Immobilization on Polyethylenimine and Chitosan Sorbents Modulates the Production of Valuable Fatty Acids by the Chlorophyte Lobosphaera sp. IPPAS C-2047
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Carrier Synthesis
2.2. Strain and Cultivation Conditions
2.3. Estimation of the Condition of the Photosynthetic Apparatus
2.4. Assay of Chlorophyll and Analysis of Cell Lipid Fatty Acid Composition
2.5. Scanning Electron Microscopy
2.6. Statistical Treatment
3. Results
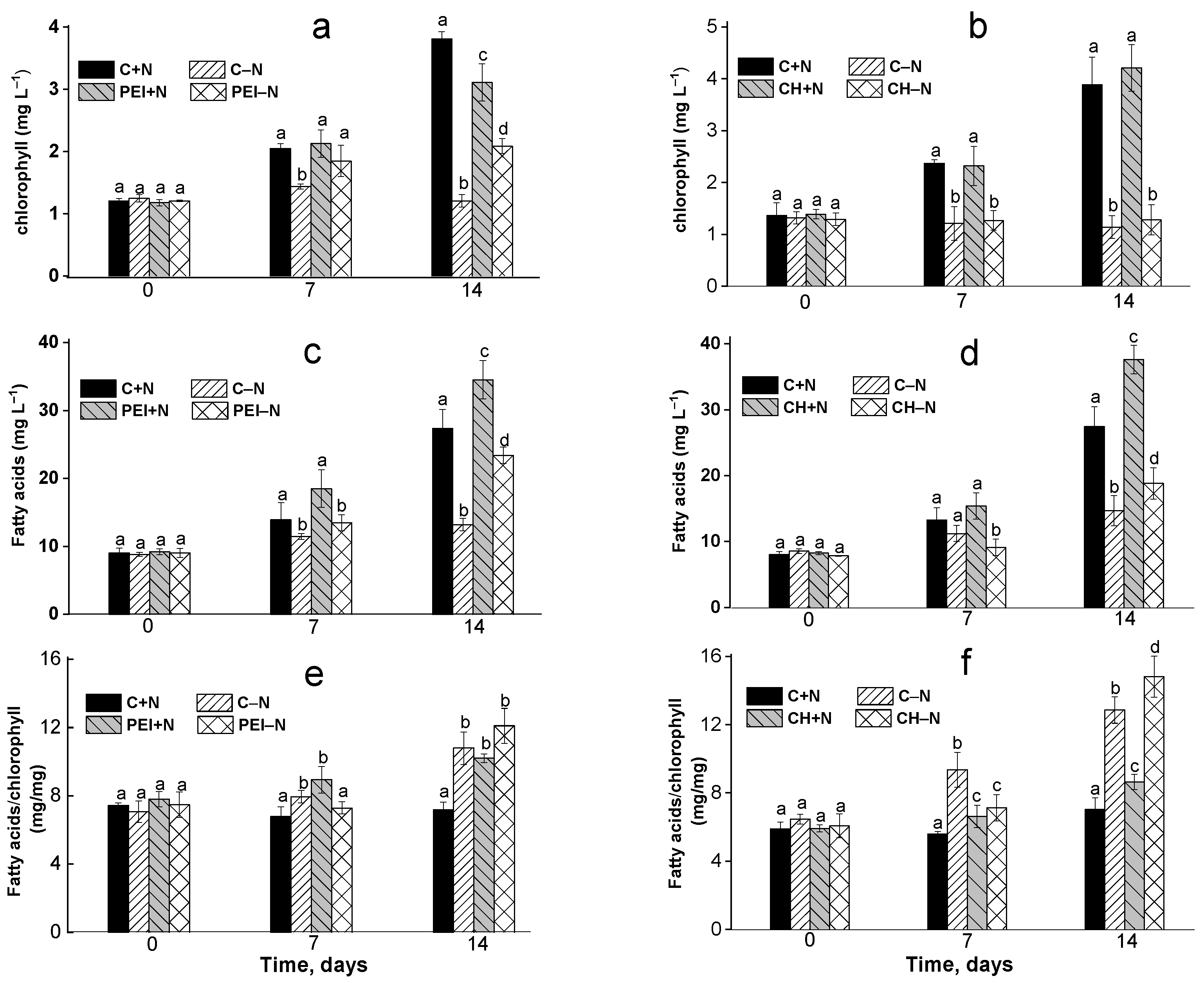
3.1. Changes in the Chlorophyll Content of the Culture
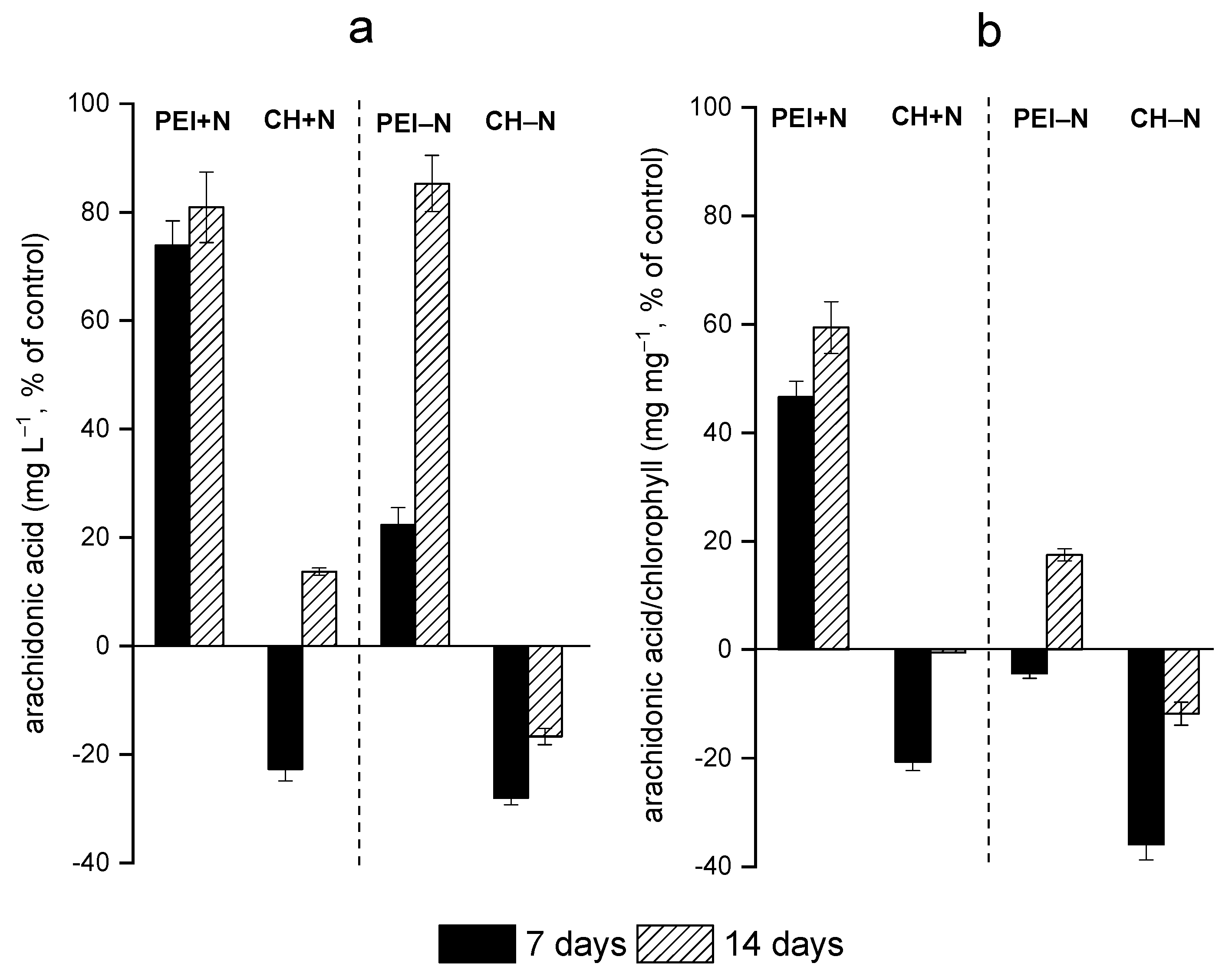
3.2. Effects of Immobilization on FA Profiles of the Cells
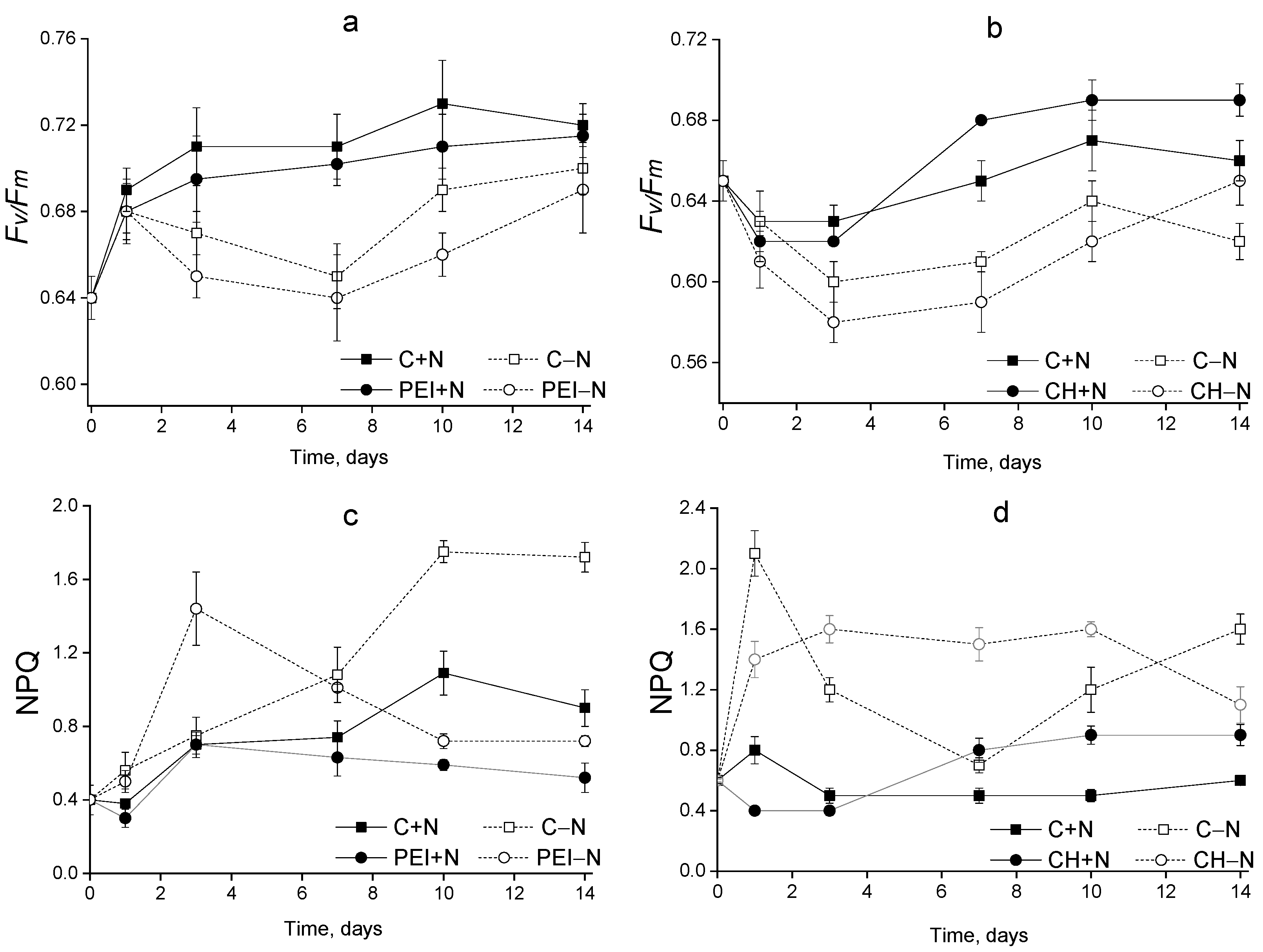
3.3. The Condition of the Photosynthetic Apparatus
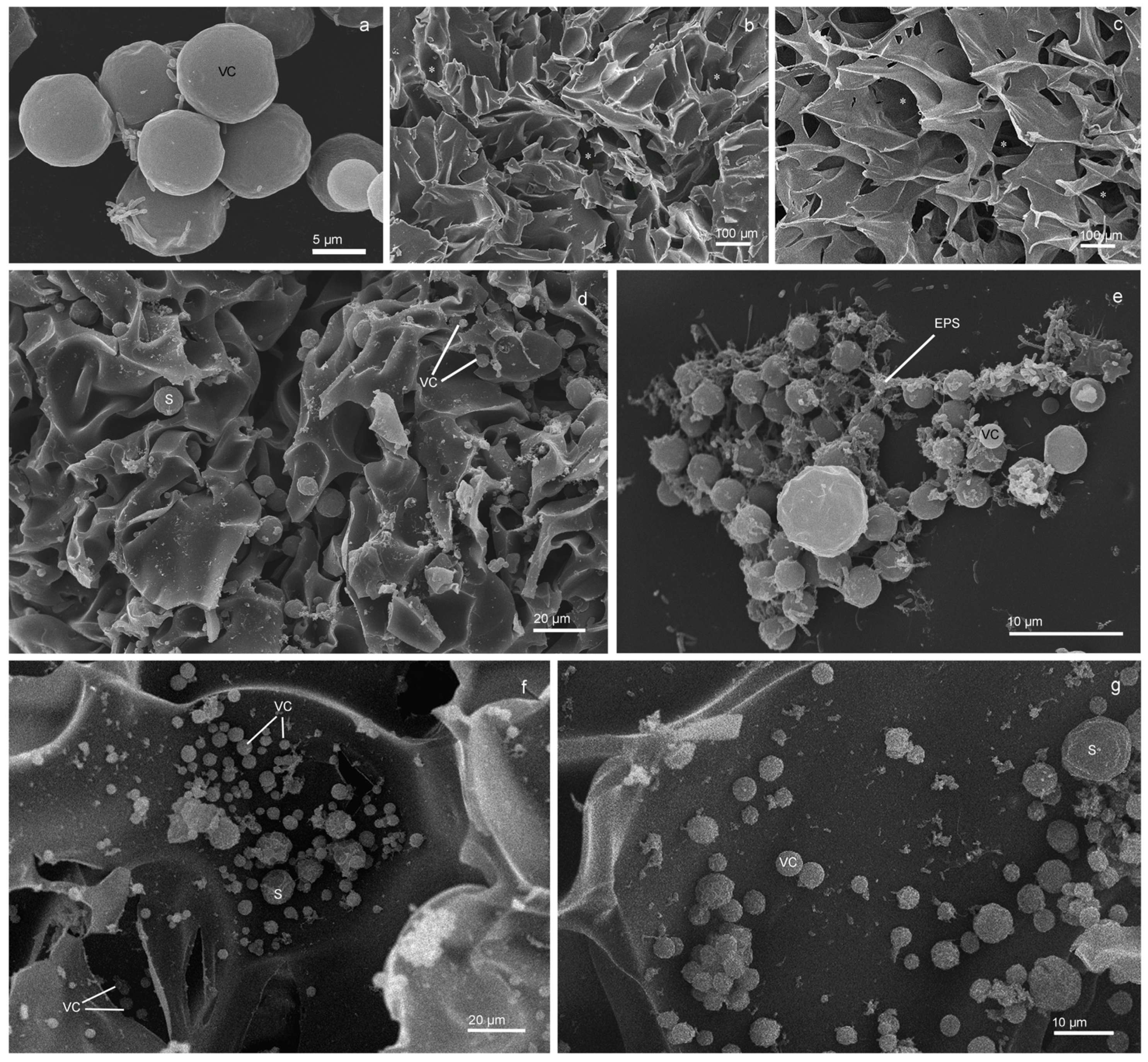
3.4. Changes in the Morphology of the Cells as Elucidated by SEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, M.J.; Janssen, M.; Sudfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Shahi Khalaf Ansar, B.; Kavusi, E.; Dehghanian, Z.; Pandey, J.; Asgari Lajayer, B.; Price, G.W.; Astatkie, T. Removal of organic and inorganic contaminants from the air, soil, and water by algae. Environ. Sci. Pollut. Res. Int 2022. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Chan, C.X.; De Clerck, O.; Cock, J.M.; Coelho, S.M.; Gachon, C.; Grossman, A.R.; Mock, T.; Raven, J.A.; Smith, A.G.; et al. The Algal Revolution. Trends Plant Sci. 2017, 22, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Khozin-Goldberg, I. Searching for PUFA-rich microalgae. In Single Cell Oils, 2 ed.; Cohen, Z., Ratledge, C., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 2010; pp. 201–224. [Google Scholar]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Dumancas, G.G. Arachidonic Acid: Dietary Sources and General Functions; Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2012. [Google Scholar]
- Dragos, A.; Kiesewalter, H.; Martin, M.; Hsu, C.Y.; Hartmann, R.; Wechsler, T.; Eriksen, C.; Brix, S.; Drescher, K.; Stanley-Wall, N.; et al. Division of Labor during Biofilm Matrix Production. Curr. Biol. 2018, 28, 1903–1913.e5. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Nozhevnikova, A.; Botchkova, E.; Plakunov, V. Multi-species biofilms in ecology, medicine, and biotechnology. Microbiology 2015, 84, 731–750. [Google Scholar] [CrossRef]
- Roeselers, G.; van Loosdrecht, M.C.; Muyzer, G. Heterotrophic pioneers facilitate phototrophic biofilm development. Microb. Ecol. 2007, 54, 578–585. [Google Scholar] [CrossRef]
- Costerton, J.W. Overview of microbial biofilms. J. Ind. Microbiol. Biotechnol. 1995, 15, 137–140. [Google Scholar] [CrossRef]
- Danaee, S.; Heydarian, S.M.; Ofoghi, H.; Varzaghani, N.B. Optimization, upscaling and kinetic study of famine technique in a microalgal biofilm-based photobioreactor for nutrient removal. Environ. Technol. Innov. 2021, 24, 102043. [Google Scholar] [CrossRef]
- Orfanos, A.G.; Manariotis, I.D. Algal biofilm ponds for polishing secondary effluent and resource recovery. J. Appl. Phycol. 2019, 31, 1765–1772. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lobakova, E.; Solovchenko, A. Biotechnological Applications of Immobilized Microalgae. Environ. Biotechnol. 2021, 3, 193–220. [Google Scholar]
- de-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Smith, S.M.; Raston, C.L. Application of Various Immobilization Techniques for Algal Bioprocesses. In Biomass and Biofuels from Microalgae; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–44. [Google Scholar]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Vasilieva, S.; Shibzukhova, K.; Morozov, A.; Solovchenko, A.; Bessonov, I.; Kopitsyna, M.; Lukyanov, A.; Chekanov, K.; Lobakova, E. Immobilization of microalgae on the surface of new cross-linked polyethylenimine-based sorbents. J. Biotechnol. 2018, 281, 31–38. [Google Scholar] [CrossRef]
- Romanova, O.; Grigor’ev, T.; Goncharov, M.; Rudyak, S.; Solov’yova, E.; Krasheninnikov, S.; Saprykin, V.; Sytina, E.; Chvalun, S.; Pal’tsev, M. Chitosan as a modifying component of artificial scaffold for human skin tissue engineering. Bull. Exp. Biol. Med. 2015, 159, 557–566. [Google Scholar] [CrossRef]
- Nuzhdina, A.V.; Morozov, A.S.; Kopitsyna, M.N.; Strukova, E.N.; Shlykova, D.S.; Bessonov, I.V.; Lobakova, E.S. Simple and versatile method for creation of non-leaching antimicrobial surfaces based on cross-linked alkylated polyethyleneimine derivatives. Mater. Sci. Eng. C 2017, 70, 788–795. [Google Scholar] [CrossRef]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Microbiol. Mol. Biol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Strasser, R.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a fluorescence: A Signature of Photosynthesis; Papageorgiou, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Merzlyak, M.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010, 46, 763–772. [Google Scholar] [CrossRef]
- Solovchenko, A.; Gorelova, O.; Selyakh, I.; Pogosyan, S.; Baulina, O.; Semenova, L.; Chivkunova, O.; Voronova, E.; Konyukhov, I.; Scherbakov, P. A novel CO2-tolerant symbiotic Desmodesmus (Chlorophyceae, Desmodesmaceae): Acclimation to and performance at a high carbon dioxide level. Algal Res. 2015, 11, 399–410. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Cohen, Z.; Merzlyak, M. Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J. Appl. Phycol. 2009, 21, 361–366. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Bigogno, C.; Shrestha, P.; Cohen, Z. Nitrogen starvation induces the accumulation of arachidonic acid in the freshwater green alga Parietochloris incisa (Trebuxiophyceae). J. Phycol. 2002, 38, 991–994. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Cohen, Z. Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (Trebuxiophyceae, Chlorophyta). Phytochemistry 2002, 60, 135–143. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Solovchenko, A. Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ. J. Plant Physiol. 2012, 59, 167–176. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Merzlyak, M.N.; Chivkunova, O.B.; Reshetnikova, I.V.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z. Effects of light irradiance and nitrogen starvation on the accumulation of arachidonic acid by the microalga Parietochloris incisa. Vestn. Mosk. Univ. Seriya 16 Biol. 2008, 63, 49. [Google Scholar]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Wacker, A.; Piepho, M.; Harwood, J.L.; Guschina, I.A.; Arts, M.T. Light-induced changes in fatty acid profiles of specific lipid classes in several freshwater phytoplankton species. Front. Plant. Sci. 2016, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Harwood, J.L. Algal Lipids and Their Metabolism. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2013; pp. 17–36. [Google Scholar]
- Kokabi, K.; Gorelova, O.; Zorin, B.; Didi-Cohen, S.; Itkin, M.; Malitsky, S.; Solovchenko, A.; Boussiba, S.; Khozin-Goldberg, I. Lipidome Remodeling and Autophagic Respose in the Arachidonic-Acid-Rich Microalga Lobosphaera incisa Under Nitrogen and Phosphorous Deprivation. Front. Plant. Sci. 2020, 11, 614846. [Google Scholar] [CrossRef] [PubMed]
- Kugler, A.; Zorin, B.; Didi-Cohen, S.; Sibiryak, M.; Gorelova, O.; Ismagulova, T.; Kokabi, K.; Kumari, P.; Lukyanov, A.; Boussiba, S. Long-chain polyunsaturated fatty acids in the green microalga Lobosphaera incisa contribute to tolerance to abiotic stresses. Plant Cell Physiol. 2019, 60, 1205–1223. [Google Scholar] [CrossRef]
- Klyachko-Gurvich, G.; Tsoglin, L.; Doucha, J.; Kopetskii, J.; Shebalina, I.; Semenenko, V. Desaturation of fatty acids as an adaptive response to shifts in light intensity 1. Plant. Physiol. 1999, 107, 240–249. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, Y.H.; Jeon, M.S.; Kim, S.; Choi, Y.-E. Polyethylenimine linked with chitosan improves astaxanthin production in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2022, 107, 569–580. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Shimada, N.; Iijima, K.; Hashizume, M.; Yoshimoto, K. Polyethyleneimine-induced astaxanthin accumulation in the green alga Haematococcus pluvialis by increased oxidative stress. J. Biosci. Bioeng. 2019, 128, 751–754. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, S.; Shibzukhova, K.; Solovchenko, A.; Chivkunova, O.; Antipova, C.; Morozov, A.; Lobakova, E. Immobilization on Polyethylenimine and Chitosan Sorbents Modulates the Production of Valuable Fatty Acids by the Chlorophyte Lobosphaera sp. IPPAS C-2047. J. Mar. Sci. Eng. 2023, 11, 865. https://doi.org/10.3390/jmse11040865
Vasilieva S, Shibzukhova K, Solovchenko A, Chivkunova O, Antipova C, Morozov A, Lobakova E. Immobilization on Polyethylenimine and Chitosan Sorbents Modulates the Production of Valuable Fatty Acids by the Chlorophyte Lobosphaera sp. IPPAS C-2047. Journal of Marine Science and Engineering. 2023; 11(4):865. https://doi.org/10.3390/jmse11040865
Chicago/Turabian StyleVasilieva, Svetlana, Karina Shibzukhova, Alexei Solovchenko, Olga Chivkunova, Christina Antipova, Alexey Morozov, and Elena Lobakova. 2023. "Immobilization on Polyethylenimine and Chitosan Sorbents Modulates the Production of Valuable Fatty Acids by the Chlorophyte Lobosphaera sp. IPPAS C-2047" Journal of Marine Science and Engineering 11, no. 4: 865. https://doi.org/10.3390/jmse11040865
APA StyleVasilieva, S., Shibzukhova, K., Solovchenko, A., Chivkunova, O., Antipova, C., Morozov, A., & Lobakova, E. (2023). Immobilization on Polyethylenimine and Chitosan Sorbents Modulates the Production of Valuable Fatty Acids by the Chlorophyte Lobosphaera sp. IPPAS C-2047. Journal of Marine Science and Engineering, 11(4), 865. https://doi.org/10.3390/jmse11040865





