0.29 | 0.68 ± 0.14 | 0.86 ± 0.21 |
| Indices | Selenium Yeast Addition Level (mg/kg) | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Before aestivation | |||||
| Crude protein | 44.25 ± 1.62 | 41.95 ± 0.23 | 42.82 ± 0.79 | 44.77 ± 1.79 | 43.38 ± 1.36 |
| Moisture | 91.75 ± 0.29 | 92.02 ± 0.20 | 92.20 ± 0.26 | 91.93 ± 0.17 | 91.86 ± 0.27 |
| Crude lipid | 0.09 ± 0.01 c | 0.10 ± 0.01 bc | 0.12 ± 0.00 a | 0.11 ± 0.00 ab | 0.10 ± 0.00 bc |
| Carbohydrate | 11.50 ± 1.17 a | 8.41 ± 0.23 b | 11.14 ± 0.50 a | 8.76 ± 0.24 b | 11.43 ± 0.09 a |
| Aestivation | |||||
| Crude protein | 45.07 ± 1.16 b | 46.67 ± 1.23 ab | 45.20 ± 0.20 b | 48.75 ± 0.47 a | 49.34 ± 0.76 a |
| Moisture | 90.62 ± 0.08 | 90.57 ± 0.13 | 90.40 ± 0.23 | 90.31 ± 0.07 | 90.34 ± 0.08 |
| Crude lipid | 0.11 ± 0.00 a | 0.11 ± 0.00 ab | 0.12 ± 0.00 a | 0.10 ± 0.01 ab | 0.09 ± 0.01 b |
| Carbohydrate | 7.34 ± 1.13 | 7.16 ± 0.48 | 8.28 ± 0.03 | 8.94 ± 0.55 | 8.30 ± 0.80 |
| After aestivation | |||||
| Crude protein | 45.56 ± 1.21 | 43.19 ± 1.15 | 45.08 ± 1.50 | 45.29 ± 0.70 | 43.03 ± 0.03 |
| Moisture | 92.64 ± 0.08 | 92.80 ± 0.11 | 92.81 ± 0.52 | 92.56 ± 0.18 | 92.72 ± 0.09 |
| Crude lipid | 0.09 ± 0.01 b | 0.11 ± 0.01 a | 0.12 ± 0.00 a | 0.12 ± 0.01 a | 0.11 ± 0.00 a |
| Carbohydrate | 11.56 ± 0.04 b | 10.51 ± 0.27 c | 12.03 ± 0.23 ab | 12.29 ± 0.28 ab | 12.35 ± 0.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, R.; Wu, X.; Wang, Z.; Zhou, X.; Chang, Y.; Yang, Z.; Huang, Z.; Ding, J. Effects of Selenium Yeast Addition on the Growth, Intestinal Health, Immune Status and Body Composition of Juvenile Sea Cucumber Apostichopus japonicus before and after Aestivation. J. Mar. Sci. Eng. 2023, 11, 601. https://doi.org/10.3390/jmse11030601
Zuo R, Wu X, Wang Z, Zhou X, Chang Y, Yang Z, Huang Z, Ding J. Effects of Selenium Yeast Addition on the Growth, Intestinal Health, Immune Status and Body Composition of Juvenile Sea Cucumber Apostichopus japonicus before and after Aestivation. Journal of Marine Science and Engineering. 2023; 11(3):601. https://doi.org/10.3390/jmse11030601
Chicago/Turabian StyleZuo, Rantao, Xiangying Wu, Ziyao Wang, Xiaohui Zhou, Yaqing Chang, Zhilong Yang, Zuqiang Huang, and Jun Ding. 2023. "Effects of Selenium Yeast Addition on the Growth, Intestinal Health, Immune Status and Body Composition of Juvenile Sea Cucumber Apostichopus japonicus before and after Aestivation" Journal of Marine Science and Engineering 11, no. 3: 601. https://doi.org/10.3390/jmse11030601
APA StyleZuo, R., Wu, X., Wang, Z., Zhou, X., Chang, Y., Yang, Z., Huang, Z., & Ding, J. (2023). Effects of Selenium Yeast Addition on the Growth, Intestinal Health, Immune Status and Body Composition of Juvenile Sea Cucumber Apostichopus japonicus before and after Aestivation. Journal of Marine Science and Engineering, 11(3), 601. https://doi.org/10.3390/jmse11030601







