A Review on the Antimicrobial Activity of Chitosan Microspheres: Milestones Achieved and Miles to Go
Abstract
:1. Introduction
2. Snapshot of Chitosan Microsphere Synthesis
3. Biomedical Applications of Chitosan MS
4. Antimicrobial Activity of Chitosan Microspheres
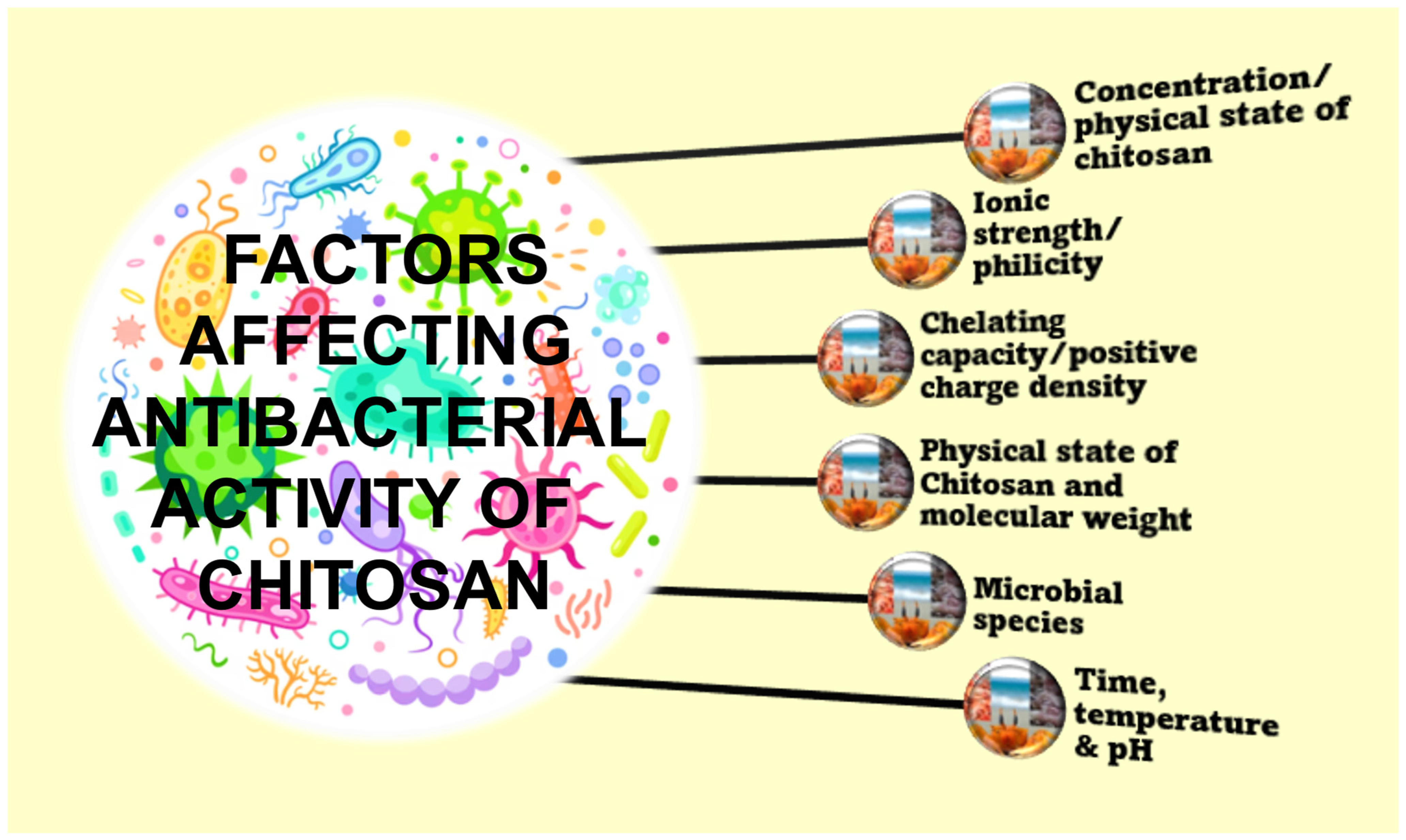
4.1. Antibacterial
4.2. Antifungal
4.3. Antiviral
5. Future Recommendations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A.K. Nifedipine Loaded Chitosan Microspheres Prepared by Emulsification Phase-Separation. Biotech. Histochem. 2003, 78, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, D.; Wauters, F.; Bouchend’Homme, S. Preparation and Characterization of Alginate Microspheres Containing a Model Antigen. Int. J. Pharm. 1998, 176, 9–19. [Google Scholar] [CrossRef]
- Hong, S.; Hsu, H.-J.; Kaunas, R.; Kameoka, J. Collagen Microsphere Production on a Chip. Lab Chip 2012, 12, 3277–3280. [Google Scholar] [CrossRef]
- Han, Y.; Radziuk, D.; Shchukin, D.; Moehwald, H. Stability and Size Dependence of Protein Microspheres Prepared by Ultrasonication. J. Mater. Chem. 2008, 18, 5162–5166. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Tadier, S.; van Garderen, N.; de Gasparo, A.; Döbelin, N.; Baroud, G. Synthesis of Spherical Calcium Phosphate Particles for Dental and Orthopedic Applications. Biomatter 2013, 3, e25103. [Google Scholar] [CrossRef] [Green Version]
- Freiberg, S.; Zhu, X.X. Polymer Microspheres for Controlled Drug Release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Hong, X.; Liu, Z.; Yuan, W. Porous Microsphere and Its Applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar]
- Li, S.; Nguyen, L.; Xiong, H.; Wang, M.; Hu, T.C.-C.; She, J.-X.; Serkiz, S.M.; Wicks, G.G.; Dynan, W.S. Porous-Wall Hollow Glass Microspheres as Novel Potential Nanocarriers for Biomedical Applications. Nanomedicine 2010, 6, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Wang, X.; Ye, Z.; Zhang, Y.; Zhou, Y.; Tan, W.-S. A Modular Approach to the Engineering of a Centimeter-Sized Bone Tissue Construct with Human Amniotic Mesenchymal Stem Cells-Laden Microcarriers. Biomaterials 2011, 32, 7532–7542. [Google Scholar] [CrossRef]
- Perez, R.A.; El-Fiqi, A.; Park, J.-H.; Kim, T.-H.; Kim, J.-H.; Kim, H.-W. Therapeutic Bioactive Microcarriers: Co-Delivery of Growth Factors and Stem Cells for Bone Tissue Engineering. Acta Biomater. 2014, 10, 520–530. [Google Scholar] [CrossRef]
- Komlev, V.S.; Barinov, S.M.; Koplik, E.V. A Method to Fabricate Porous Spherical Hydroxyapatite Granules Intended for Time-Controlled Drug Release. Biomaterials 2002, 23, 3449–3454. [Google Scholar] [CrossRef]
- Liu, D.M. Fabrication and Characterization of Porous Hydroxyapatite Granules. Biomaterials 1996, 17, 1955–1957. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Park, J.-H.; Mordan, N.J.; Salih, V.; Wall, I.B.; Kim, H.-W.; King, S.P.; Hanna, J.V.; Martin, R.A.; Addison, O.; et al. Titanium Phosphate Glass Microspheres for Bone Tissue Engineering. Acta Biomater. 2012, 8, 4181–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sene, F.F.; Martinelli, J.R.; Okuno, E. Synthesis and Characterization of Phosphate Glass Microspheres for Radiotherapy Applications. J. Non-Cryst. Solids 2008, 354, 4887–4893. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Santana, M.H.A.; Ré, M.I. Spray-Dried Chitosan Microspheres Cross-Linked with d, l-Glyceraldehyde as a Potential Drug Delivery System: Preparation and Characterization. Braz. J. Chem. Eng. 2005, 22, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A.; Vieira, R.S.; Beppu, M.M.; Arruda, E.J.; Santana, C.C. Production of Chemically Modified Chitosan Microspheres by a Spraying and Coagulation Method. Mater. Res. 2007, 10, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Akamatsu, K.; Kaneko, D.; Sugawara, T. Three Preparation Methods for Monodispersed Chitosan Microspheres Using the Shirasu Porous Glass Membrane Emulsification Technique and Mechanisms of Microsphere Formation. Ind. Eng. Chem. Res. 2010, 49, 3236–3241. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Silva, C.; Ferreira, D.; Veiga, F. Chitosan-Reinforced Alginate Microspheres Obtained through the Emulsification/Internal Gelation Technique. Eur. J. Pharm. Sci. 2005, 25, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.T.; Martin, G.P.; Berry, D.J.; Brown, M.B. Preparation and Evaluation of the in Vitro Drug Release Properties and Mucoadhesion of Novel Microspheres of Hyaluronic Acid and Chitosan. J. Control. Release 2000, 66, 281–292. [Google Scholar] [CrossRef]
- Aggarwal, A.; Kaur, S.; Tiwary, A.K.; Gupta, S. Chitosan Microspheres Prepared by an Aqueous Process: Release of Indomethacin. J. Microencapsul. 2001, 18, 819–823. [Google Scholar] [CrossRef]
- Pavanetto, F.; Perugini, P.; Conti, B.; Modena, T.; Genta, I. Evaluation of Process Parameters Involved in Chitosan Microsphere Preparation by the o/w/o Multiple Emulsion Method. J. Microencapsul. 1996, 13, 679–688. [Google Scholar] [CrossRef]
- Orienti, I.; Aiedeh, K.; Gianasi, E.; Bertasi, V.; Zecchi, V. Indomethacin Loaded Chitosan Microspheres. Correlation between the Erosion Process and Release Kinetics. J. Microencapsul. 1996, 13, 463–472. [Google Scholar] [CrossRef]
- Ohya, Y.; Takei, T.; Kobayashi, H.; Ouchi, T. Release Behaviour of 5-Fluorouracil from Chitosan-Gel Microspheres Immobilizing 5-Fluorouracil Derivative Coated with Polysaccharides and Their Cell Specific Recognition. J. Microencapsul. 1993, 10, 1–9. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wong, T.-B.; Jang, S.-F.; Lee, S.-T.; Lu, K.-T. Chitosan-Polyelectrolyte Complexation for the Preparation of Gel Beads and Controlled Release of Anticancer Drug. II. Effect of PH-Dependent Ionic Crosslinking or Interpolymer Complex Using Tripolyphosphate or Polyphosphate as Reagent. J. Appl. Polym. Sci. 1999, 74, 1093–1107. [Google Scholar] [CrossRef]
- Varshosaz, J. The Promise of Chitosan Microspheres in Drug Delivery Systems. Expert Opin. Drug Deliv. 2007, 4, 263–273. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Novel Interpenetrating Network Chitosan-Poly(Ethylene Oxide-g-Acrylamide) Hydrogel Microspheres for the Controlled Release of Capecitabine. Int. J. Pharm. 2006, 324, 103–115. [Google Scholar] [CrossRef]
- Liu, L.-S.; Liu, S.-Q.; Ng, S.Y.; Froix, M.; Ohno, T.; Heller, J. Controlled Release of Interleukin-2 for Tumour Immunotherapy Using Alginate/Chitosan Porous Microspheres. J. Control. Release 1997, 43, 65–74. [Google Scholar] [CrossRef]
- Grant, J.; Blicker, M.; Piquette-Miller, M.; Allen, C. Hybrid Films from Blends of Chitosan and Egg Phosphatidylcholine for Localized Delivery of Paclitaxel. J. Pharm. Sci. 2005, 94, 1512–1527. [Google Scholar] [CrossRef] [PubMed]
- Nsereko, S.; Amiji, M. Localized Delivery of Paclitaxel in Solid Tumors from Biodegradable Chitin Microparticle Formulations. Biomaterials 2002, 23, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.V.; Udupa, N. Methotrexate Loaded Chitosan and Chitin Microspheres—In Vitro Characterization and Pharmacokinetics in Mice Bearing Ehrlich Ascites Carcinoma. J. Microencapsul. 1998, 15, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, S.E.; Kwon, I.C.; Ahn, H.J.; Cho, H.; Lee, S.-H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of a Chitosan Scaffold Containing TGF-Beta1 Encapsulated Chitosan Microspheres on in Vitro Chondrocyte Culture. Artif. Organs 2004, 28, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.-H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the Controlled-Released TGF-Beta 1 from Chitosan Microspheres on Chondrocytes Cultured in a Collagen/Chitosan/Glycosaminoglycan Scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef]
- Jiang, T.; Abdel-Fattah, W.I.; Laurencin, C.T. In Vitro Evaluation of Chitosan/Poly(Lactic Acid-Glycolic Acid) Sintered Microsphere Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 4894–4903. [Google Scholar] [CrossRef]
- Han, G.; Chen, H.-L.; Sun, X.-Y.; Gao, J.-Q.; Liang, W.-Q. Preparation and release behavior of chitosan scaffolds encapsulating proteins loaded in PLGA microspheres. Yao Xue Xue Bao Acta Pharm. Sin. 2006, 41, 493–497. [Google Scholar]
- Cevher, E.; Orhan, Z.; Mülazimoğlu, L.; Sensoy, D.; Alper, M.; Yildiz, A.; Ozsoy, Y. Characterization of Biodegradable Chitosan Microspheres Containing Vancomycin and Treatment of Experimental Osteomyelitis Caused by Methicillin-Resistant Staphylococcus Aureus with Prepared Microspheres. Int. J. Pharm. 2006, 317, 127–135. [Google Scholar] [CrossRef]
- Blanco, M.D.; Gómez, C.; Olmo, R.; Muñiz, E.; Teijón, J.M. Chitosan Microspheres in PLG Films as Devices for Cytarabine Release. Int. J. Pharm. 2000, 202, 29–39. [Google Scholar] [CrossRef]
- Machida, Y.; Nagai, T.; Abe, M.; Sannan, T. Use of Chitosan and Hydroxypropylchitosan in Drug Formulations to Effect Sustained Release. Drug Des. Deliv. 1986, 1, 119–130. [Google Scholar]
- Elçin, Y.M.; Dixit, V.; Gitnick, G. Controlled Release of Endothelial Cell Growth Factor from Chitosan-Albumin Microspheres for Localized Angiogenesis: In Vitro and in Vivo Studies. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996, 24, 257–271. [Google Scholar] [CrossRef]
- Çelik, Ö.; Akbuğa, J. Preparation of Superoxide Dismutase Loaded Chitosan Microspheres: Characterization and Release Studies. Eur. J. Pharm. Biopharm. 2007, 66, 42–47. [Google Scholar] [CrossRef]
- Schally, A.V. LH-RH Analogues: I. Their Impact on Reproductive Medicine. Gynecol. Endocrinol. 1999, 13, 401–409. [Google Scholar] [CrossRef]
- Xue, Z.-X.; Yang, G.-P.; Zhang, Z.-P.; He, B.-L. Application of Chitosan Microspheres as Carriers of LH-RH Analogue TX46. React. Funct. Polym. 2006, 66, 893–901. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Gu, Y.-H.; Zhou, Q.-Z.; Ma, G.-H.; Wan, Y.-H.; Su, Z.-G. Preparation and Characterization of Uniform-Sized Chitosan Microspheres Containing Insulin by Membrane Emulsification and a Two-Step Solidification Process. Colloids Surf. B Biointerfaces 2006, 50, 126–135. [Google Scholar] [CrossRef]
- Kofuji, K.; Murata, Y.; Kawashima, S. Sustained Insulin Release with Biodegradation of Chitosan Gel Beads Prepared by Copper Ions. Int. J. Pharm. 2005, 303, 95–103. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, J.Y.; Lee, J.H.; Chung, H.Y.; Park, J.W.; Roh, K.H.; Kim, G.U.; Kwon, I.C.; Jang, K.H.; Lee, D.-S.; et al. The Bone Regenerative Effect of Chitosan Microsphere-Encapsulated Growth Hormone on Bony Consolidation in Mandibular Distraction Osteogenesis in a Dog Model. J. Craniofac. Surg. 2004, 15, 299–311; discussion 312–313. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a Novel Nasal Delivery System for Vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Park, I.-K.; Shin, N.-R.; Kang, S.-G.; Yoo, H.-S.; Kim, S.-I.; Suh, S.-B.; Akaike, T.; Cho, C.-S. In Vitro Study of the Immune Stimulating Activity of an Athrophic Rhinitis Vaccine Associated to Chitosan Microspheres. Eur. J. Pharm. Biopharm. 2004, 58, 471–476. [Google Scholar] [CrossRef]
- Jaganathan, K.S.; Rao, Y.U.B.; Singh, P.; Prabakaran, D.; Gupta, S.; Jain, A.; Vyas, S.P. Development of a Single Dose Tetanus Toxoid Formulation Based on Polymeric Microspheres: A Comparative Study of Poly(d,l-Lactic-Co-Glycolic Acid) versus Chitosan Microspheres. Int. J. Pharm. 2005, 294, 23–32. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Chen, C.T.; Schoung, J.Y. Porous Chitosan Microsphere for Controlling the Antigen Release of Newcastle Disease Vaccine: Preparation of Antigen-Adsorbed Microsphere and in Vitro Release. Biomaterials 1999, 20, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- van der Lubben, I.M.; van Opdorp, F.A.C.; Hengeveld, M.R.; Onderwater, J.J.M.; Koerten, H.K.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Transport of Chitosan Microparticles for Mucosal Vaccine Delivery in a Human Intestinal M-Cell Model. J. Drug Target. 2002, 10, 449–456. [Google Scholar] [CrossRef]
- Van Der Lubben, I.M.; Konings, F.A.; Borchard, G.; Verhoef, J.C.; Junginger, H.E. In Vivo Uptake of Chitosan Microparticles by Murine Peyer’s Patches: Visualization Studies Using Confocal Laser Scanning Microscopy and Immunohistochemistry. J. Drug Target. 2001, 9, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cha, D.S.; Park, H.J. Survival of Freeze-Dried Lactobacillus Bulgaricus KFRI 673 in Chitosan-Coated Calcium Alginate Microparticles. J. Agric. Food Chem. 2004, 52, 7300–7305. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and Characterisation of Chitosan Microspheres for Antioxidant Delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Harikarnpakdee, S.; Lipipun, V.; Sutanthavibul, N.; Ritthidej, G.C. Spray-Dried Mucoadhesive Microspheres: Preparation and Transport through Nasal Cell Monolayer. AAPS PharmSciTech 2006, 7, E12. [Google Scholar] [CrossRef]
- Cerchiara, T.; Luppi, B.; Chidichimo, G.; Bigucci, F.; Zecchi, V. Chitosan and Poly(Methyl Vinyl Ether-Co-Maleic Anhydride) Microparticles as Nasal Sustained Delivery Systems. Eur. J. Pharm. Biopharm. 2005, 61, 195–200. [Google Scholar] [CrossRef]
- Martinac, A.; Filipović-Grcić, J.; Perissutti, B.; Voinovich, D.; Pavelić, Z. Spray-Dried Chitosan/Ethylcellulose Microspheres for Nasal Drug Delivery: Swelling Study and Evaluation of in Vitro Drug Release Properties. J. Microencapsul. 2005, 22, 549–561. [Google Scholar] [CrossRef]
- Krauland, A.H.; Guggi, D.; Bernkop-Schnürch, A. Thiolated Chitosan Microparticles: A Vehicle for Nasal Peptide Drug Delivery. Int. J. Pharm. 2006, 307, 270–277. [Google Scholar] [CrossRef]
- Gavini, E.; Hegge, A.B.; Rassu, G.; Sanna, V.; Testa, C.; Pirisino, G.; Karlsen, J.; Giunchedi, P. Nasal Administration of Carbamazepine Using Chitosan Microspheres: In Vitro/in Vivo Studies. Int. J. Pharm. 2006, 307, 9–15. [Google Scholar] [CrossRef]
- Varshosaz, J.; Sadrai, H.; Alinagari, R. Nasal Delivery of Insulin Using Chitosan Microspheres. J. Microencapsul. 2004, 21, 761–774. [Google Scholar] [CrossRef]
- Jain, S.K.; Chourasia, M.K.; Jain, A.K.; Jain, R.K.; Shrivastava, A.K. Development and Characterization of Mucoadhesive Microspheres Bearing Salbutamol for Nasal Delivery. Drug Deliv. 2004, 11, 113–122. [Google Scholar] [CrossRef]
- Illum, L.; Watts, P.; Fisher, A.N.; Hinchcliffe, M.; Norbury, H.; Jabbal-Gill, I.; Nankervis, R.; Davis, S.S. Intranasal Delivery of Morphine. J. Pharmacol. Exp. Ther. 2002, 301, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Turkoglu, O.F.; Eroglu, H.; Okutan, O.; Burul, E.; Sargon, M.F.; Özer, N.; Öner, L.; Beskonaklı, E. The Efficiency of Dexamethasone Sodium Phosphate–Encapsulated Chitosan Microspheres after Cold Injury. Surg. Neurol. 2005, 64, S11–S16. [Google Scholar] [CrossRef]
- Jiang, D.-S.; Long, S.-Y.; Huang, J.; Xiao, H.-Y.; Zhou, J.-Y. Immobilization of Pycnoporus Sanguineus Laccase on Magnetic Chitosan Microspheres. Biochem. Eng. J. 2005, 25, 15–23. [Google Scholar] [CrossRef]
- Shentu, J.; Wu, J.; Song, W.; Jia, Z. Chitosan Microspheres as Immobilized Dye Affinity Support for Catalase Adsorption. Int. J. Biol. Macromol. 2005, 37, 42–46. [Google Scholar] [CrossRef]
- Martins, A.O.; da Silva, E.L.; Carasek, E.; Laranjeira, M.C.M.; de Fávere, V.T. Sulphoxine Immobilized onto Chitosan Microspheres by Spray Drying: Application for Metal Ions Preconcentration by Flow Injection Analysis. Talanta 2004, 63, 397–403. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shi, T.S.; Pu, Y.L.; Zhu, J.G.; Zhao, Y.L. Studies on hepatic arterial embolization with cisplatin-chitosan-microspheres in dogs. Yao Xue Xue Bao 1995, 30, 891–895. [Google Scholar]
- Misirli, Y.; Oztürk, E.; Kurşaklioğlu, H.; Denkbaş, E.B. Preparation and Characterization of Mitomycin-C Loaded Chitosan-Coated Alginate Microspheres for Chemoembolization. J. Microencapsul. 2005, 22, 167–178. [Google Scholar] [CrossRef]
- Eroğlu, M.; Kurşaklioğlu, H.; Misirli, Y.; Iyisoy, A.; Acar, A.; Işin Doğan, A.; Denkbaş, E.B. Chitosan-Coated Alginate Microspheres for Embolization and/or Chemoembolization: In Vivo Studies. J. Microencapsul. 2006, 23, 367–376. [Google Scholar] [CrossRef]
- Denkbaş, E.B.; Kiliçay, E.; Birlikseven, C.; Öztürk, E. Magnetic Chitosan Microspheres: Preparation and Characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Yang, W.; Wang, C.; Fu, S. Preparation and Characterization of Chitosan–Poly(Acrylic Acid) Polymer Magnetic Microspheres. Polymer 2006, 47, 5287–5294. [Google Scholar] [CrossRef]
- Moore, A.; Basilion, J.P.; Chiocca, E.A.; Weissleder, R. Measuring Transferrin Receptor Gene Expression by NMR Imaging. Biochim. Biophys. Acta 1998, 1402, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Zhang, Y.; Kehr, J.; Klason, T.; Bjelke, B.; Muhammed, M. Characterization and MRI Study of Surfactant-Coated Superparamagnetic Nanoparticles Administered into the Rat Brain. J. Magn. Magn. Mater. 2001, 225, 256–261. [Google Scholar] [CrossRef]
- Morales, M.P.; Bomati-Miguel, O.; Pérez de Alejo, R.; Ruiz-Cabello, J.; Veintemillas-Verdaguer, S.; O’Grady, K. Contrast Agents for MRI Based on Iron Oxide Nanoparticles Prepared by Laser Pyrolysis. J. Magn. Magn. Mater. 2003, 266, 102–109. [Google Scholar] [CrossRef]
- Kim, E.H.; Ahn, Y.; Lee, H.S. Biomedical Applications of Superparamagnetic Iron Oxide Nanoparticles Encapsulated within Chitosan. J. Alloys Compd. 2007, 434–435, 633–636. [Google Scholar] [CrossRef]
- Lee, H.S.; Hee Kim, E.; Shao, H.; Kook Kwak, B. Synthesis of SPIO-Chitosan Microspheres for MRI-Detectable Embolotherapy. J. Magn. Magn. Mater. 2005, 293, 102–105. [Google Scholar] [CrossRef]
- Guliyeva, U.; Oner, F.; Ozsoy, S.; Haziroğlu, R. Chitosan Microparticles Containing Plasmid DNA as Potential Oral Gene Delivery System. Eur. J. Pharm. Biopharm. 2006, 62, 17–25. [Google Scholar] [CrossRef]
- Yun, Y.H.; Jiang, H.; Chan, R.; Chen, W. Sustained Release of PEG-g-Chitosan Complexed DNA from Poly(Lactide-Co-Glycolide). J. Biomater. Sci. Polym. Ed. 2005, 16, 1359–1378. [Google Scholar] [CrossRef]
- Lameiro, M.H.; Malpique, R.; Silva, A.C.; Alves, P.M.; Melo, E. Encapsulation of Adenoviral Vectors into Chitosan–Bile Salt Microparticles for Mucosal Vaccination. J. Biotechnol. 2006, 126, 152–162. [Google Scholar] [CrossRef]
- Aral, C.; Akbuga, J. Preparation and in Vitro Transfection Efficiency of Chitosan Microspheres Containing Plasmid DNA:Poly(L-Lysine) Complexes. J. Pharm. Pharm. Sci. 2003, 6, 321–326. [Google Scholar]
- Ozbas-Turan, S.; Aral, C.; Kabasakal, L.; Keyer-Uysal, M.; Akbuga, J. Co-Encapsulation of Two Plasmids in Chitosan Microspheres as a Non-Viral Gene Delivery Vehicle. J. Pharm. Pharm. Sci. 2003, 6, 27–32. [Google Scholar]
- Hejazi, R.; Amiji, M. Stomach-Specific Anti-H. Pylori Therapy; Part III: Effect of Chitosan Microspheres Crosslinking on the Gastric Residence and Local Tetracycline Concentrations in Fasted Gerbils. Int. J. Pharm. 2004, 272, 99–108. [Google Scholar] [CrossRef]
- El-Gibaly, I. Development and in Vitro Evaluation of Novel Floating Chitosan Microcapsules for Oral Use: Comparison with Non-Floating Chitosan Microspheres. Int. J. Pharm. 2002, 249, 7–21. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Stomach-Specific Anti-H. Pylori Therapy. I: Preparation and Characterization of Tetracyline-Loaded Chitosan Microspheres. Int. J. Pharm. 2002, 235, 87–94. [Google Scholar] [CrossRef]
- Portero, A.; Remuñán-López, C.; Criado, M.T.; Alonso, M.J. Reacetylated Chitosan Microspheres for Controlled Delivery of Anti-Microbial Agents to the Gastric Mucosa. J. Microencapsul. 2002, 19, 797–809. [Google Scholar] [CrossRef]
- Williams, R.O., III; Barron, M.K.; José Alonso, M.; Remuñán-López, C. Investigation of a PMDI System Containing Chitosan Microspheres and P134a. Int. J. Pharm. 1998, 174, 209–222. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Yeh, M.-K.; Cheng, S.-N.; Chiang, C.-H. The Characteristics of Betamethasone-Loaded Chitosan Microparticles by Spray-Drying Method. J. Microencapsul. 2003, 20, 459–472. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chiang, C.-H.; Yeh, M.-K. Optimizing Formulation Factors in Preparing Chitosan Microparticles by Spray-Drying Method. J. Microencapsul. 2003, 20, 247–260. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yeh, M.K.; Chiang, C.H. Formulation Factors in Preparing BTM–Chitosan Microspheres by Spray Drying Method. Int. J. Pharm. 2002, 242, 239–242. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated Chitosan Nanoparticles for Lung Protein Delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-Modified PLGA Nanosphere with Chitosan Improved Pulmonary Delivery of Calcitonin by Mucoadhesion and Opening of the Intercellular Tight Junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; van Meijgaarden, K.E.; Franken, K.L.M.C.; Junginger, H.E.; Borchard, G.; Ottenhoff, T.H.M.; Geluk, A. Pulmonary Delivery of Chitosan-DNA Nanoparticles Enhances the Immunogenicity of a DNA Vaccine Encoding HLA-A*0201-Restricted T-Cell Epitopes of Mycobacterium Tuberculosis. Vaccine 2004, 22, 1609–1615. [Google Scholar] [CrossRef]
- Varshosaz, J.; Jaffarian Dehkordi, A.; Golafshan, S. Colon-Specific Delivery of Mesalazine Chitosan Microspheres. J. Microencapsul. 2006, 23, 329–339. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Kruenate, J.; Prahsarn, C. Preparation and in Vitro Evaluation of Mucoadhesive Properties of Alginate/Chitosan Microparticles Containing Prednisolone. Int. J. Pharm. 2006, 312, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Jain, S.K.; Agrawal, S.; Bhadra, S.; Pancholi, S.S.; Agrawal, G.P. Chitosan Hydrochloride Based Microspheres of Albendazole for Colonic Drug Delivery. Pharmazie 2005, 60, 131–134. [Google Scholar]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Lauwo, J.A.K.; Agrawal, D.K.; Emenike, I.V. Some Pharmaceutical Studies on Sustained Release Coprbcipitates of Ampicillin Trihydrat with Acrylic Resin (Eudragit)(R)-RS. Drug Dev. Ind. Pharm. 1990, 16, 1375–1389. [Google Scholar] [CrossRef]
- Bodmeier, R.; Chen, H.; Tyle, P.; Jarosz, P. Pseudoephedrine HCl Microspheres Formulated into an Oral Suspension Dosage Form. J. Control. Release 1991, 15, 65–77. [Google Scholar] [CrossRef]
- Giunchedi, P.; Genta, I.; Conti, B.; Muzzarelli, R.A.; Conte, U. Preparation and Characterization of Ampicillin Loaded Methylpyrrolidinone Chitosan and Chitosan Microspheres. Biomaterials 1998, 19, 157–161. [Google Scholar] [CrossRef]
- Yin, C.; Hou, C.; Jiang, L.; Gu, Q. Research on Carboxymethyl Chitosan Acting as the Adjuvant for Implantable Degradable Microspheres. J. Biomed. Eng. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2004, 21, 350–354. [Google Scholar]
- Orhan, Z.; Cevher, E.; Mülazimoglu, L.; Gürcan, D.; Alper, M.; Araman, A.; Ozsoy, Y. The Preparation of Ciprofloxacin Hydrochloride-Loaded Chitosan and Pectin Microspheres: Their Evaluation in an Animal Osteomyelitis Model. J. Bone Joint Surg. Br. 2006, 88, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Cerchiara, T.; Luppi, B.; Bigucci, F.; Petrachi, M.; Orienti, I.; Zecchi, V. Controlled Release of Vancomycin from Freeze-Dried Chitosan Salts Coated with Different Fatty Acids by Spray-Drying. J. Microencapsul. 2003, 20, 473–478. [Google Scholar] [CrossRef]
- Majithiya, R.J.; Murthy, R.S.R. Chitosan-Based Mucoadhesive Microspheres of Clarithromycin as a Delivery System for Antibiotic to Stomach. Curr. Drug Deliv. 2005, 2, 235–242. [Google Scholar] [CrossRef]
- Govender, S.; Pillay, V.; Chetty, D.J.; Essack, S.Y.; Dangor, C.M.; Govender, T. Optimisation and Characterisation of Bioadhesive Controlled Release Tetracycline Microspheres. Int. J. Pharm. 2005, 306, 24–40. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Sala, N.; Bondi, M. Alginate Microparticles for Polymyxin B Peyer’s Patches Uptake: Microparticles for Antibiotic Oral Administration. J. Microencapsul. 2004, 21, 829–839. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L. Surface Fabrication of Hollow Microspheres from N-Methylated Chitosan Cross-Linked with Gultaraldehyde. Langmuir 2005, 21, 1091–1095. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Chemotherapeutic Potential of Alginate–Chitosan Microspheres as Anti-Tubercular Drug Carriers. J. Antimicrob. Chemother. 2004, 53, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Takishima, J.; Onishi, H.; Machida, Y. Prolonged Intestinal Absorption of Cephradine with Chitosan-Coated Ethylcellulose Microparticles in Rats. Biol. Pharm. Bull. 2002, 25, 1498–1502. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Jin, B.; Qi, Y.-C.; Jiang, Q.-Y.; Gao, X.-F. Carboxylated Chitosan/Silver-Hydroxyapatite Hybrid Microspheres with Improved Antibacterial Activity and Cytocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 589–597. [Google Scholar] [CrossRef]
- Fonseca, D.R.; Moura, A.; Leiro, V.; Silva-Carvalho, R.; Estevinho, B.N.; Seabra, C.L.; Henriques, P.C.; Lucena, M.; Teixeira, C.; Gomes, P.; et al. Grafting MSI-78A onto Chitosan Microspheres Enhances Its Antimicrobial Activity. Acta Biomater. 2022, 137, 186–198. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Xia, K.; Li, Y.; Han, J.; Zhao, C. Enhanced Antibacterial Activity of Silver Doped Titanium Dioxide-Chitosan Composites under Visible Light. Materials 2018, 11, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.K.S.; Reis, D.T.; Barbosa, K.M.; Scheidt, G.N. Antibacterial Effects and Ibuprofen Release Potential Using Chitosan Microspheres Loaded with Silver Nanoparticles. Carbohydrate 2020, 488, 107891. [Google Scholar] [CrossRef]
- Thaya, R.; Vaseeharan, B.; Sivakamavalli, J.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Synthesis of Chitosan-Alginate Microspheres with High Antimicrobial and Antibiofilm Activity against Multi-Drug Resistant Microbial Pathogens. Microb. Pathog. 2018, 114, 17–24. [Google Scholar] [CrossRef]
- Fuentes, S.; Alviña, R.; Zegarra, K.; Pérez, B.; Pozo, P. Antibacterial Activities of Copper Nanoparticles in Hybrid Microspheres. J. Nanosci. Nanotechnol. 2019, 19, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently Antibacterial Alginate-Chitosan Hydrogel Dressing Integrated Gelatin Microspheres Containing Tetracycline Hydrochloride for Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Ji, Z.; Wang, D.; Luo, Q.; Li, X. Preparation and Characterization of Uniform-Sized Chitosan/Silver Microspheres with Antibacterial Activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 33–41. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Hassan, K.T.; Hassan, O.M. Assessment of Antimicrobial Activity of Chitosan/Silver Nanoparticles Hydrogel and Cryogel Microspheres. Int. J. Biol. Macromol. 2023, 233, 123580. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K. Design, Synthesis, Spectroscopic Characterization, in-Vitro Antibacterial Evaluation and in-Silico Analysis of Polycaprolactone Containing Chitosan-Quercetin microspheres. J. Biomol. Struct. Dyn. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Liu, Y.; Peng, D. Cefepime Loaded O-Carboxymethyl Chitosan Microspheres with Sustained Bactericidal Activity and Enhanced Biocompatibility. J. Biomater. Sci. Polym. Ed. 2017, 28, 79–92. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Wu, C.; Sun, T.; Li, X. Preparation, Characterization and Antibacterial Mechanism of the Chitosan Coatings Modified by Ag/ZnO Microspheres. J. Sci. Food Agric. 2020, 100, 5527–5538. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J. Antibacterial Mechanism of Chitosan Microspheres in a Solid Dispersing System against E. coli. Colloids Surf. B Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and Wound Healing Properties of Chitosan/Poly(Vinyl Alcohol)/Zinc Oxide Beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef]
- Tiburcio, E.; García-Junceda, E.; Garrido, L.; Fernández-Mayoralas, A.; Revuelta, J.; Bastida, A. Preparation and Characterization of Aminoglycoside-Loaded Chitosan/Tripolyphosphate/Alginate Microspheres against E. coli. Polymers 2021, 13, 3326. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, N.; Wan, W.; Gao, Y.; Zhu, S. Selective Capture of Toxic Anionic Dyes of a Novel Prepared DMDAAC-Grafted Chitosan/Genipin/Cellulose Hydrogel Beads with Antibacterial Activity. Int. J. Biol. Macromol. 2021, 189, 722–733. [Google Scholar] [CrossRef]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, Characterisation and Biomedical Applications of Curcumin Conjugated Chitosan Microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef]
- Chen, M.-M.; Cao, H.; Liu, Y.-Y.; Liu, Y.; Song, F.-F.; Chen, J.-D.; Zhang, Q.-Q.; Yang, W.-Z. Sequential Delivery of Chlorhexidine Acetate and BFGF from PLGA-Glycol Chitosan Core-Shell Microspheres. Colloids Surf. B Biointerfaces 2017, 151, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Na, R.; Wang, X.; Liu, H.; Zhao, L.; Sun, X.; Ma, G.; Cui, F. Fabrication of Antimicrobial Peptide-Loaded PLGA/Chitosan Composite Microspheres for Long-Acting Bacterial Resistance. Molecules 2017, 22, 1637. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A Rose Bengal/Graphene Oxide/PVA Hybrid Hydrogel with Enhanced Mechanical Properties and Light-Triggered Antibacterial Activity for Wound Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111447. [Google Scholar] [CrossRef]
- Jyothi, S.S.; Seethadevi, A.; Prabha, K.S.; Muthuprasanna, P.; Pavitra, P. Microencapsulation: A Review. Int. J. Pharm. Biol. Sci. 2012, 3, 509–531. [Google Scholar]
- Albertini, B.; Passerini, N.; Di Sabatino, M.; Vitali, B. Polymer–Lipid Based Mucoadhesive Microspheres Prepared by Spray-Congealing for the Vaginal Delivery of Econazole Nitrate. Eur. J. Pharm. Sci. 2009, 36, 591–601. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Lu, Z.; Xing, R.; Yao, X.; Jin, Z.; Wang, Y.; Yu, F. Citral-Loaded Chitosan/Carboxymethyl Cellulose Copolymer Hydrogel Microspheres with Improved Antimicrobial Effects for Plant Protection. Int. J. Biol. Macromol. 2020, 164, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Synthesis and Characterization of Semi-Interpenetrating Polymer Network Microspheres of Acrylamide Grafted Dextran and Chitosan for Controlled Release of Acyclovir. Carbohydr. Polym. 2007, 67, 605–613. [Google Scholar] [CrossRef]
- Genta, I.; Conti, B.; Perugini, P.; Pavanetto, F.; Spadaro, A.; Puglisi, G. Bioadhesive Microspheres for Ophthalmic Administration of Acyclovir. J. Pharm. Pharmacol. 1997, 49, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Ciejka, J.; Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K. Biopolymeric Nano/Microspheres for Selective and Reversible Adsorption of Coronaviruses. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 735–742. [Google Scholar] [CrossRef]


| Chitosan MS/ Composite Type | Microbe | Activity |
|---|---|---|
| Tetracycline has also been loaded into CMS | Staphylococcus aureus | Antibacterial activity-Growth inhibition |
| Carboxylated chitosan/silver-hydroxyapatite (CMCS/Ag-HA) hybrid MS | Staphylococcus aureus | Antibacterial activity by synergistic effect of Ag+ and CMCS |
| MSI-78A with a C-terminal cysteine was grafted onto chitosan MS (AMP-ChMic) | Helicobacter pylori | Bactericidal-membrane disruption and cytoplasmic release |
| Ag and TiO2 nanoparticles on the cross-linked chitosan MS | E. coli, P. aeruginosa and S. aureus | Antibacterial-enhancement of both electron-hole separations, oxidizing hydroxyl release, |
| Epichlorohydrin-crosslinked chitosan MS loaded with silver nanoparticles | E. coli and S. aureus | Antibacterial |
| Chitosan-alginate (CS/ALG) MS | Gram-positive Staphylococcus aureus, Enterococcus faecalis and Gram-negative Pseudomonas aeruginosa and Proteus vulgaris | Antibacterial, antibiofilm |
| Cephalosporins loaded in magnetic chitosan MS | Staphylococcus aureus and Escherichia coli | Antibacterial |
| CuNPs coated with chitosan | Gram-negative bacterium E. coli and the Gram-positive bacterium E. faecalis | Antibacterial |
| Tetracycline hydrochloride (TH) loaded gelatin MS (GMS) integrated into the OAlg-CMCS hydrogel | Escherichia coli and Staphylococcus aureus | Powerful bacterial growth inhibition |
| Chitosan/silver MS (CAgMS) | E. coli, S. aureus, Rhizopus and Mucor | Antibacterial and antifungal |
| Hydrogel and cryogel MS doped with green synthesized silver nanoparticles (CS-AgNPs) | Gram-positive and gram-negative bacteria | Antibacterial |
| Chitosan-quercetin (CTS-QT) | E. coli, S. aureus and P. aeruginosa | Bactericidal |
| Cefepime loaded O-carboxymethyl chitosan MS | Staphylococcus aureus | Long lasting bactericidal activity |
| Ag/ZnO-CS | Shewanella putrefaciens and Pseudomonas aeruginosa | Bacterial cell membrane |
| Oleoyl-CMS (OCMS) | E. coli. | Antibacterial |
| Chitosan/poly(vinyl alcohol)/zinc oxide (CS/PVA/ZnO) | Escherichia coli, and Staphylococcus aureus | Antibacterial |
| Aminoglycoside-Loaded Chitosan/Tripolyphosphate/Alginate MS | Escherichia coli | Growth inhibition |
| Dimethyldiallylammonium chloride (DMDAAC) grafted chitosan/genipin/cellulose hydrogel beads (CCBG-g-PDMDAAC) | S. aureus and E. coli | Antibacterial |
| Curcumin conjugated chitosan MS (CCCMS) | Staphylococcus aureus and Escherichia coli | Antibacterial |
| PLGA-glycol chitosan (GC) core-shell MS | Staphylococcus aureus | Antibacterial |
| KSL-W-loaded PLGA/chitosan composite MS (KSL/PLGA/CS MSs) | Oral bacterial pathogens | Antibacterial |
| Rose bengal/graphene oxide/PVA hybrid hydrogel immobilized with CMS | Hyperthermia generated ROS | Antibacterial |
| Chitosan, sodium carboxymethylcellulose and poloxamers | Candida albicans | Antifungal |
| Chitosan/carboxymethyl cellulose (CS/CMC) | Botrytis cinerea in Solanum lycopersicum | Antifungal |
| Crosslinking of chitosan (CHIT) with genipin | Human coronavirus NL63 (HCoV-NL63), mouse hepatitis virus (MHV), and human coronavirus HCoV-OC43 | Antiviral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, M.; Pushparaj, S.S.C.; Gopal, J.; Sivanesan, I. A Review on the Antimicrobial Activity of Chitosan Microspheres: Milestones Achieved and Miles to Go. J. Mar. Sci. Eng. 2023, 11, 1480. https://doi.org/10.3390/jmse11081480
Muthu M, Pushparaj SSC, Gopal J, Sivanesan I. A Review on the Antimicrobial Activity of Chitosan Microspheres: Milestones Achieved and Miles to Go. Journal of Marine Science and Engineering. 2023; 11(8):1480. https://doi.org/10.3390/jmse11081480
Chicago/Turabian StyleMuthu, Manikandan, Suraj Shiv Charan Pushparaj, Judy Gopal, and Iyyakkannu Sivanesan. 2023. "A Review on the Antimicrobial Activity of Chitosan Microspheres: Milestones Achieved and Miles to Go" Journal of Marine Science and Engineering 11, no. 8: 1480. https://doi.org/10.3390/jmse11081480







