The Effects of Non-Indigenous Macrophytes on Native Biodiversity: Case Studies from Sicily
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global Ecological Impacts of Invasive Species in Aquatic Ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Mačić, V.; Albano, P.G.; Almpanidou, V.; Claudet, J.; Corrales, X.; Essl, F.; Evagelopoulos, A.; Giovos, I.; Jimenez, C.; Kark, S.; et al. Biological Invasions in Conservation Planning: A Global Systematic Review. Front. Mar. Sci. 2018, 5, 178. [Google Scholar] [CrossRef] [Green Version]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the Global Threat of Invasive Species to Marine Biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A Global Review of the Distribution, Taxonomy, and Impacts of Introduced Seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef] [Green Version]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine Invasive Alien Species: A Threat to Global Biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine Alien Species in Italy: A Contribution to the Implementation of Descriptor D2 of the Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2019, 18, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Occhipinti-Ambrogi, A.; Galil, B. Marine Alien Species as an Aspect of Global Change. Adv. Oceanogr. Limnol. 2010, 1, 199–218. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D.; Guerra García, J.M.; Lubinevsky, H.; Galil, B.S. Desalination Effluents and the Establishment of the Non-Indigenous Skeleton Shrimp Paracaprella pusilla Mayer, 1890 in the South-Eastern Mediterranean. BioInvasions Rec. 2019, 8, 661–669. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien Marine Species in the Mediterranean—The 100 “worst Invasives” and Their Impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef] [Green Version]
- Zenetos, A.; Galanidi, M. Mediterranean Non Indigenous Species at the Start of the 2020s: Recent Changes. Mar. Biodivers. Rec. 2020, 13, 10. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global Climate Change Amplifies the Entry of Tropical Species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Winters, G.; Beer, S.; Willette, D.A.; Viana, I.G.; Chiquillo, K.L.; Beca-Carretero, P.; Villamayor, B.; Azcárate-García, T.; Shem-Tov, R.; Mwabvu, B.; et al. The Tropical Seagrass Halophila stipulacea: Reviewing What We Know From Its Native and Invasive Habitats, Alongside Identifying Knowledge Gaps. Front. Mar. Sci. 2020, 7, 300. [Google Scholar] [CrossRef]
- Willette, D.A.; Ambrose, R.F. Effects of the Invasive Seagrass Halophila stipulacea on the Native Seagrass, Syringodium filiforme, and Associated Fish and Epibiota Communities in the Eastern Caribbean. Aquat. Bot. 2012, 103, 74–82. [Google Scholar] [CrossRef]
- Maggi, E.; Benedetti-Cecchi, L.; Castelli, A.; Chatzinikolaou, E.; Crowe, T.P.; Ghedini, G.; Kotta, J.; Lyons, D.A.; Ravaglioli, C.; Rilov, G.; et al. Ecological Impacts of Invading Seaweeds: A Meta-analysis of Their Effects at Different Trophic Levels. Diversity Distrib. 2015, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ramsay-Newton, C.; Drouin, A.; Hughes, A.R.; Bracken, M.E.S. Species, Community, and Ecosystem-Level Responses Following the Invasion of the Red Alga Dasysiphonia japonica to the Western North Atlantic Ocean. Biol. Invasions 2017, 19, 537–547. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Byers, J.E.; Schiel, D.R.; Bruno, J.F.; Olden, J.D.; Wernberg, T.; Silliman, B.R. Impacts of Marine Invaders on Biodiversity Depend on Trophic Position and Functional Similarity. Mar. Ecol. Prog. Ser. 2014, 495, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Ruitton, S.; Verlaque, M. Large-Scale Disturbances, Regime Shift and Recovery in Littoral Systems Subject to Biological Invasions. In Proceedings of the Unesco-Roste/BAS Workshop on Regime Shifts, Varnam, Bulgaria, 14–16 June 2005; pp. 85–101. [Google Scholar]
- Geburzi, J.C.; McCarthy, M.L. How Do They Do It?—Understanding the Success of Marine Invasive Species. In YOUMARES 8—Oceans Across Boundaries: Learning fromEeach Other; Springer International Publishing: Cham, Switzerland, 2018; pp. 109–124. ISBN 9783319932842. [Google Scholar]
- Dijkstra, J.A.; Harris, L.G.; Mello, K.; Litterer, A.; Wells, C.; Ware, C. Invasive Seaweeds Transform Habitat Structure and Increase Biodiversity of Associated Species. J. Ecol. 2017, 105, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- Engelen, A.H.; Primo, A.L.; Cruz, T.; Santos, R. Faunal Differences between the Invasive Brown Macroalga Sargassum muticum and Competing Native Macroalgae. Biol. Invasions 2013, 15, 171–183. [Google Scholar] [CrossRef]
- Veiga, P.; Torres, A.C.; Besteiro, C.; Rubal, M. Mollusc Assemblages Associated with Invasive and Native Sargassum Species. Cont. Shelf Res. 2018, 161, 12–19. [Google Scholar] [CrossRef]
- Veiga, P.; Rubal, M.; Sousa-Pinto, I. Structural Complexity of Macroalgae Influences Epifaunal Assemblages Associated with Native and Invasive Species. Mar. Environ. Res. 2014, 101, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.P.; Agostaro, R.D.; Milazzo, M.; Badalamenti, F. The Invasive Seaweed Asparagopsis taxiformis Erodes the Habitat Structure and Biodiversity of Native Algal Forests in the Mediterranean Sea. Mar. Environ. Res. 2022, 173, 105515. [Google Scholar] [CrossRef] [PubMed]
- Gestoso, I.; Olabarria, C.; Troncoso, J.S. Variability of Epifaunal Assemblages Associated with Native and Invasive Macroalgae. Mar. Freshw. Res. 2010, 61, 724–731. [Google Scholar] [CrossRef]
- Buschbaum, C.; Chapman, A.S.; Saier, B. How an Introduced Seaweed Can Affect Epibiota Diversity in Different Coastal Systems. Mar. Biol. 2006, 148, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Wernberg, T.; Thomsen, M.S.; Staehr, P.A.; Pedersen, M.F. Epibiota Communities of the Introduced and Indigenous Macroalgal Relatives Sargassum muticum and Halidrys siliquosa in Limfjorden (Denmark). Helgol. Mar. Res. 2004, 58, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Rubal, M.; Costa-Garcia, R.; Besteiro, C.; Sousa-Pinto, I.; Veiga, P. Mollusc Diversity Associated with the Non-Indigenous Macroalga Asparagopsis armata Harvey, 1855 along the Atlantic Coast of the Iberian Peninsula. Mar. Environ. Res. 2018, 136, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Benedetti-Cecchi, L. Facilitation of the Introduced Green Alga Caulerpa racemosa by Resident Algal Turfs: Experimental Evaluation of Underlying Mechanisms. Mar. Ecol. Prog. Ser. 2008, 364, 77–86. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Bouma, T.J.; Morris, E.P.; Duarte, C.M. Effects of Seagrasses and Algae of the Caulerpa Family on Hydrodynamics and Particle-Trapping Rates. Mar. Biol. 2010, 157, 473–481. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential Effects of an Invasive Seaweed (Caulerpa cylindracea Sonder) on Sedimentary Organic Matter and Microbial Metabolic Activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef] [Green Version]
- Bando, K.J. The Roles of Competition and Disturbance in a Marine Invasion. Biol. Invasions 2006, 8, 755–763. [Google Scholar] [CrossRef]
- Rotini, A.; Mejia, A.Y.; Costa, R.; Migliore, L.; Winters, G. Ecophysiological Plasticity and Bacteriome Shift in the Seagrass Halophila stipulacea along a Depth Gradient in the Northern Red Sea. Front. Plant Sci. 2017, 7, 2015. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, G.; Acunto, S.; Famà, P.; Maltagliati, F. Structural, Morphological and Genetic Variability in Halophila stipulacea (Hydrocharitaceae) Populations in the Western Mediterranean. Mar. Biol. 1999, 135, 181–189. [Google Scholar] [CrossRef]
- Mejia, A.Y.; Rotini, A.; Lacasella, F.; Bookman, R.; Thaller, M.C.; Shem-Tov, R.; Winters, G.; Migliore, L. Assessing the Ecological Status of Seagrasses Using Morphology, Biochemical Descriptors and Microbial Community Analyses. A Study in Halophila stipulacea (Forsk.) Aschers Meadows in the Northern Red Sea. Ecol. Indic. 2016, 60, 1150–1163. [Google Scholar] [CrossRef]
- Schwarz, A.M.; Hellblom, F. The Photosynthetic Light Response of Halophila stipulacea Growing along a Depth Gradient in the Gulf of Aqaba, the Red Sea. Aquat. Bot. 2002, 74, 263–272. [Google Scholar] [CrossRef]
- Andreakis, N.; Procaccini, G.; Kooistra, W.H.C.F. Asparagopsis taxiformis and Asparagopsis armata (Bonnemaisoniales, Rhodophyta): Genetic and Morphological Identification of Mediterranean Populations. Eur. J. Phycol. 2004, 39, 273–283. [Google Scholar] [CrossRef]
- Mannino, A.M.; Balistreri, P. An Updated Overview of Invasive Caulerpa Taxa in Sicily and CircumSicilian Islands, Strategic Zones within the NW Mediterranean Sea. Flora Mediterr. 2017, 27, 221–240. [Google Scholar] [CrossRef]
- Mannino, A.M.; Balistreri, P. Citizen Science: A Successful Tool for Monitoring Invasive Alien Species (IAS) in Marine Protected Areas. The Case Study of the Egadi Islands MPA (Tyrrhenian Sea, Italy). Biodiversity 2018, 19, 42–48. [Google Scholar] [CrossRef]
- Mannino, A.M.; Borfecchia, F.; Micheli, C. Tracking Marine Alien Macroalgae in the Mediterranean Sea: The Contribution of Citizen Science and Remote Sensing. J. Mar. Sci. Eng. 2021, 9, 288. [Google Scholar] [CrossRef]
- Horton, T.; Lowry, J.; De Broyer, C.; Bellan-Santini, D.; Coleman, C.O.; Corbari, L.; Costello, M.J.; Daneliya, M.; Dauvin, J.-C.; Fišer, C.; et al. World Amphipoda Database 2021. Available online: https://www.marinespecies.org/amphipoda/index.php (accessed on 10 January 2023).
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Milazzo, M.; Chemello, R. Decreasing in Patch-Size of Cystoseira Forests Reduces the Diversity of Their Associated Molluscan Assemblage in Mediterranean Rocky Reefs. Estuar. Coast. Shelf Sci. 2021, 250, 107163. [Google Scholar] [CrossRef]
- Mannino, A.M.; Balistreri, P.; Mancuso, F.P.; Bozzeda, F.; Pinna, M. Searching for the Competitive Ability of the Alien Seagrass Halophila stipulacea with the Autochthonous Species Cymodocea nodosa. NeoBiota 2023, 83, 155–177. [Google Scholar] [CrossRef]
- Mannino, A.M.; Di Giovanni, D. Competition among Introduced and Indigenous Submerged Macrophytes in a Southern Mediterranean Shallow System. In Proceedings of the Congresso Società Botanica Italiana, Genova, Italy, 21–24 September 2011; Volume 73, p. 21. [Google Scholar]
- Mannino, A.M.; Mancuso, F.P.; Toccaceli, M. Spreading of the Alien Seagrass Halophila stipulacea (Hydrocharitaceae) along the Sicilian Coast (Western Mediterranean Sea). In Proceedings of the Mediterranean Seagrass Workshop 09, Hvar, Croatia, 7–11 September 2009; p. 66. [Google Scholar]
- Mannino, A.M.; Balistreri, P. Invasive Alien Species in Mediterranean Marine Protected Areas: The Egadi Islands (Italy) Case Study. Biodiversity 2021, 22, 13–23. [Google Scholar] [CrossRef]
- Piazzi, L.; Meinesz, A.; Verlaque, M.; Akcali, B.; Antolić, B.; Argyrou, M.; Balata, D.; Ballesteros, E.; Ballesteros, E.; Calvo, S.; et al. Invasion of Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea: An Assessment of the Spread. Cryptogam. Algol. 2005, 26, 189–202. [Google Scholar]
- Ceccherelli, G.; Piazzi, L. Dispersal of Caulerpa racemosa Fragments in the Mediterranean: Lack of Detachment Time Effect on Establishment. Bot. Mar. 2001, 44, 209–213. [Google Scholar] [CrossRef]
- Panayotidis, P.; Žuljević, A. Sexual Reproduction of the Invasive Green Alga Caulerpa racemosa var. occidentalis in the Mediterranean Sea. Oceanol. Acta 2001, 24, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Holmer, M.; Marbà, N.; Lamote, M.; Duarte, C.M. Deterioration of Sediment Quality in Seagrass Meadows (Posidonia oceanica) Invaded by Macroalgae (Caulerpa sp.). Estuaries Coasts 2009, 32, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Arias, A.; Giangrande, A.; Gambi, M.C.; Anadón, N. Biology and New Records of the Invasive Species Branchiomma bairdi (Annelida: Sabellidae) in the Mediterranean Sea. Mediterr. Mar. Sci. 2013, 14, 162–171. [Google Scholar] [CrossRef]
- Mollo, E.; Gavagnin, M.; Carbone, M.; Castelluccio, F.; Pozone, F.; Roussis, V.; Templado, J.; Ghiselin, M.T.; Cimino, G. Factors Promoting Marine Invasions: A Chemoecological Approach. Proc. Natl. Acad. Sci. USA 2008, 105, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Issaris, Y.; Poursanidis, D.; Thessalou-Legaki, M. Vulnerability of Marine Habitats to the Invasive Green Alga Caulerpa racemosa var. cylindracea within a Marine Protected Area. Mar. Environ. Res. 2010, 70, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Iacarella, J.C.; Lyons, D.A.; Burke, L.; Davidson, I.C.; Therriault, T.W.; Dunham, A.; DiBacco, C. Climate Change and Vessel Traffic Create Networks of Invasion in Marine Protected Areas. J. Appl. Ecol. 2020, 57, 1793–1805. [Google Scholar] [CrossRef]
- Mannino, A.M.; Parasporo, M.; Crocetta, F.; Balistreri, P. An Updated Overview of the Marine Alien and Cryptogenic Species from the Egadi Islands Marine Protected Area (Italy). Mar. Biodivers. 2017, 47, 469–480. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Florido, M.; Ros, M.; González-Romero, P.; Guerra-García, J.M. Impoverished Mobile Epifaunal Assemblages Associated with the Invasive Macroalga Asparagopsis taxiformis in the Mediterranean Sea. Mar. Environ. Res. 2018, 141, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Guerra-García, J.M.; Ros, M.; Izquierdo, D.; Soler-Hurtado, M.M. The Invasive Asparagopsis armata versus the Native Corallina elongata: Differences in Associated Peracarid Assemblages. J. Exp. Mar. Bio. Ecol. 2012, 416–417, 121–128. [Google Scholar] [CrossRef]
- Janiak, D.S.; Whitlatch, R.B. Epifaunal and Algal Assemblages Associated with the Native Chondrus crispus (Stackhouse) and the Non-Native Grateloupia turuturu (Yamada) in Eastern Long Island Sound. J. Exp. Mar. Bio. Ecol. 2012, 413, 38–44. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Muñoz-Gómez, B.; Saiz, D.; Ros, M.; Guerra-García, J.M.; Altamirano, M.; Ostalé-Valriberas, E.; Moreira, J. Can Invasive Habitat-Forming Species Play the Same Role as Native Ones? The Case of the Exotic Marine Macroalga Rugulopteryx okamurae in the Strait of Gibraltar. Biol. Invasions 2019, 21, 3319–3334. [Google Scholar] [CrossRef]
- Roberts, D.A.; Poore, A.G.B.B. Habitat Configuration Affects Colonisation of Epifauna in a Marine Algal Bed. Biol. Conserv. 2006, 127, 18–26. [Google Scholar] [CrossRef]
- Lanham, B.S.; Gribben, P.E.; Poore, A.G.B. Beyond the Border: Effects of an Expanding Algal Habitat on the Fauna of Neighbouring Habitats. Mar. Environ. Res. 2015, 106, 10–18. [Google Scholar] [CrossRef]
- Taylor, R.B. Short-Term Dynamics of a Seaweed Epifaunal Assemblage. J. Exp. Mar. Bio. Ecol. 1998, 227, 67–82. [Google Scholar] [CrossRef]
- Martin, T.H.; Crowder, L.B.; Dumas, C.F.; Burkholder, J.M. Indirect Effects of Fish on Macrophytes in Bays Mountain Lake: Evidence for a Littoral Trophic Cascade. Oecologia 1992, 89, 476–481. [Google Scholar] [CrossRef]
- Heck, K.L.; Hays, G.; Orth, R.J.; Heck Hay, K.L.; Hays, G.; Orth, R.J. Critical Evaluation of the Nursery Role Hypothesis for Seagrass Meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Chemello, R.; Cìuna, I.; Pandolfo, A.; Riggio, S. Molluscan Assemblages Associated with Intertidal Vermetid Formations: A Morpho-Functional Approach. Boll. Malacol. 1997, 3, 105–114. [Google Scholar]
- Mancuso, F.P. Decreasing in Patch-Size of Cystoseira Forests Reduce the Diversity of Their Associated Molluscan Assemblages in Mediterranean Rocky Reefs—Data and Scripts. Mendeley Data 2021, V1. [Google Scholar] [CrossRef]
- Apostolaki, E.T.; Vizzini, S.; Santinelli, V.; Kaberi, H.; Andolina, C.; Papathanassiou, E. Exotic Halophila stipulacea is an Introduced Carbon Sink for the Eastern Mediterranean Sea. Sci. Rep. 2019, 9, 9643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sghaier, Y.R.; Zakhama-Sraieb, R.; Charfi-Cheikhrouha, F. Effects of the Invasive Seagrass Halophila stipulacea on the Native Seagrass Cymodocea nodosa. In Proceedings of the 5ème Symposium Méditerranéen sur la Végétation Marine, Portorož, Slovénie, 27–28 October 2014; pp. 27–28. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; van Katwijk, M.M.; Bouma, T.J.; van der Heide, T.; Govers, L.L.; Leuven, R.S.E.W. Non-Native Seagrass Halophila stipulacea Forms Dense Mats under Eutrophic Conditions in the Caribbean. J. Sea Res. 2016, 115, 1–5. [Google Scholar] [CrossRef]
- Cinelli, F. La Prateria di Posidonia oceanica ed Altre Storie. In Nel Mare Di Ustica. Vita e Ambienti Tra Coste e Fondali; Editore, V.L., Ed.; Villaggio Letterario: Palermo, Italy, 2022; pp. 337–347. ISBN 978-88-945489-5-2. [Google Scholar]
- Sghaier, Y.R.; Zakhama-Sraieb, R.; Benamer, I.; Charfi-Cheikhrouha, F. Occurrence of the Seagrass Halophila stipulacea (Hydrocharitaceae) in the Southern Mediterranean Sea. Bot. Mar. 2011, 54, 575–582. [Google Scholar] [CrossRef]
- Willette, D.A.; Ambrose, R.F. The Distribution and Expansion of the Invasive Seagrass Halophila stipulacea in Dominica, West Indies, with a Preliminary Report from St. Lucia. Aquat. Bot. 2009, 91, 137–142. [Google Scholar] [CrossRef]
- Riera, L.; Ramalhosa, P.; Canning-Clode, J.; Gestoso, I. Variability in the Settlement of Non-Indigenous Species in Benthic Communities from an Oceanic Island. Helgol. Mar. Res. 2018, 72, 15. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward Understanding the Ecological Impacts of Nonnative Species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Atkinson, S.K. Distinctiveness Magnifies the Impact of Biological Invaders in Aquatic Ecosystems. Ecol. Lett. 2004, 7, 781–784. [Google Scholar] [CrossRef]
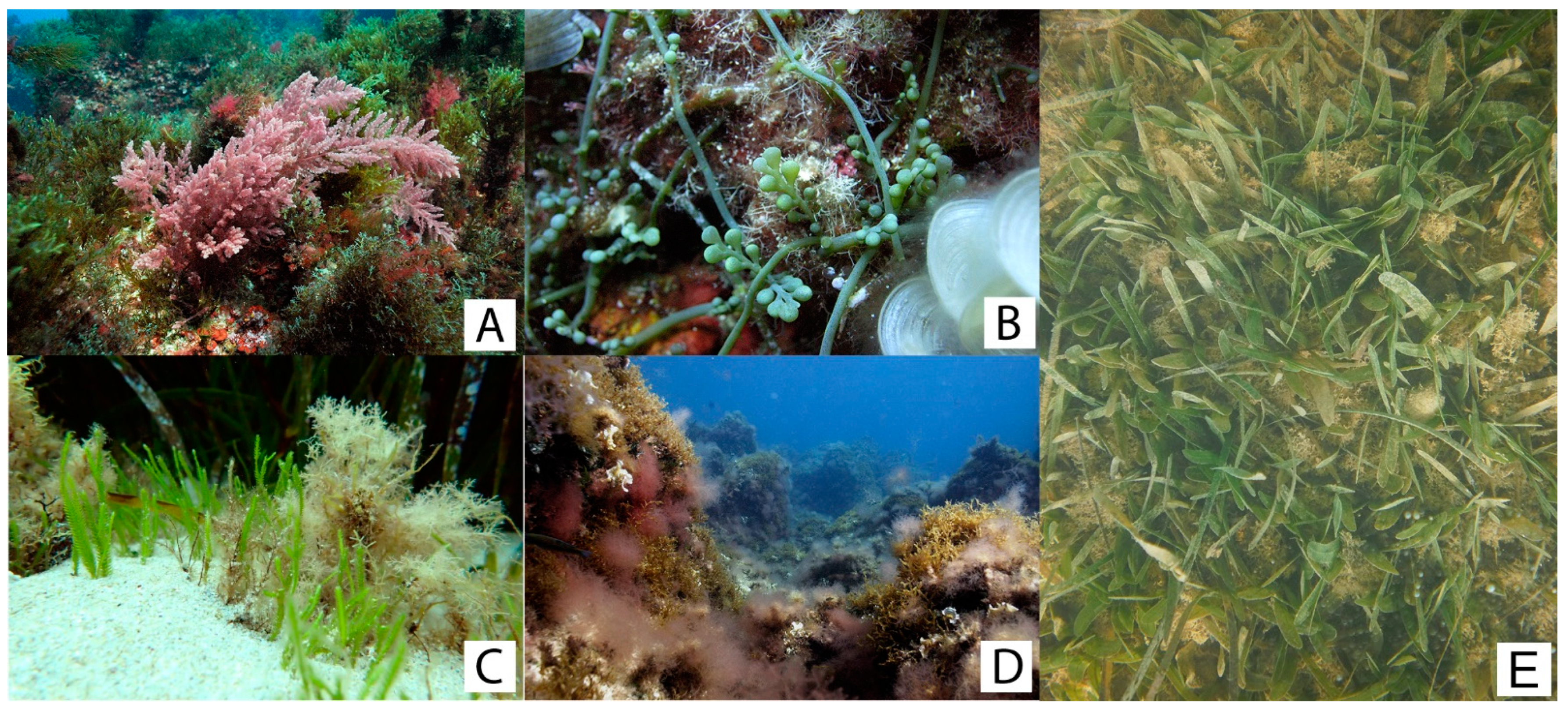
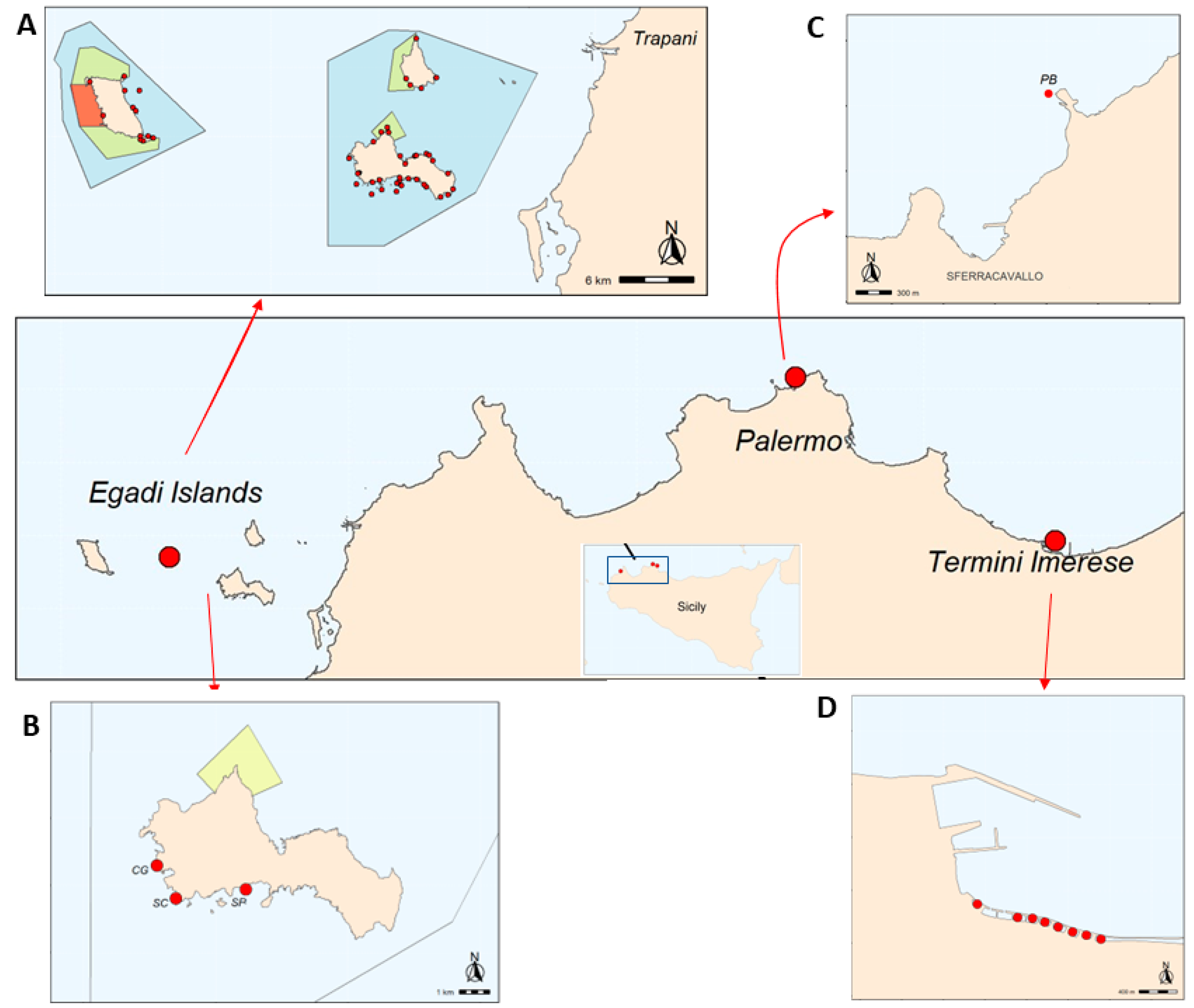
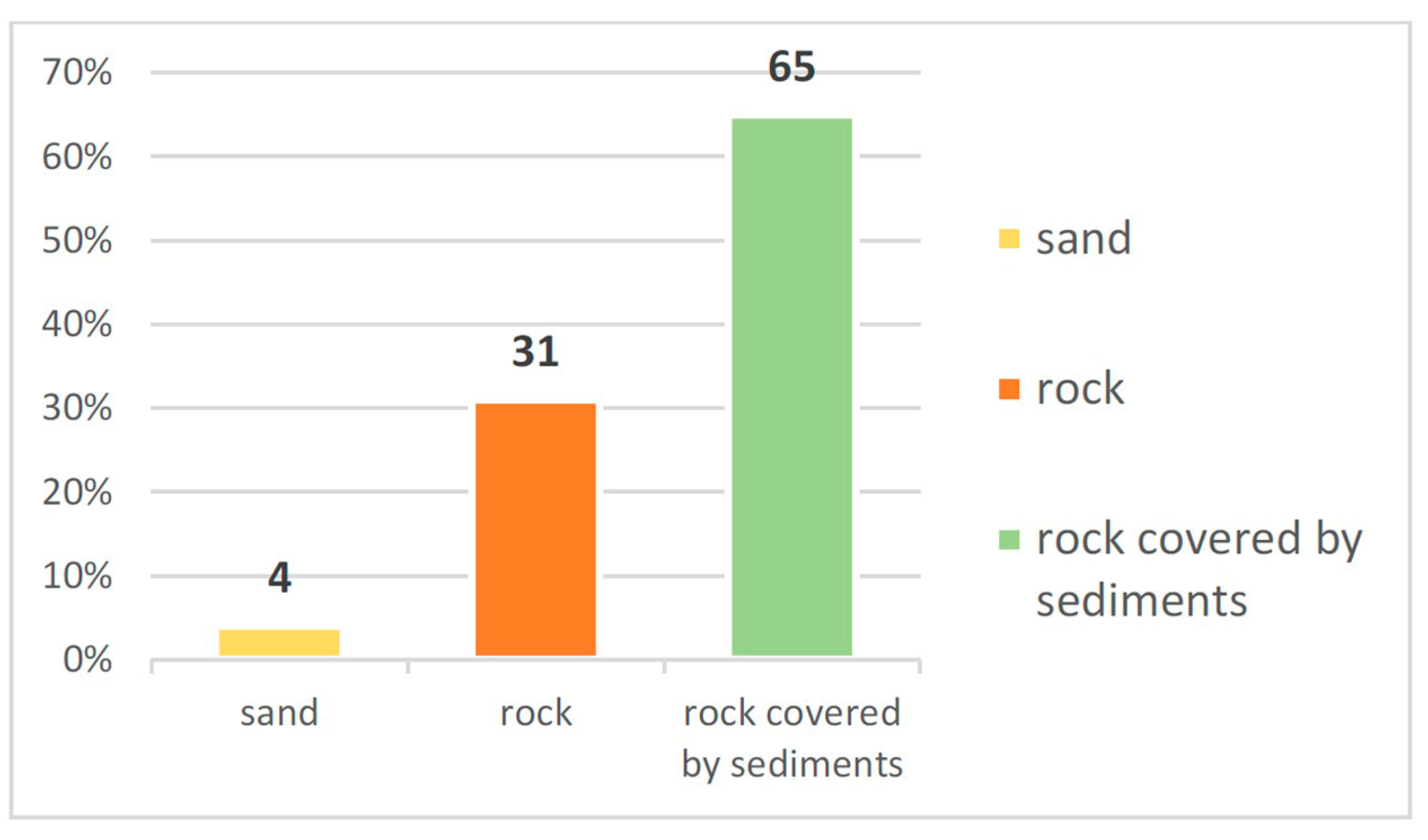

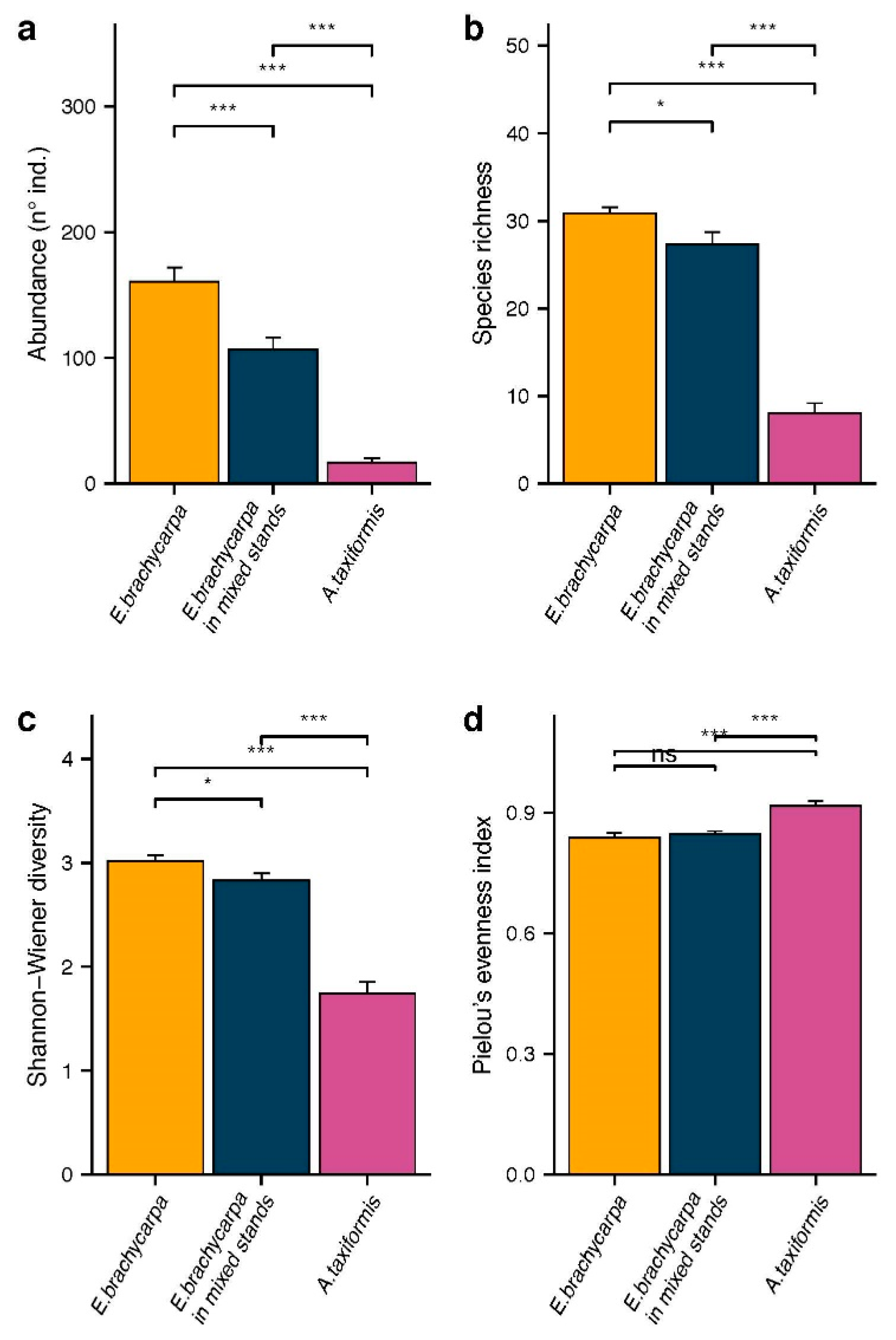
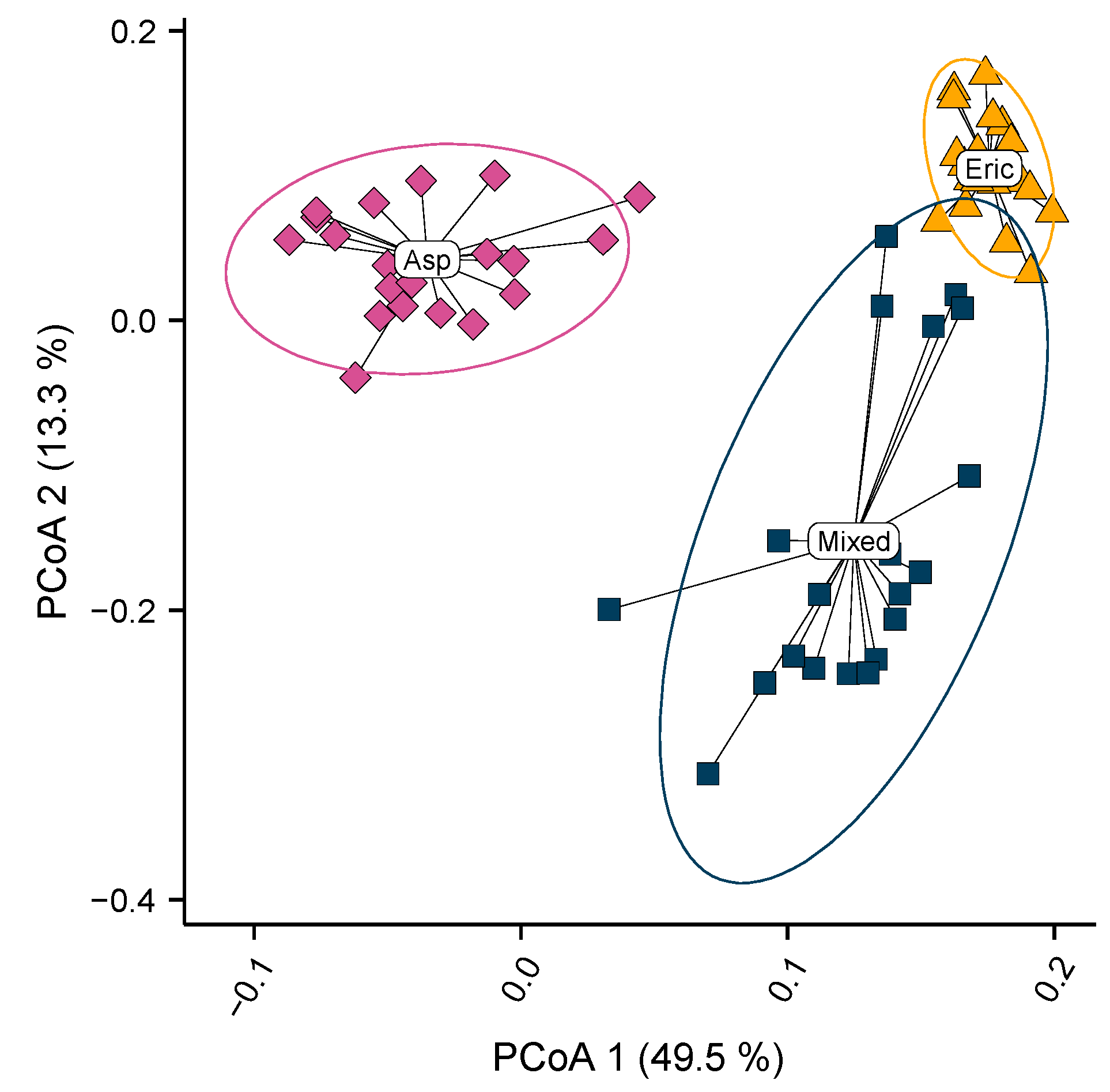
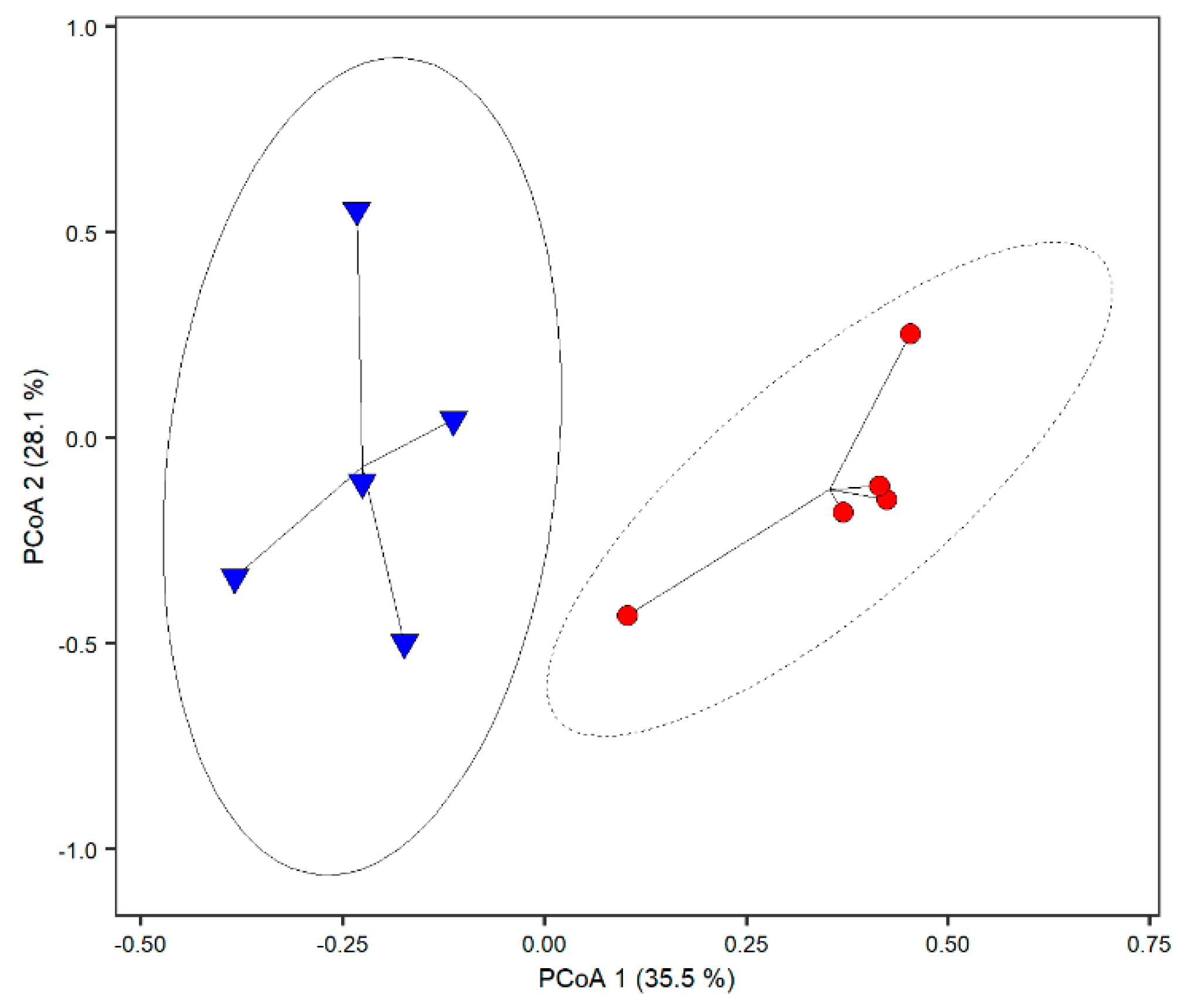
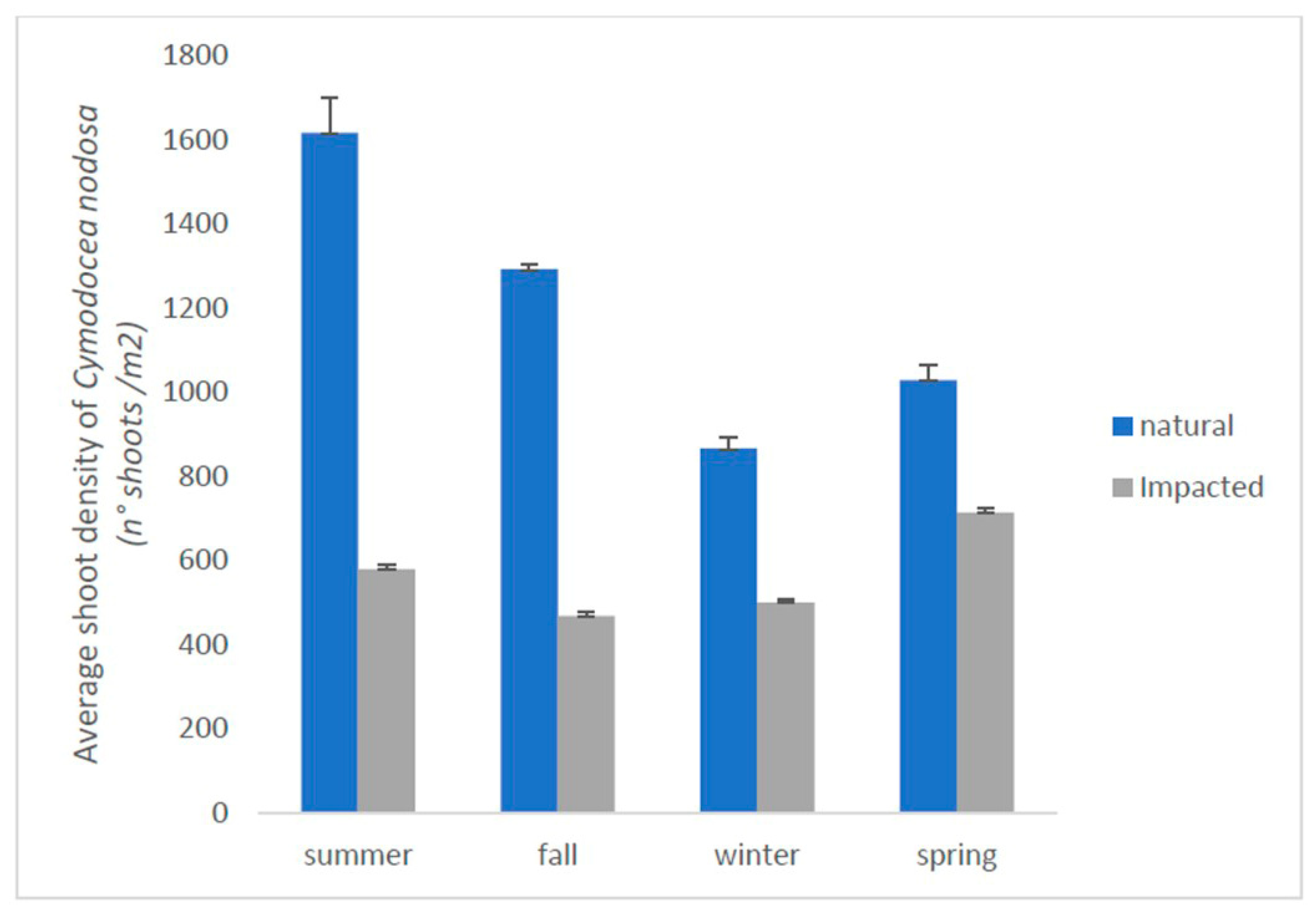
| Source of Variation | ||||
|---|---|---|---|---|
| df | MS | Model-F | ||
| Presence or absence L. lallemandii | 1 | 0.871 | 3.802 | ** |
| Residuals | 8 | 0.229 | ||
| Total | 9 | |||
| Average Abundance | ||||
|---|---|---|---|---|
| Species | With L. lallemandii | Without L. lallemandii | δi/SD(δi) | Cum_δi% |
| Barleeia unifasciata | 5.8 | 0.0 | 2.46 | 0.29 |
| Alvania hirta | 2.6 | 0.0 | 0.68 | 0.39 |
| Alvania lineata | 1.4 | 1.0 | 1.28 | 0.47 |
| Rissoa variabilis | 0.6 | 1.2 | 1.11 | 0.54 |
| Columbella rustica | 1.4 | 1.2 | 1.20 | 0.60 |
| Rissoa guerinii | 1.0 | 0.8 | 1.04 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, F.P.; Chemello, R.; Mannino, A.M. The Effects of Non-Indigenous Macrophytes on Native Biodiversity: Case Studies from Sicily. J. Mar. Sci. Eng. 2023, 11, 1389. https://doi.org/10.3390/jmse11071389
Mancuso FP, Chemello R, Mannino AM. The Effects of Non-Indigenous Macrophytes on Native Biodiversity: Case Studies from Sicily. Journal of Marine Science and Engineering. 2023; 11(7):1389. https://doi.org/10.3390/jmse11071389
Chicago/Turabian StyleMancuso, Francesco Paolo, Renato Chemello, and Anna Maria Mannino. 2023. "The Effects of Non-Indigenous Macrophytes on Native Biodiversity: Case Studies from Sicily" Journal of Marine Science and Engineering 11, no. 7: 1389. https://doi.org/10.3390/jmse11071389






