Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strains
2.2. Cultivation Medium
2.3. Cultivation Conditions
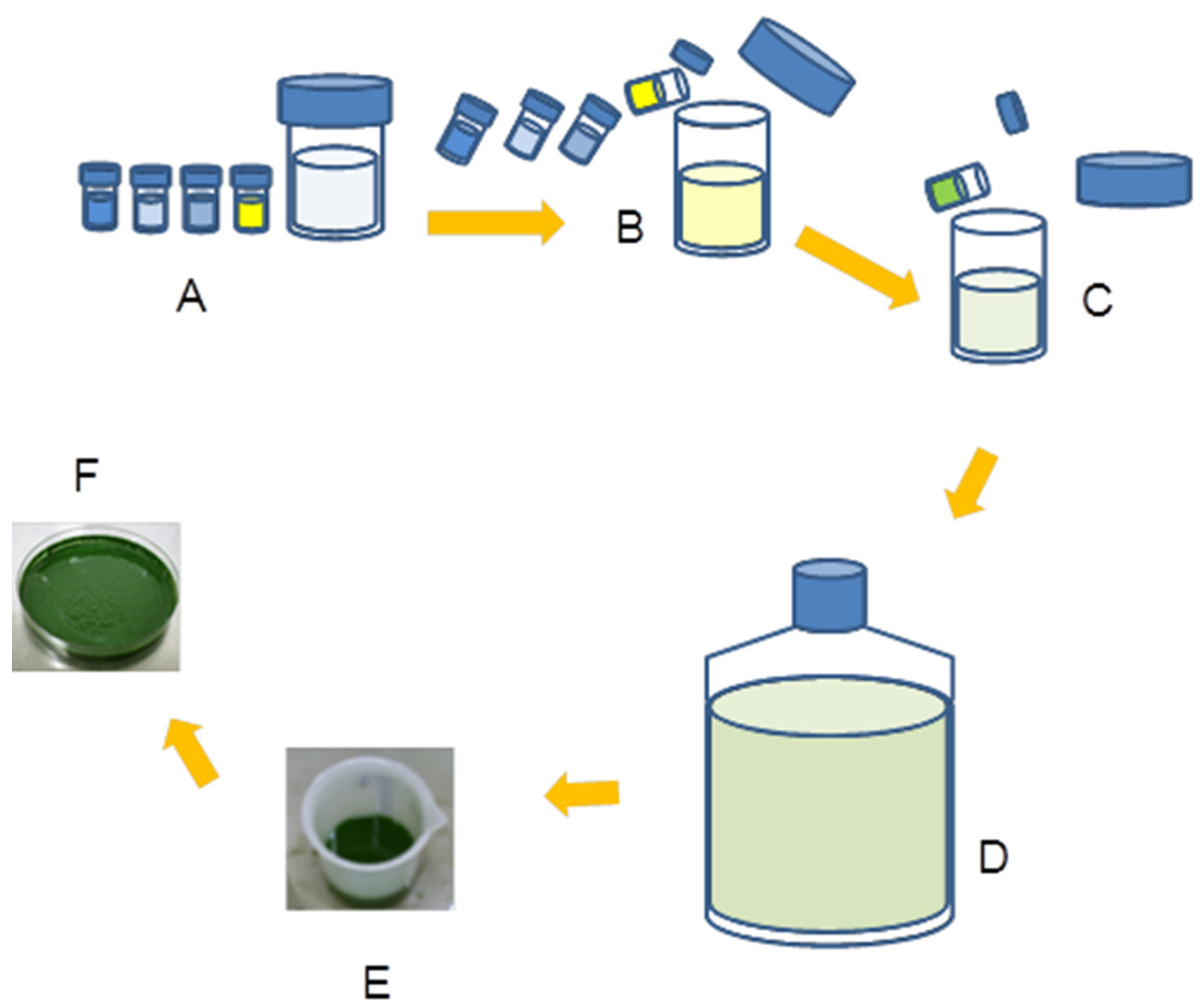
2.4. Cells Harvesting
2.5. Extraction of C-phycocyanin from Dry Biomass of Three Microalgae Strains
2.6. Protein Content and Concentration of the Extracted C-phycocyanin
2.7. The Template Nucleotide Sequence of A. platensis Used in This Study
2.8. The Template Amino Acid Sequence of A. platensis Used in This Study
2.9. Analysis of the Nucleotide and Protein Sequences
3. Results
3.1. Extraction of C-phycocyanin
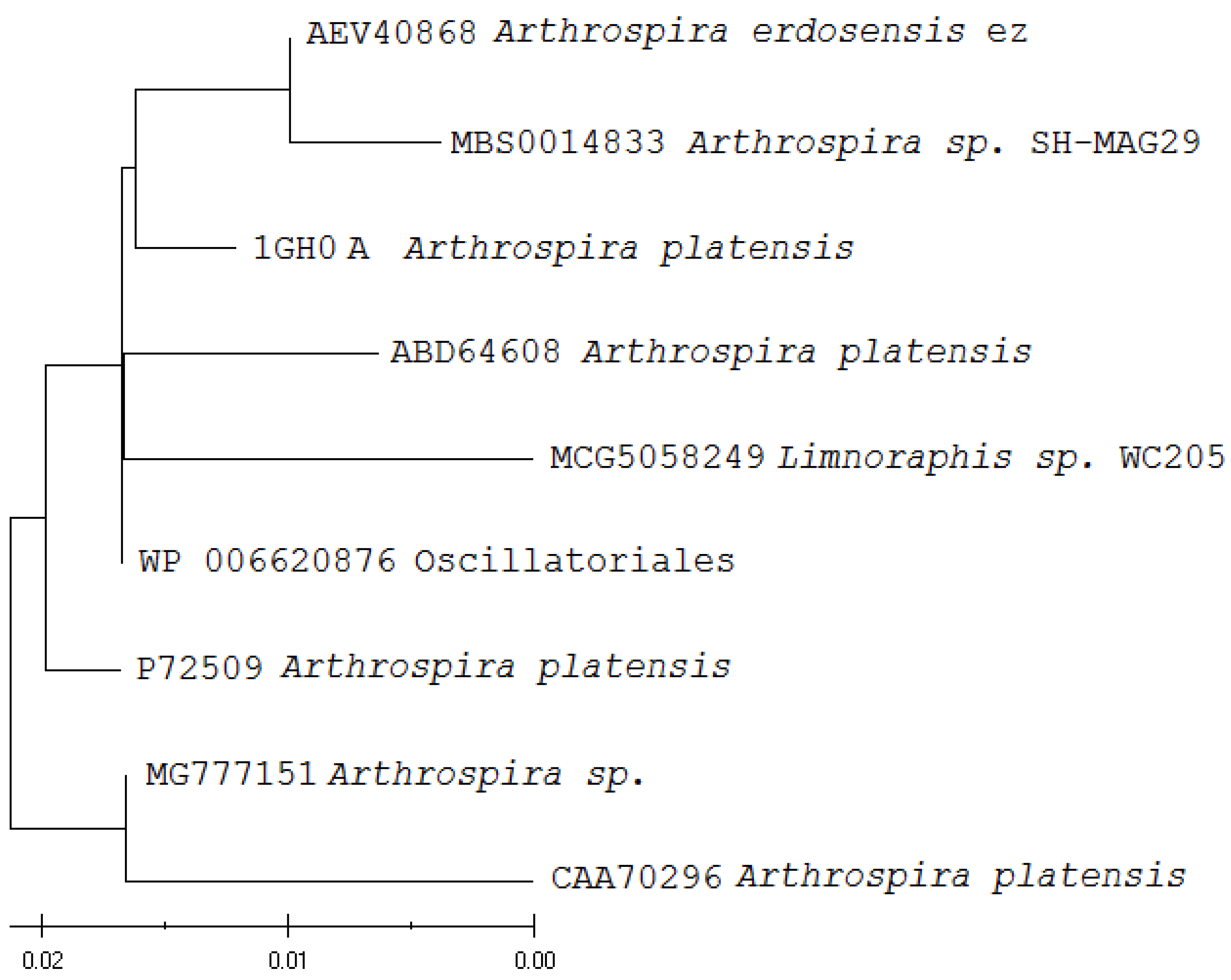
3.2. Bioinformatics Analysis of the Nucleotide Sequences of C-phycocyanin Subunit Alpha
3.3. Bioinformatics Analysis of the Amino Acid Sequences of C-phycocyanin Subunit Alpha
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Steinbüchel, A. New medium for pharmaceutical grade Arthrospira. Int. J. Bacteriol. 2013, 2013, 203432. [Google Scholar]
- Kebede, E. Response of Spirulina platensis (=Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J. Appl. Phycol. 1997, 9, 551–558. [Google Scholar]
- Turpin, P.J.F. Spirulina oscillarioide. Dict. Sci. 1827, 50, 309–310. [Google Scholar]
- Rich, F. Notes on Arthrospira platetnsis. Rev. Algol. 1931, 6, 75–79. [Google Scholar]
- Dangeard, P. Sur une algue bleue alimentaire pour l’homme: Arthrospira platensis (Nordst.) Gomont. Act. Soc. Linn. Bordx. 1940, 91, 39–41. [Google Scholar]
- Shimamatsu, H. Mass production of Spirulina, an edible microalga. Hydrobiologia 2004, 512, 39–44. [Google Scholar] [CrossRef]
- Mühling, M.; Belay, A.; Whitton, B.A. Screening Arthrospira (Spirulina) strains for heterotrophy. J. Appl. Phycol. 2005, 17, 129–135. [Google Scholar] [CrossRef]
- Busnel, A.; Samhat, K.; Gérard, E.; Kazbar, A.; Marec, H.; Dechandol, E.; Le Gouic, B.; Hauser, J.-L.; Pruvost, J. Development and validation of a screening system for characterizing and modeling biomass production from cyanobacteria and microalgae: Application to Arthrospira platensis and Haematococcus pluvialis. Algal Res. 2021, 58, 102386. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies affecting the growth and cultivation of genus Spirulina: An investigative review on current trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Capelli, B.; Cysewski, G.R. Potential health benefits of Spirulina microalgae: A review of the existing literature. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Chapter 19-Nutritional and pharmaceutical properties of microalgal Spirulina. In Handbook of Marine Microalgae; Kim, S.K., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 299–308. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- García, A.B.; Longo, E.; Bermejo, R. The application of a phycocyanin extract obtained from Arthrospira platensis as a blue natural colorant in beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Li, S.; Doumandji, A.; Markou, G.; Nasri, M. Stability, bio-functionality and bio-activity of crude phycocyanin from a two-phase cultured Saharian Arthrospira sp. Strain. Algal Res. 2018, 35, 395–406. [Google Scholar] [CrossRef]
- Sharaf, M.; Amara, A.; Aboul-Enein, A.; Helmi, S.; Ballot, A.; Astani, A.; Schnitzler, P. Molecular authentication and characterization of the antiherpetic activity of the cyanobacterium Arthrospira fusiformis. Pharmazie 2010, 65, 132–136. [Google Scholar]
- Haverkamp, T.H.; Schouten, D.; Doeleman, M.; Wollenzien, U.; Huisman, J.; Stal, L.J. Colorful microdiversity of Synechococcus strains (picocyanobacteria) isolated from the Baltic Sea. ISME J. 2009, 3, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Wan Maznah, W.O.; Faradina Merican, M.S.M.; Convey, P.; Najimudin, N.; Alias, S.A. A comparative study of phycobilliprotein production in two strains of Pseudanabaena isolated from Arctic and tropical regions in relation to different light wavelengths and photoperiods. Polar Sci. 2019, 20, 3–8. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid Green Extractions of C-Phycocyanin from Arthrospira maxima for Functional Applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, H.; Dinh, T.B.; Choi, S.; De Saeger, J.; Depuydt, S.; Brown, M.T.; Han, T. Commercial Potential of the Cyanobacterium Arthrospira maxima: Physiological and Biochemical Traits and the Purification of Phycocyanin. Biology 2022, 11, 628. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Siegelman, H.; Kycia, J.H. Alga biliproteins. In Handbook of Phycological Methods: Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: Cambridge, UK, 1978; pp. 72–78. [Google Scholar]
- Sharmaa, G.; Sarana, S.; Puri, N.; Jasuja, N.D.; Kumar, M. Optimization, purification and characterization of phycocyanin from Spirulina platensis. Int. J. Appl. Pure Sci. Agric. 2016, 2, 15–20. [Google Scholar]
- Madden, T. The BLAST Sequence analysis tool (Chapter 16). In The NCBI Handbook [Internet]; McEntyre, J., Ostell, J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2002; p. 15. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, L.N.; Chang, W.R.; Zhang, J.P.; Gui, L.L.; Guo, B.J.; Liang, D.C. Structure of C-phycocyanin from Spirulina platensis at 2.2 A resolution: A novel monoclinic crystal form for phycobiliproteins in phycobilisomes. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 784–792. [Google Scholar] [CrossRef]
- Castro-Severyn, J.; Pardo-Esté, C.; Mendez, K.N.; Fortt, J.; Marquez, S.; Molina, F.; Castro-Nallar, E.; Remonsellez, F.; Saavedra, C.P. Living to the High Extreme: Unraveling the Composition, Structure, and Functional Insights of Bacterial Communities Thriving in the Arsenic-Rich Salar de Huasco Altiplanic Ecosystem. Microbiol. Spectr. 2021, 9, e0044421. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.J.; Castelle, C.J.; Singh, A.; Brown, C.T.; Anantharaman, K.; Sharon, I.; Hug, L.A.; Burstein, D.; Emerson, J.B.; Thomas, B.C.; et al. Genomic resolution of a cold subsurface aquifer community provides metabolic insights for novel microbes adapted to high CO2 concentrations. Environ. Microbiol. 2017, 19, 459–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Carrascal, O.M.; Tromas, N.; Terrat, Y.; Moreno, E.; Giani, A.; Marques, L.C.B.; Fortin, N.; Shapiro, B.J. Single-colony sequencing reveals microbe-by-microbiome phylosymbiosis between the cyanobacterium Microcystis and its associated bacteria. Microbiome 2021, 9, 194. [Google Scholar] [CrossRef]
- Dalcin Martins, P.; Danczak, R.E.; Roux, S.; Frank, J.; Borton, M.A.; Wolfe, R.A.; Burris, M.N.; Wilkins, M.J. Viral and metabolic controls on high rates of microbial sulfur and carbon cycling in wetland ecosystems. Microbiome 2018, 6, 138. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Olm, M.R.; Probst, A.J.; Anantharaman, K.; Power, M.E.; Banfield, J.F. Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network. ISME J. 2019, 13, 1618–1634. [Google Scholar] [CrossRef] [Green Version]
- Alcorta, J.; Alarcón-Schumacher, T.; Salgado, O.; Díez, B. Taxonomic Novelty and Distinctive Genomic Features of Hot Spring Cyanobacteria. Front. Genet. 2020, 11, 568223. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, A.N.; Bryant, D.A. Allophycocyanin B (λmax 671, 618 nm) Arch. Microbiol. 1975, 104, 15–22. [Google Scholar]
- Kulshreshtha, A.; Jacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.; Bisen, P.S. Spirulina in health care management. Curr. Pharmaceut. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Hamad, G.M.; Abd El-Baky, N.; Sharaf, M.M.; Amara, A.A. Volatile compounds, fatty acids constituents, and antimicrobial activity of cultured Spirulina (Arthrospira fusiformis) isolated from Lake Mariout in Egypt. Sci. World J. 2023, 2023, 9919814. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Production of phycocyanin–A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochem. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Hu, E.Z.; Lan, X.R.; Liu, Z.L.; Gao, J.; Niu, D.-K. A positive correlation between GC content and growth temperature in prokaryotes. BMC Genom. 2022, 23, 110. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kevin, A.; Indira, R.; Taralyn, T. Structure & Function—Amino Acids—Biology LibreTexts. Available online: https://bio.libretexts.org/Bookshelves/Biochemistry/Book:_Biochemistry_Free_For_All_(Ahern,_Rajagopal,_and_Tan)/2:_Structure_and_Function/2.2:_Structure_and_Function_-_Amino_Acids (accessed on 20 November 2020).
- Li, J.; Zhou, J.; Wu, Y.; Yang, S.; Tian, D. GC-content of synonymous codons profoundly influences amino acid usage. G3 (Bethesda) 2015, 5, 2027–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, M.E.; Fitz-Gibbon, S.T.; Oren, A.; House, C.H. Amino acid signatures of salinity on an environmental scale with a focus on the Dead Sea. Environ. Microbiol. 2010, 12, 2613–2623. [Google Scholar] [CrossRef] [PubMed]






| Strain | Total Protein Concentration (mg/g Dried Biomass) |
|---|---|
| Egyptian A. platensis | 1.233 |
| French A. platensis | 1.188 |
| Chinese A. platensis | 1.028 |
| Strain | C-phycocyanin Concentration (mg/mL) | C-phycocyanin Purity (Dividing A615 by A280) |
|---|---|---|
| Egyptian A. platensis | 0.323 | 0.84 |
| French A. platensis | 0.333 | 0.69 |
| Chinese A. platensis | 0.327 | 0.84 |
| Accession and Species | T(U) | C | A | G | Total | T-1 | C-1 | A-1 | G-1 | Pos #1 | T-2 | C-2 | A-2 | G-2 | Pos #2 | T-3 | C-3 | A-3 | G-3 | Pos #3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG777151, Arthrospira sp. CCALA 030 | 18.9 | 25.1 | 24.5 | 31.5 | 486.0 | 11.7 | 20.4 | 31.5 | 36.4 | 162.0 | 22.2 | 23.5 | 31.5 | 22.8 | 162.0 | 22.8 | 31.5 | 10.5 | 35.2 | 162.0 |
| P72509, Arthrospira platensis | 19.1 | 25.1 | 24.7 | 31.1 | 486.0 | 11.7 | 20.4 | 32.1 | 35.8 | 162.0 | 22.8 | 24.1 | 31.5 | 21.6 | 162.0 | 22.8 | 30.9 | 10.5 | 35.8 | 162.0 |
| WP 006620876, Oscillatoriales | 19.3 | 24.7 | 24.5 | 31.5 | 486.0 | 11.7 | 20.4 | 31.5 | 36.4 | 162.0 | 23.5 | 23.5 | 31.5 | 21.6 | 162.0 | 22.8 | 30.2 | 10.5 | 36.4 | 162.0 |
| 1GH0 A, Arthrospira platensis | 19.1 | 24.7 | 24.3 | 31.9 | 486.0 | 11.7 | 20.4 | 30.9 | 37.0 | 162.0 | 23.5 | 23.5 | 31.5 | 21.6 | 162.0 | 22.2 | 30.2 | 10.5 | 37.0 | 162.0 |
| AEV40868, Arthrospira erdosensis ez | 19.3 | 24.7 | 24.7 | 31.3 | 486.0 | 11.7 | 20.4 | 32.1 | 35.8 | 162.0 | 23.5 | 22.8 | 31.5 | 22.2 | 162.0 | 22.8 | 30.9 | 10.5 | 35.8 | 162.0 |
| MBS0014833, Arthrospira sp. SH-MAG29 | 19.3 | 24.7 | 24.9 | 31.1 | 486.0 | 11.7 | 20.4 | 32.7 | 35.2 | 162.0 | 23.5 | 22.2 | 31.5 | 22.8 | 162.0 | 22.8 | 31.5 | 10.5 | 35.2 | 162.0 |
| ABD64608, Arthrospira platensis | 19.3 | 25.1 | 24.7 | 30.9 | 486.0 | 11.7 | 20.4 | 32.7 | 35.2 | 162.0 | 23.5 | 24.1 | 31.5 | 21.0 | 162.0 | 22.8 | 30.9 | 9.9 | 36.4 | 162.0 |
| MCG5058249, Limnoraphis sp. WC205 | 18.9 | 25.3 | 24.3 | 31.5 | 486.0 | 11.7 | 21.0 | 30.9 | 36.4 | 162.0 | 22.8 | 24.1 | 31.5 | 21.6 | 162.0 | 22.2 | 30.9 | 10.5 | 36.4 | 162.0 |
| CAA70296, Arthrospira platensis | 20.6 | 23.5 | 24.5 | 31.5 | 486.0 | 12 | 20.4 | 31.5 | 36.4 | 162.0 | 22 | 23.5 | 31.5 | 22.8 | 162.0 | 28 | 26.5 | 10.5 | 35.2 | 162.0 |
| Average % | 19.3 | 24.8 | 24.6 | 31.3 | 486.0 | 12 | 20.4 | 31.8 | 36.1 | 162.0 | 23 | 23.5 | 31.5 | 22.0 | 162.0 | 23 | 30.4 | 10.4 | 35.9 | 162.0 |
| Accession | GC Content (%) | AT Content (%) |
|---|---|---|
| MG777151 | 56.58 | 43.42 |
| P72509 | 56.17 | 43.83 |
| WP 006620876 | 56.17 | 43.83 |
| 1GH0 A | 56.58 | 43.42 |
| AEV40868 | 55.97 | 44.03 |
| MBS0014833 | 55.76 | 44.24 |
| ABD64608 | 55.97 | 44.03 |
| MCG5058249 | 56.79 | 43.21 |
| CAA70296 | 54.94 | 45.06 |
| Phycocyanin Subunit Alpha | A | C | D | E | F | G | H | I | K | L | M | N | P | Q | R | S | T | V | W | Y | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABD64608.1:1-162 Arthrospira platensis | 14.8 | 1.2 | 5.6 | 4.3 | 3.1 | 7.4 | 0.6 | 6.8 | 5.6 | 8.0 | 2.5 | 4.3 | 3.1 | 4.3 | 4.3 | 7.4 | 6.2 | 3.1 | 0.6 | 6.8 | 162 |
| 1GH0 A:1-162 Arthrospira platensis | 14.8 | 1.2 | 5.6 | 4.9 | 3.1 | 8.0 | 0.6 | 6.2 | 5.6 | 8.0 | 2.5 | 3.7 | 3.1 | 4.3 | 4.3 | 7.4 | 5.6 | 3.7 | 0.6 | 6.8 | 162 |
| AEV40868.1:1-162 Arthrospira erdosensis ez | 14.2 | 1.2 | 5.6 | 4.9 | 3.1 | 8.0 | 0.6 | 6.8 | 5.6 | 8.0 | 2.5 | 3.7 | 3.1 | 4.3 | 4.3 | 8.0 | 5.6 | 3.1 | 0.6 | 6.8 | 162 |
| MBS0014833.1:1-162 Arthrospira sp. SH-MAG29 | 13.6 | 1.2 | 5.6 | 4.9 | 3.1 | 8.0 | 0.6 | 6.8 | 5.6 | 8.0 | 2.5 | 3.7 | 3.1 | 4.3 | 4.3 | 8.6 | 5.6 | 3.1 | 0.6 | 6.8 | 162 |
| CAA70296.1:1-162 Arthrospira platensis | 14.8 | 1.2 | 5.6 | 4.9 | 3.1 | 8.6 | 0.6 | 6.8 | 5.6 | 7.4 | 2.5 | 3.7 | 3.1 | 4.3 | 4.9 | 7.4 | 5.6 | 2.5 | 0.6 | 6.8 | 162 |
| NJO70792.1:1-162 Oscillatoriales cyanobacterium RM1 1 9 | 15.4 | 1.2 | 5.6 | 4.9 | 3.1 | 8.0 | 0.6 | 5.6 | 5.6 | 8.0 | 3.1 | 3.7 | 3.1 | 4.3 | 4.3 | 6.2 | 7.4 | 3.1 | 0.6 | 6.2 | 162 |
| OIP70521.1:1-162 Oscillatoriales cyanobacterium CG2 30 40 61 | 14.8 | 1.2 | 4.3 | 5.6 | 3.1 | 8.0 | 0.6 | 5.6 | 5.6 | 7.4 | 1.9 | 3.7 | 3.1 | 4.3 | 4.3 | 6.8 | 8.0 | 4.3 | 0.6 | 6.8 | 162 |
| WP 026793960.1:1-162 Planktothrix mougeotii | 14.2 | 1.2 | 4.3 | 5.6 | 3.1 | 8.0 | 0.6 | 4.9 | 5.6 | 8.0 | 1.9 | 4.3 | 3.1 | 3.7 | 4.3 | 8.6 | 6.8 | 4.3 | 0.6 | 6.8 | 162 |
| WP 083621658.1:1-162 Planktothrix paucivesiculata | 14.8 | 1.2 | 4.3 | 5.6 | 3.1 | 8.6 | 0.6 | 4.9 | 5.6 | 8.6 | 1.2 | 3.7 | 3.1 | 4.3 | 4.3 | 6.8 | 7.4 | 4.3 | 0.6 | 6.8 | 162 |
| PSN18344.1:1-162 filamentous cyanobacterium CCP5 | 14.8 | 1.2 | 4.9 | 5.6 | 3.1 | 8.6 | 0.6 | 3.7 | 4.9 | 8.6 | 3.1 | 3.7 | 3.1 | 4.9 | 3.7 | 8.0 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| WP 194023545.1:1-162 Nodosilinea sp. LEGE 07298 | 14.8 | 1.2 | 4.9 | 5.6 | 3.1 | 8.6 | 0.6 | 3.1 | 4.9 | 8.0 | 3.1 | 3.7 | 3.1 | 4.3 | 3.7 | 8.0 | 6.8 | 4.9 | 0.6 | 6.8 | 162 |
| MCA2686410.1:1-162 Microcystis sp. M046S2 | 15.4 | 1.2 | 5.6 | 4.3 | 3.1 | 7.4 | 0.6 | 6.2 | 4.3 | 7.4 | 2.5 | 4.9 | 3.1 | 4.3 | 4.9 | 7.4 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| WP 015143844.1:1-162 MULTISPECIES: Pleurocapsales | 15.4 | 1.9 | 4.9 | 4.9 | 2.5 | 8.0 | 0.6 | 6.2 | 4.9 | 8.0 | 3.1 | 5.6 | 3.1 | 4.9 | 3.7 | 6.8 | 5.6 | 2.5 | 0.6 | 6.8 | 162 |
| WP 147071652.1:1-162 Microcystis aeruginosa | 15.4 | 1.2 | 5.6 | 4.3 | 3.1 | 8.0 | 0.6 | 6.2 | 4.3 | 7.4 | 2.5 | 3.7 | 3.1 | 4.3 | 4.9 | 8.0 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| RPH86579.1:1-162 Chroococcales cyanobacterium metabat2.561 | 16.0 | 1.2 | 4.9 | 4.9 | 3.1 | 7.4 | 0.6 | 6.2 | 4.3 | 7.4 | 2.5 | 4.3 | 3.1 | 4.3 | 4.9 | 7.4 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| WP 002771746.1:1-162 Microcystis aeruginosa | 16.0 | 1.2 | 5.6 | 4.3 | 3.1 | 6.8 | 0.6 | 6.2 | 4.9 | 7.4 | 2.5 | 3.7 | 3.1 | 4.3 | 4.3 | 8.6 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| WP 079207951.1:1-162 Microcystis aeruginosa | 15.4 | 1.2 | 5.6 | 4.3 | 3.1 | 8.6 | 0.6 | 6.2 | 4.3 | 7.4 | 2.5 | 3.7 | 3.1 | 4.3 | 4.9 | 7.4 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| WP 035990438.1:1-162 Leptolyngbya sp. KIOST-1 | 14.8 | 1.2 | 4.9 | 5.6 | 3.1 | 8.6 | 0.6 | 3.7 | 4.9 | 8.0 | 3.1 | 3.7 | 2.5 | 4.3 | 3.7 | 9.3 | 6.2 | 4.3 | 0.6 | 6.8 | 162 |
| MBF2078792.1:1-162 Synechococcales cyanobacterium T60 A2020 003 | 14.8 | 1.2 | 5.6 | 4.9 | 3.1 | 7.4 | 0.6 | 6.2 | 4.9 | 7.4 | 2.5 | 3.7 | 3.1 | 6.2 | 4.3 | 7.4 | 5.6 | 3.7 | 0.6 | 6.8 | 162 |
| NJN32399.1:1-162 Synechococcales cyanobacterium RM1 1 8 | 14.8 | 1.2 | 5.6 | 4.3 | 3.1 | 8.0 | 0.6 | 4.3 | 5.6 | 8.6 | 2.5 | 3.7 | 3.7 | 4.3 | 3.1 | 8.6 | 6.8 | 3.7 | 0.6 | 6.8 | 162 |
| WP 190519399.1:1-162 MULTISPECIES: unclassified Nodosilinea | 16.7 | 1.9 | 4.9 | 5.6 | 2.5 | 8.6 | 0.6 | 3.1 | 4.9 | 8.6 | 2.5 | 4.3 | 2.5 | 3.7 | 3.7 | 7.4 | 6.2 | 4.9 | 0.6 | 6.8 | 162 |
| WP 010871860.1:1-162 MULTISPECIES: Synechocystis | 14.2 | 1.2 | 6.8 | 3.7 | 3.1 | 8.0 | 0.6 | 4.9 | 4.3 | 9.9 | 0.6 | 6.2 | 2.5 | 5.6 | 4.9 | 6.2 | 6.8 | 3.7 | 0.6 | 6.2 | 162 |
| WP 194029021.1:1-162 Lusitaniella coriacea | 14.8 | 1.2 | 4.9 | 6.2 | 2.5 | 9.3 | 1.2 | 4.3 | 4.9 | 8.6 | 2.5 | 3.7 | 2.5 | 3.1 | 3.7 | 9.9 | 5.6 | 4.3 | 0.6 | 6.2 | 162 |
| TAG95764.1:1-162 Oscillatoriales cyanobacterium | 16.0 | 1.2 | 4.3 | 4.9 | 3.1 | 8.0 | 0.6 | 4.3 | 4.3 | 8.6 | 2.5 | 3.7 | 3.1 | 4.9 | 4.3 | 6.2 | 8.6 | 3.7 | 0.6 | 6.8 | 162 |
| HIK44559.1:1-162 Leptolyngbyaceae cyanobacterium M65 K2018 010 | 16.7 | 1.9 | 4.9 | 5.6 | 2.5 | 8.0 | 0.6 | 4.3 | 3.7 | 8.6 | 2.5 | 4.3 | 2.5 | 3.7 | 4.9 | 8.0 | 6.2 | 3.7 | 0.6 | 6.8 | 162 |
| Average % | 15.1 | 1.3 | 5.2 | 5.0 | 3.0 | 8.1 | 0.6 | 5.3 | 5.0 | 8.1 | 2.4 | 4.0 | 3.0 | 4.4 | 4.3 | 7.7 | 6.4 | 3.7 | 0.6 | 6.7 | 162 |
| Strain | Characteristics of Isolate | Accession | Reference |
|---|---|---|---|
| Arthrospira platensis | - Phycocyanin alpha chain - Country: India - Submitted by Plant Molecular Biology and Biotechnology, Tamil Nadu Agricultural University, India | ABD64608 | Unpublished |
| Arthrospira platensis (Gomont) Geitler 1925 | - Crystal structure of C-phycocyanin - Isolation source: urban reservoir in Poland, Central Europe. | 1GH0 A | [29] |
| Arthrospira erdosensis ez | - Phycocyanin alpha chain - Submitted by College of Life Science and Technology, Inner Mongolia Normal University, P.R. China - Isolation source: alkaline lake 1200–1600 m above sea level | AEV40868 | Unpublished |
| Arthrospira sp. SH-MAG29 | - Phycocyanin subunit alpha - Isolation source: shallow sediments of the arsenic-rich Salar de Huasco lagoon - Submitted by Departamento de Ingenieria Quimica, Universidad Catolica del Norte, Chile | MBS0014833 | [30] |
| Arthrospira platensis | - Phycocyanin alpha subunit - Submitted by W. Jeamton, School of Bioresources and Technology, King Mongkut’s Institute of Technology Thonburi (KMITT), Thailand | CAA70296 | Unpublished |
| Oscillatoriales cyanobacterium RM1_1_9 | - Phycocyanin subunit alpha - Environmental sample - Country: South Africa, Cape Recife | NJO70792 | Unpublished |
| Oscillatoriales cyanobacterium CG2_30_40_61 | - Phycocyanin subunit alpha - Isolation source: Crystal Geyser (Utah, USA), a site where deeply sourced CO2 -saturated fluids are erupted at the surface | OIP70521 | [31] |
| Planktothrix mougeotii | Phycocyanin subunit alpha | WP_026793960 | Unpublished |
| Planktothrix paucivesiculata | Phycocyanin subunit alpha | WP_083621658 | Unpublished |
| Filamentous cyanobacterium CCP5 | - Phycocyanin subunit alpha- Submitted by Earth, Atmospheric and Planetary Sciences, Massachusetts Institute of Technology, USA - Isolation source: salt marsh | PSN18344 | Unpublished |
| Nodosilinea sp. LEGE 07298 | Phycocyanin subunit alpha | WP_194023545 | Unpublished |
| Microcystis sp. M046S2 | - Phycocyanin subunit alpha - Isolation source: fresh water surface (50 cm depth) | MCA2686410 | [32] |
| Pleurocapsales | Phycocyanin subunit alpha | WP_015143844 | Unpublished |
| Microcystis aeruginosa | Phycocyanin subunit alpha | WP_147071652 | Unpublished |
| Chroococcales cyanobacterium metabat2.561 | - Phycocyanin subunit alpha - Isolation source: Prairie Pothole Region wetland sediments | RPH86579 | [33] |
| Microcystis aeruginosa | Phycocyanin subunit alpha | WP_002771746 | Unpublished |
| Microcystis aeruginosa | Phycocyanin subunit alpha | WP_079207951 | Unpublished |
| Leptolyngbya sp. KIOST-1 | Phycocyanin subunit alpha | WP_035990438 | Unpublished |
| Synechococcales cyanobacterium T60_A2020_003 | - Phycocyanin subunit alpha - Isolation source: nonacidic hot spring microbial mat - Submitted by Departamento de Genetica Molecular y Microbiologia, Pontificia Universidad Catolica de Chile, Chile | MBF2078792 | Unpublished |
| Synechococcales cyanobacterium RM1_1_8 | -Phycocyanin subunit alpha -Isolation source: stromatolite - Submitted by Pharmaceutical Sciences, University of Wisconsin, USA | NJN32399 | Unpublished |
| Unclassified Nodosilinea | Phycocyanin subunit alpha | WP_190519399 | Unpublished |
| Synechocystis | Phycocyanin subunit alpha | WP_010871860 | Unpublished |
| Lusitaniella coriacea | Phycocyanin subunit alpha | WP_194029021 | Unpublished |
| Oscillatoriales cyanobacterium | - Phycocyanin subunit alpha - Isolation source: microbial mat material - Environmental sample - Country: USA, California, Eel River, Elder Creek | TAG95764 | [34] |
| Leptolyngbyaceae cyanobacterium M65_K2018_010 | -Phycocyanin subunit alpha -Isolation source: Hot spring_65deg. | HIK44559 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Baky, N.A.; Rezk, N.M.F.; Amara, A.A. Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions. J. Mar. Sci. Eng. 2023, 11, 663. https://doi.org/10.3390/jmse11030663
El-Baky NA, Rezk NMF, Amara AA. Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions. Journal of Marine Science and Engineering. 2023; 11(3):663. https://doi.org/10.3390/jmse11030663
Chicago/Turabian StyleEl-Baky, Nawal Abd, Neama Mahmoud Fattouh Rezk, and Amro A. Amara. 2023. "Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions" Journal of Marine Science and Engineering 11, no. 3: 663. https://doi.org/10.3390/jmse11030663
APA StyleEl-Baky, N. A., Rezk, N. M. F., & Amara, A. A. (2023). Arthrospira platensis Variants: A Comparative Study Based on C-phycocyanin Gene and Protein, Habitat, and Growth Conditions. Journal of Marine Science and Engineering, 11(3), 663. https://doi.org/10.3390/jmse11030663








