Microplastic–Pharmaceuticals Interaction in Water Systems
Abstract
:1. Introduction
2. Pharmaceuticals in Aquatic Systems
3. MPs in Aquatic Systems
4. Association of PHs–MPs
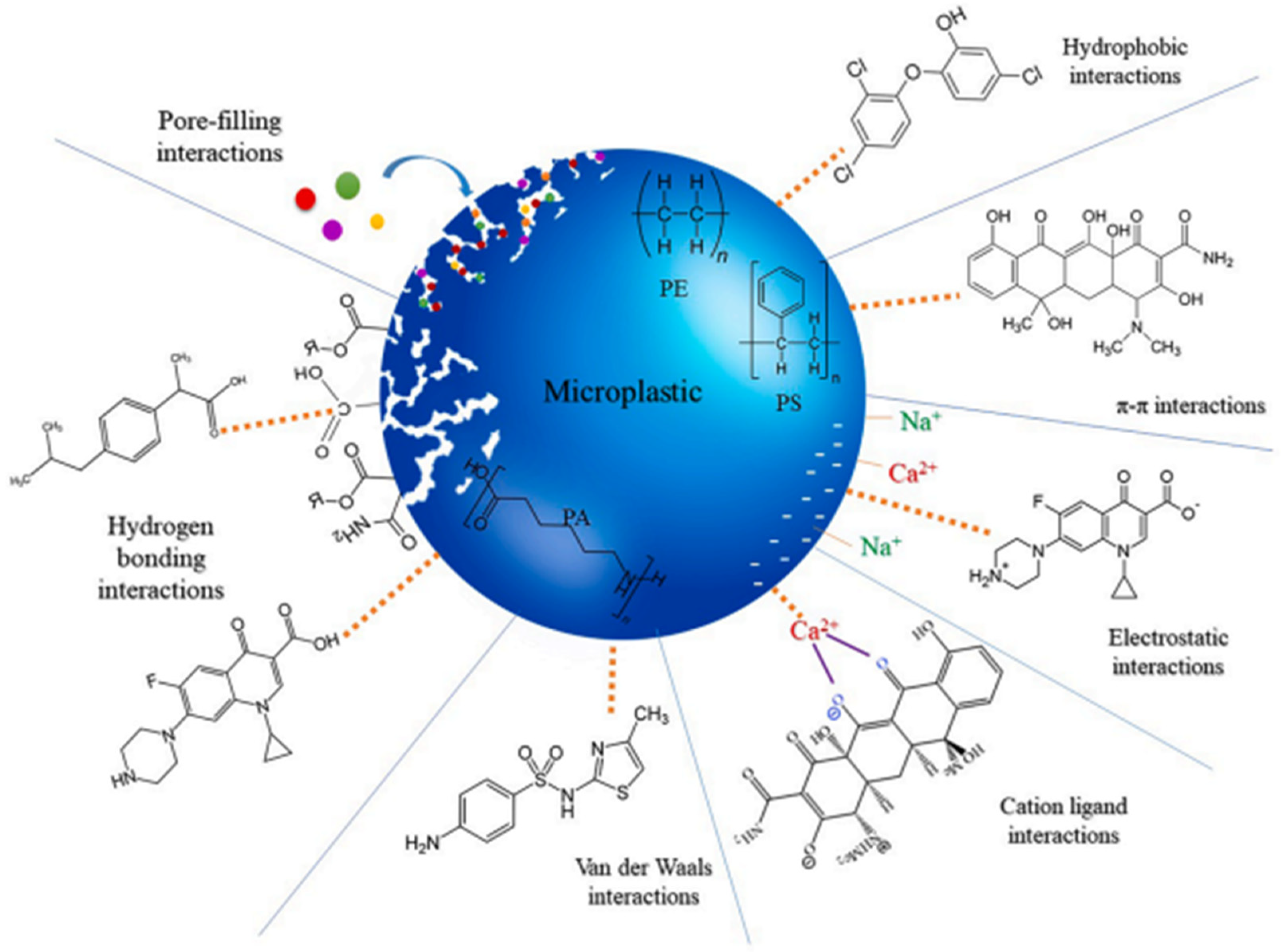
4.1. Mechanisms of Interaction
4.1.1. Hydrophobic Interactions
4.1.2. Electrostatic Interactions
4.1.3. π–π Interactions
5. Factors Driving the Interaction
5.1. Polymer Type
5.2. Particle Size
5.3. Structure and Cristallinity
5.4. Surface Charge
5.5. Physical–Chemical Properties of Drugs
6. Environmental Factors
6.1. Effect of Temperature
6.2. Effect of pH
6.3. Effect of Ionic Strength
6.4. Effect of Dissolved Organic Matter
6.5. Effect of Biofouling
6.6. Effect of MP Ageing
7. Sorption Models
8. Bioaccumulation
9. Toxicity
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD Organization for Economic Coooperation and Development. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Ritchie, H.; Roser, M. Plastic Pollution. Our World in Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 22 May 2023).
- Geyer, R. Chapter 2—Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment- A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Möhlenkamp, P.; Purser, A.; Thomsen, L. Plastic microbeads from cosmetic products: An experimental study of their hydrodynamic behaviour, vertical transport and resuspension in phytoplankton and sediment aggregates. Elementa 2018, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheavly, S.B.; Register, K.M. Marine debris & plastics: Environmental concerns, sources, impacts and solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar] [CrossRef]
- Coyle, R.; Hardiman, G.; Driscoll, K.O. Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud. Chem. Environ. Eng. 2020, 2, 100010. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Brown, R.J.C.; Kim, K.H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mat. 2021, 403, 123910. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mat. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [Green Version]
- Loraine, G.A.; Pettigrove, M.E. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef]
- Puckowski, A.; Cwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Cui, H.L.; Su, J.Q.; Penuelas, J.; Zhu, Y.G. Antibiotic resistomes in plant microbiomes. Trends Plant Sci. 2019, 24, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, M.; Wang, L.; Lou, Y.; Shi, L.; Jiang, S. Sorption of three synthetic musks by microplastics. Mar. Pollut. Bull. 2018, 126, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, A.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Internat. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Jasna, M.; Jelena, L.; Pero, T.; Dubravka, B.V.; Jasna, S.; Josko, P. Levels of trace metals on microplastic particles in beach sediments of the island of Vis, Adriatic Sea, Croatia. Mar. Pollut. Bull. 2018, 137, 231–236. [Google Scholar] [CrossRef]
- Wang, M.; Lee, J. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont Rotifer Brachionus koreanus via multi-xenobiotic resistance (MXR) disruption. Environ. Sci. Technol. 2018, 52, 11411–11418. [Google Scholar] [CrossRef]
- Leon, V.M.; Garcia, I.; Gonzalez, E.; Sampler, R.; Fernandez-Gonzalez, V.; Muniategui- Lorenzo, S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ. Pollut. 2018, 236, 442–453. [Google Scholar] [CrossRef]
- Turner, A. Black plastics: Linear and circular economies, hazardous additives and marine pollution. Environ. Int. 2018, 117, 308–318. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M. The dual role of microplastics in marine environment: Sink and vectors of pollutants. J. Marine Sci Eng. 2021, 9, 642. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M. Research progress in transfer, accumulation, and effects of microplastics in the oceans. J. Mar. Sci. Eng. 2021, 9, 433. [Google Scholar] [CrossRef]
- MacKeown, H.; Benedetti, B.; Di Carro, M.; Magi, E. The study of polar emerging contaminants in seawater by passive sampling: A review. Chemosphere 2022, 299, 134448. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, L.; Liu, S.; Guo, Z.; Hua, X. Antibiotics in water and sediments from Liao River in Jilin Province, China: Occurrence, distribution, and risk assessment. Environ. Earth Sci. 2016, 75, 1202. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S. Health effects and risks associated with the occurrence of pharmaceuticals and their metabolites in marine organisms and seafood. Sci. Total. Environ. 2021, 837, 155780. [Google Scholar] [CrossRef]
- Mohana, A.A.; Rahman, M.; Sarker, S.K.; Haque, N.; Gao, L.; Pramanik, B.K. Nano/microplastics: Fragmentation, interaction with co-existing pollutants and their removal from wastewater using membrane processes. Chemosphere 2022, 309, 136682. [Google Scholar] [CrossRef]
- da Silva, L.F.; Nobre, C.R.; Moreno, B.B.; Seabra Pereira, C.D.; de Souza Abessa, D.M.; Brasil Choueri, R.; Gusso-Choueri, P.K.; Cesar, A. Non-destructive biomarkers can reveal effects of the association of microplastics and pharmaceuticals or personal care products. Mar. Pollut. Bull. 2022, 177, 113469. [Google Scholar] [CrossRef]
- Verdú, I.; Amariei, G.; Rueda-Varela, C.; González-Pleiter, M.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Biofilm formation strongly influences the vector transport of triclosan-loaded polyethylene microplastics. Sci. Total Environ. 2023, 859, 160231. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- O’Connor, I.A.; Golsteijn, L.; Hendriks, A.J. Review of the partitioning of chemicals into different plastics: Consequences for the risk assessment of marine plastic debris. Mar. Pollut. Bull. 2016, 113, 17–24. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Velázquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2000, 12, 3313. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions. A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Godoy, V.; Martín-Lara, M.A.; Calero, M.; Blázquez, G. The relevance of interaction of chemicals/pollutants and microplastic samples as route for transporting contaminants. Process Saf. Environ. Prot. 2020, 138, 312–323. [Google Scholar] [CrossRef]
- Mofakhami, E.; Tencé-Girault, S.; Perrin, J.; Scheel, M.; Gervat, L.; Ovalle, C.; Laiarinandrasana, L.; Fayolle, B.; Miquelard-Garnier, G. Microstructure-mechanical properties relationships in vibration welded glass-fiber-reinforced polyamide 66: A high-resolution X-ray microtomography study. Polym. Test. 2020, 85, 106454. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Alex, S.A.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Interactive effects of micro/nanoplastics and nanomaterials/pharmaceuticals: Their ecotoxicological consequences in the aquatic systems. Aquat. Toxicol. 2021, 232, 105747. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ. Pollut. 2019, 252, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.; Wang, B.; Zhang, Y.; Yin, L.; Yu, G.; Deng, S.; Huang, J.; Wang, Y. Effects of microplastics on the uptake, distribution and biotransformation of chiral antidepressant venlafaxine in aquatic ecosystem. J. Hazard. Mater. 2018, 359, 104–112. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Chen, S.; Cai, Y.; Cui, M. Highly enhanced adsorption of antibiotics on aged polyamide microplastics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130690. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Akhdhar, A.; Elwakeel, K.Z. Microplastics prevalence, interactions, and remediation in the aquatic environment: A critical review. J. Environ. Chem. Eng. 2021, 9, 106224. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Wang, J. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Zhao, Y.; Shi, H.; Huang, C.H. Hydrophobic sorption behaviors of 17B-Estradiol on environmental microplastics. Chemosphere 2019, 226, 726–735. [Google Scholar] [CrossRef]
- Wang, W.; Qi, M.; Jia, X.; Jin, J.; Zhou, Q.; Zhang, M.; Zhou, W.; Li, A. Differential adsorption of zwitterionic PPCPs by multifunctional resins: The influence of the hydrophobicity and electrostatic potential of PPCPs. Chemosphere 2020, 24, 1125023. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Liu, G.Z.; Zhu, Z.L.; Wang, S.C.; Zhao, F.F. Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 2019, 214, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Wijesekara, H.; Werellagama, D.; Jinadasa, K.; Bolan, N.S.; Vithanage, M. Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water. Environ. Technol. Innov. 2020, 19, 100971. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The influence of nanoplastics on the toxic effects, bioaccumulation, biodegradation and enantioselectivity of ibuprofen in freshwater algae Chlorella pyrenoidosa. Environ. Pollut. 2020, 263, 114593. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Kotdawala, R.R.; Kazantzis, N.; Thompson, R.W. Analysis of binary adsorption of polar and nonpolar molecules in narrow slit-pores by mean-field perturbation theory. J. Chem. Phys. 2005, 123, 244709. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef]
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of perfluoroalkyl substances on polyamide microplastics: Effect of sorbent and influence of environmental factors. Environ. Res. 2023, 216, 114834. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of fourhydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Chen, G.; Bao, J.; Song, M.; Li, Y.; Luo, C. Interactions between microplastics and organic compounds in aquatic environments: A mini review. Sci. Total Environ. 2020, 736, 139472. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Q.; Yang, S.Z.; Guo, L.; Xu, X.; Yao, T.; Xie, F. Microscopic investigation on the adsorption of lubrication oil on microplastics. J. Mol. Liq. 2017, 227, 351–355. [Google Scholar] [CrossRef]
- Velez, J.F.M.; Shashoua, Y.; Syberg, K.; Khan, F.R. Considerations on the use of equilibrium models for the characterisation of HOC-microplastic interactions invector studies. Chemosphere 2018, 210, 359–365. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Man. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Cao, R.; Fan, Y.; Xu, P.; Chen, J. Release and transformation of BTBPE during the thermal treatment of flame-retardant ABS plastics. Environ. Sci. Technol. 2019, 53, 185–193. [Google Scholar] [CrossRef]
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Sorption of antibiotics onto aged microplastics in freshwater and seawater. Mar. Pollut. Bull. 2019, 149, 110511. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.V.; Carter, L.J.; Agatz, A.; Boxall, A.B.A. Novel approach for characterizing pH-dependent uptake of ionizable chemicals in aquatic organisms. Environ. Sci. Technol. 2017, 51, 6965–6971. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wu, J.; Wu, J.; Zhang, C.; Luo, Y. Adsorption and desorption of steroid hormones by microplastics in seawater. Bull. Environ. Contam. Toxicol. 2020, 107, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. On points of zero charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behaviour of per fluorooctanesulfonate (PFOS) and per fluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef] [PubMed]
- Marion, G.M.; Millero, F.J.; Camoes, M.F.; Spitzer, P.; Feistel, R.; Chen, C.T.A. PH of seawater. Mar. Chem. 2011, 126, 89–96. [Google Scholar] [CrossRef]
- McDougall, L.; Thomson, L.; Brand, S.; Wagstaff, A.; Lawton, L.A.; Petrie, P. Adsorption of a diverse range of pharmaceuticals to polyethylene microplastics in wastewater and their desorption in environmental matrices. Sci. Total Environ. 2022, 808, 152071. [Google Scholar] [CrossRef]
- Wan, T.; Lu, S.; Cheng, W.; Ren, J.; Wang, M.; Hu, B.; Jia, Z.; Li, Y.; Sun, Y. A spectroscopic and theoretical investigation of interaction mechanisms of tetracycline and polystyrene nanospheres under different conditions. Environ. Pollut. 2019, 249, 398–405. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef]
- MacKay, A.A.; Canterbury, B. Oxytetracycline sorption to organic matter by metal-bridging. J. Environ. Qual. 2005, 34, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, Z.Y.; Qian, C.; Yu, H.Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environ. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, L.; Zhang, W.; Huang, R.; Lv, X.; Jing, X.; Yang, Z.; Wang, J.; Qiu, Y. Role of surface functionalities of nanoplastics on their transport in seawater saturated sea sand. Environ. Pollut. 2019, 255, 113177. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, S.; Zarfl, C.; Cirpka, O.A.; Fellenberg, G.; Grathwohl, P. Shift in mass transfer of wastewater contaminants from microplastics in the presence of dissolved substances. Environ. Sci. Technol. 2017, 51, 12254–12263. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugherty, M. Adsorption of Organic Pollutants to Microplastics. The Effects of Dissolved Organic Matter. Ph.D. Thesis, Northwest University, Evanston, IL, USA, 2016; pp. 1–27. [Google Scholar]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Aristilde, L.; Sposito, G. Binding of ciprofloxacin by humic substances: A molecular dynamics study. Environ. Toxicol. Chem. 2010, 29, 90–98. [Google Scholar] [CrossRef]
- Glaser, A.J. The Importance of Biofilms to the Fate and Effects of Microplastics. In Bacterial Biofilms; Dincer, S., Özdenefe, M.S., Arkut, A., Eds.; IntechOpen: London, UK, 2020; p. 360. [Google Scholar] [CrossRef]
- Endo, S.; Koelmans, A.A. Sorption of Hydrophobic Organic Compounds to Plastics in the Marine Environment: Equilibrium. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 185–204. [Google Scholar] [CrossRef]
- Writer, J.H.; Ryan, J.N.; Barber, L.B. Role of biofilms in sorptive removal of steroidal hormones and 4-nonylphenol compounds from streams. Environ. Sci. Technol. 2011, 45, 7275–7283. [Google Scholar] [CrossRef]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, J.; Razanajatovo, R.M.; Jiang, H.; Zou, H.; Zhu, W. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 648, 1431–1439. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Lavorante, B.R.; Maria da Conceição, B.S.M.; Guilhermino, L. Influence of microplastics on the toxicity of the pharmaceutical’s procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquat. Toxicol. 2018, 197, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.; Wang, B.; Yang, J.; Duan, L.; Yu, G. Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. J. Hazard. Mater. 2019, 370, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; D’Almeida, M.; Magueresse, A.; Le Grand, A.; Duval, H.; César, G.; Sire, O.; Bruzaud, S.; Tilly, V.L. Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants. Mar. Pollut. Bull. 2018, 127, 684–694. [Google Scholar] [CrossRef]
- Mao, H.; Yang, H.; Xu, Z.; Yang, Y.; Zhang, X.; Huang, F.; Wei, L.; Li, Z. Microplastics and co-pollutant with ciprofloxacin affect interactions between free-floating macrophytes. Environ. Pollut. 2023, 316, 120546. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Razanajatovo, M.R.; Ding, J.; Zhang, S.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Svobodova, Z.; Dolezelova, P.; Vosmerova, P.; Faggio, C. Effects of waterborne antidepressants on non-target animals living in the aquatic environment: A review. Sci. Total Environ. 2018, 631–632, 789–794. [Google Scholar] [CrossRef]
- Guilhermino, L.; Vieira, L.R.; Ribeiro, D.; Tavares, A.S.; Cardoso, V.; Alves, A.; Almeida, J.M. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci. Total Environ. 2018, 622, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Cormier, Z. A Toxic Trojan Horse: Tiny Plastic Particles Pack a Major punch. Special to the Globe Mail. 2007. Available online: https://www.theglobeandmail.com/technology/science/a-toxic-trojan-horse-tiny-plastic-particles-pack-a-major-punch/article698293/ (accessed on 20 April 2023).
- Zhou, W.; Han, Y.; Tang, Y.; Shi, W.; Du, X.; Sun, S.; Liu, G. Microplastics aggravate the bioaccumulation of two waterborne veterinary antibiotics in an edible bivalve species: Potential mechanisms and implications for human health. Environ. Sci. Technol. 2020, 54, 8115–8122. [Google Scholar] [CrossRef]
- Yan, X.; Yang, X.; Tang, Z.; Fu, J.; Chen, F.; Zhao, Y.; Ruan, L.; Yang, Y. Downward transport of naturally aged light microplastics in natural loamy sand and the implication to the dissemination of antibiotic resistance genes. Environ. Pollut. 2020, 262, 114270. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.S.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Wagstaff, A.; Petrie, B. Enhanced desorption of fluoxetine from polyethylene terephthalate microplastics in gastric fluids and sea water. Environ. Chem. Lett. 2022, 20, 975–982. [Google Scholar] [CrossRef]
- Schmieg, H.; Burmester, J.K.Y.; Krais, S.; Ruhl, A.S.; Tisler, S.; Zwiener, C.; Kohler, H.R.; Triebskorn, R. Interacting effects of polystyrene microplastics and the antidepressant amitriptyline on early life stages of Brown trout (Salmo trutta f. fario). Water 2020, 12, 2361. [Google Scholar] [CrossRef]
- Jaafar, N.; Azfaralariff, A.; Musa, S.M.; Mohamed, M.; Yusoff, A.H.; Lazim, A.M. Occurrence, distribution and characteristics of microplastics in gastrointestinal tract and gills of commercial marine fish from Malaysia. Sci. Total Environ. 2021, 799, 149457. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chevre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers, and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef]
- Almeida, Â.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Presence of the pharmaceutical drug carbamazepine in coastal systems: Effects on bivalves. Aquat. Toxicol. 2014, 156, 74–87. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotic’s ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the platisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct community structure and microbial functions of biogfilms colonizing micrplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.E.; Luo, Y.; Yang, Y.; Shi, H.; Hollert, H. Marine microplastics bound dioxin-like chemicals: Model explanation and risk assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sheng, G.D.; O’Connor, P. Microplastics combined with tetracycline in soils facilitate the formation of antibiotic resistance in the Enchytraeus crypticus microbiome. Environ. Pollut. 2020, 264, 114689. [Google Scholar] [CrossRef]
| Pharmaceutical | Interaction Mechanism | Reference | |
|---|---|---|---|
| Polyamide | Sulfadiazine, amoxicillin, tetracycline, ciprofloxacin, trimethoprim | Hydrogen bonding, hydrophobic interaction, van der Waals force, and electrostatic interaction | [35] |
| Polyethylene | Tetracycline | Electrostatic interactions, hydrophobic interactions, π–π interactions, and polar interactions | [49] |
| Polyethylene | Ciprofloxacin | Hydrophobic and electrostatic interactions | [59] |
| Polyethylene | Sulfadiazine, amoxicillin, tetracycline, ciprofloxacin, and trimethoprim | Hydrogen bonding, hydrophobic interaction, van der Waals force, and electrostatic interactions | [35] |
| Polypropylene | Tetracycline | Electrostatic interactions, hydrophobic interactions, π–π interactions, and polar interactions | [49] |
| Polypropylene | Sulfadiazine amoxicillin, tetracycline, ciprofloxacin, and trimethoprim | Hydrogen bonding, hydrophobic interaction, van der Waals force, and electrostatic interactions | [35] |
| Polystyrene | Oxytetracycline | Electrostatic interaction, multivalent cationic bridging mechanisms, and H-bonding interaction | [60] |
| Polystyrene | Tylosin | Electrostatic interaction, surface complexation, and hydrophobic interactions | [54] |
| Polyvinyl chloride | Ciprofloxacin | Intermolecular hydrogen bonding, partitioning, and electrostatic interactions | [58] |
| Drug | MPs | Organism | Effect | Reference |
|---|---|---|---|---|
| Roxithromycin | PS (10–100 µg/L) | Red tilapia | Reduced inhibitory effect | [98] |
| Cephalexin | No-specified (0.184 mg/L) | Polatoschistus microps | Increased inhibitory effect | [99] |
| Procainamide | No-specified (0.75–48 mg/L) | algae | Increased inhibitory effect | [100] |
| Venlafaxine | PVC (50 mg/L) | loach | Increased inhibitory effect | [101] |
| Propranolol | PS (10 µg/L) | Red tilapia | Reduced inhibitory effect | [97] |
| Sulfamethoxazole | PS (50 µg/L) | Red tilapia | Increased inhibitory effect | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arienzo, M.; Donadio, C. Microplastic–Pharmaceuticals Interaction in Water Systems. J. Mar. Sci. Eng. 2023, 11, 1437. https://doi.org/10.3390/jmse11071437
Arienzo M, Donadio C. Microplastic–Pharmaceuticals Interaction in Water Systems. Journal of Marine Science and Engineering. 2023; 11(7):1437. https://doi.org/10.3390/jmse11071437
Chicago/Turabian StyleArienzo, Michele, and Carlo Donadio. 2023. "Microplastic–Pharmaceuticals Interaction in Water Systems" Journal of Marine Science and Engineering 11, no. 7: 1437. https://doi.org/10.3390/jmse11071437
APA StyleArienzo, M., & Donadio, C. (2023). Microplastic–Pharmaceuticals Interaction in Water Systems. Journal of Marine Science and Engineering, 11(7), 1437. https://doi.org/10.3390/jmse11071437







