Metal Accumulations in Two Extreme-Environment Amphipods, Hadal Eurythenes gryllus and Antarctic Pseudorchomene plebs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparations
2.2. Quality Assurance
2.3. Statistical Analysis
3. Results
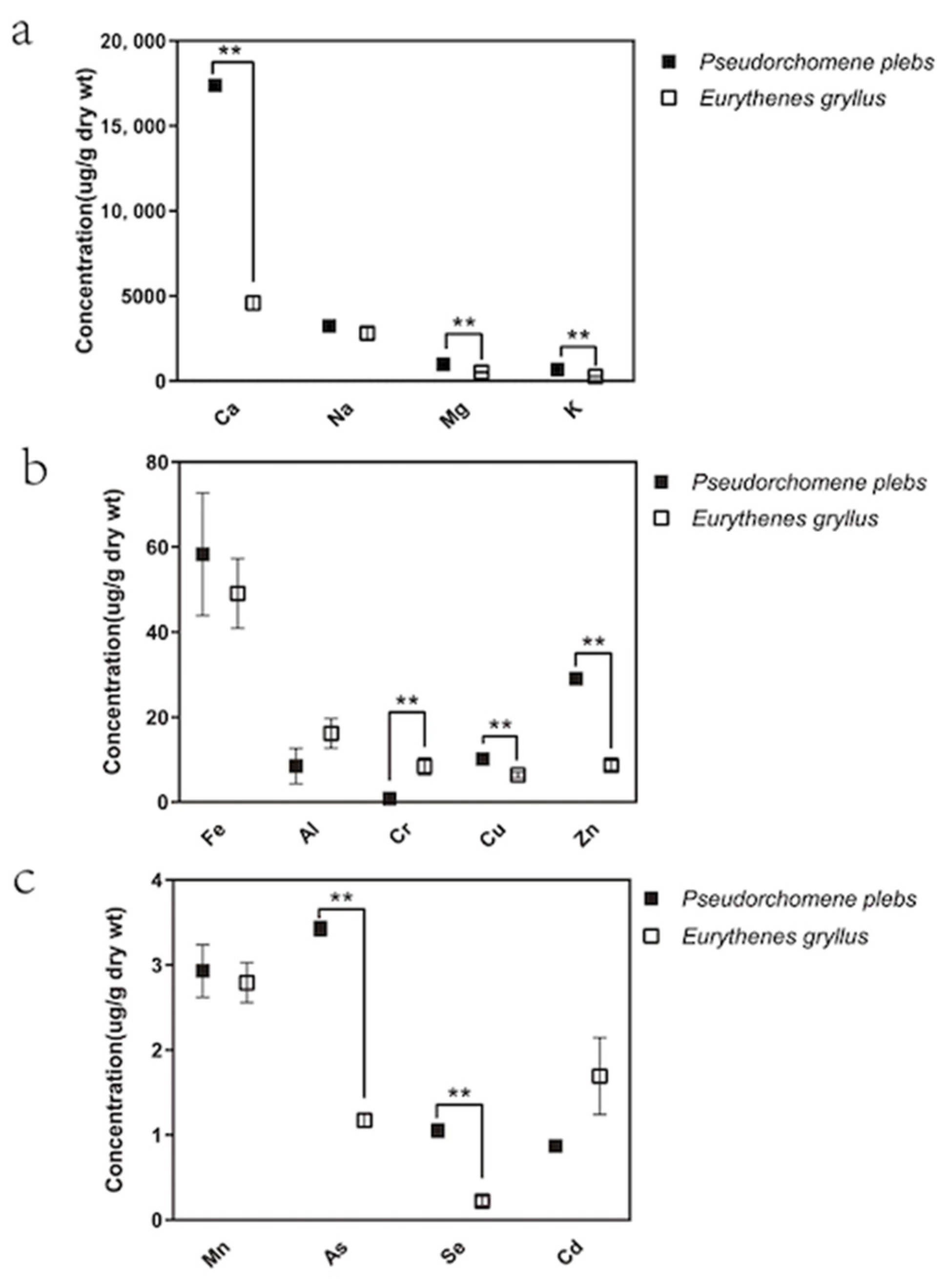
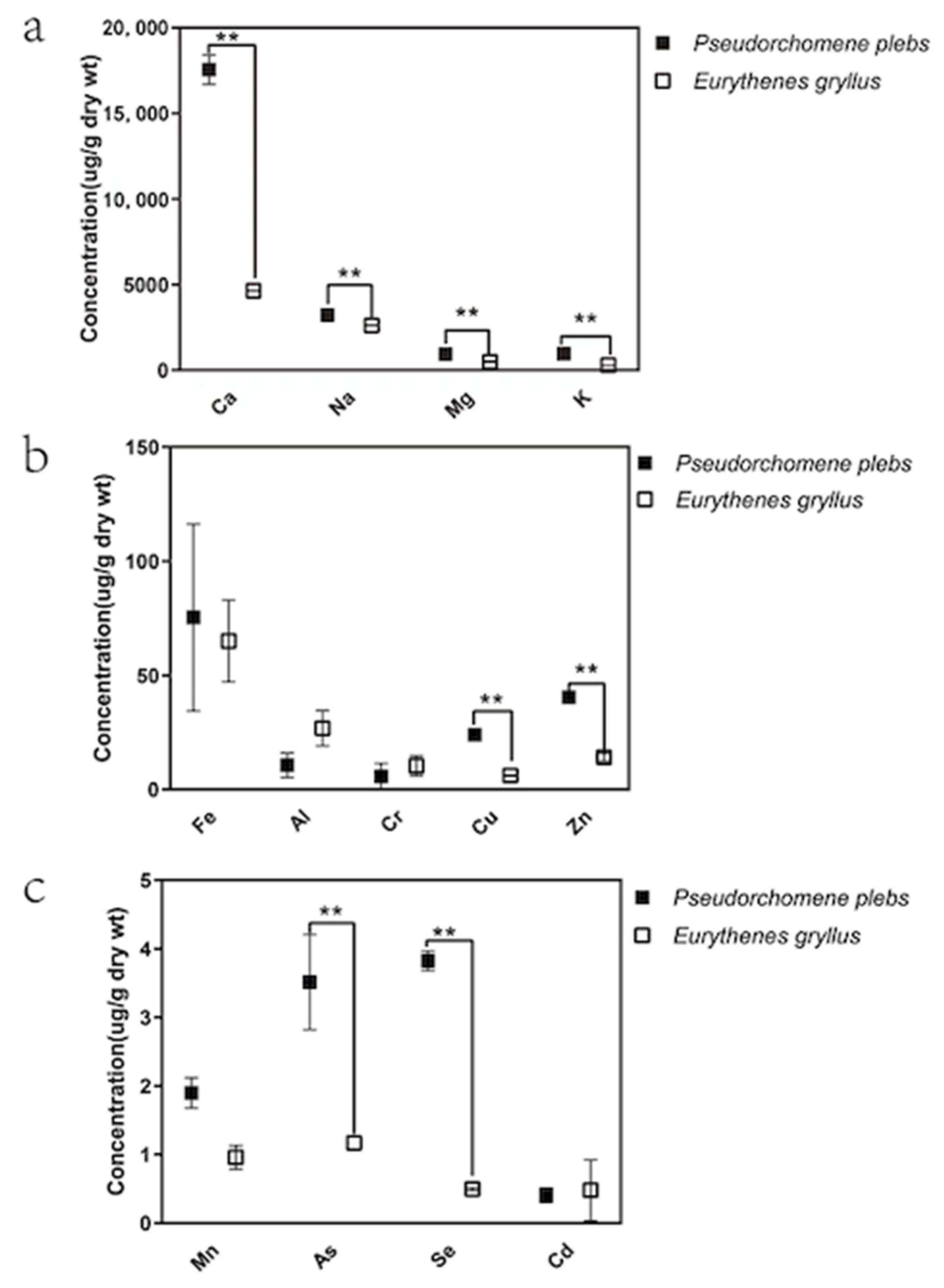
3.1. Regional Variations in the Amphipods’ Metal Concentrations
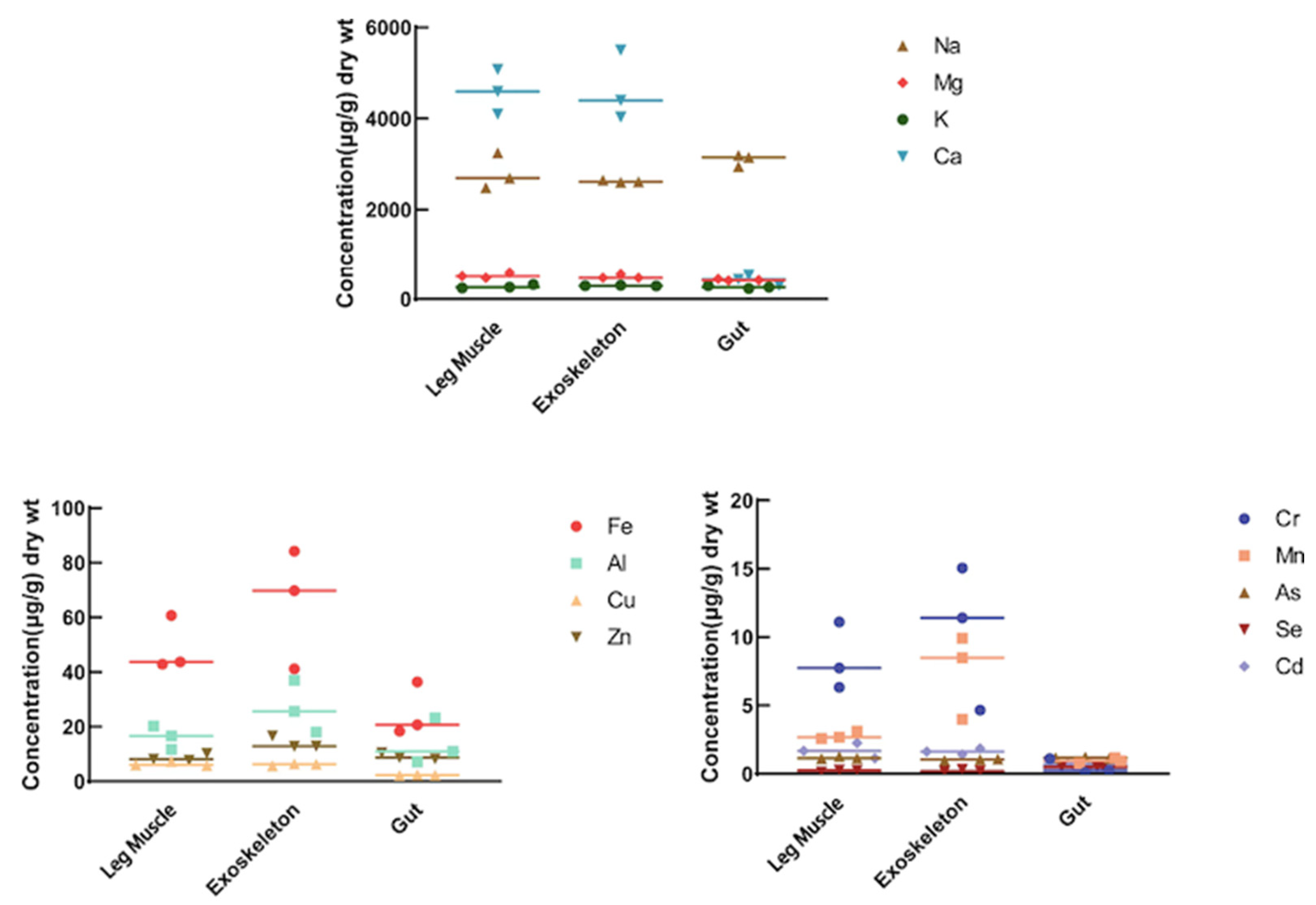
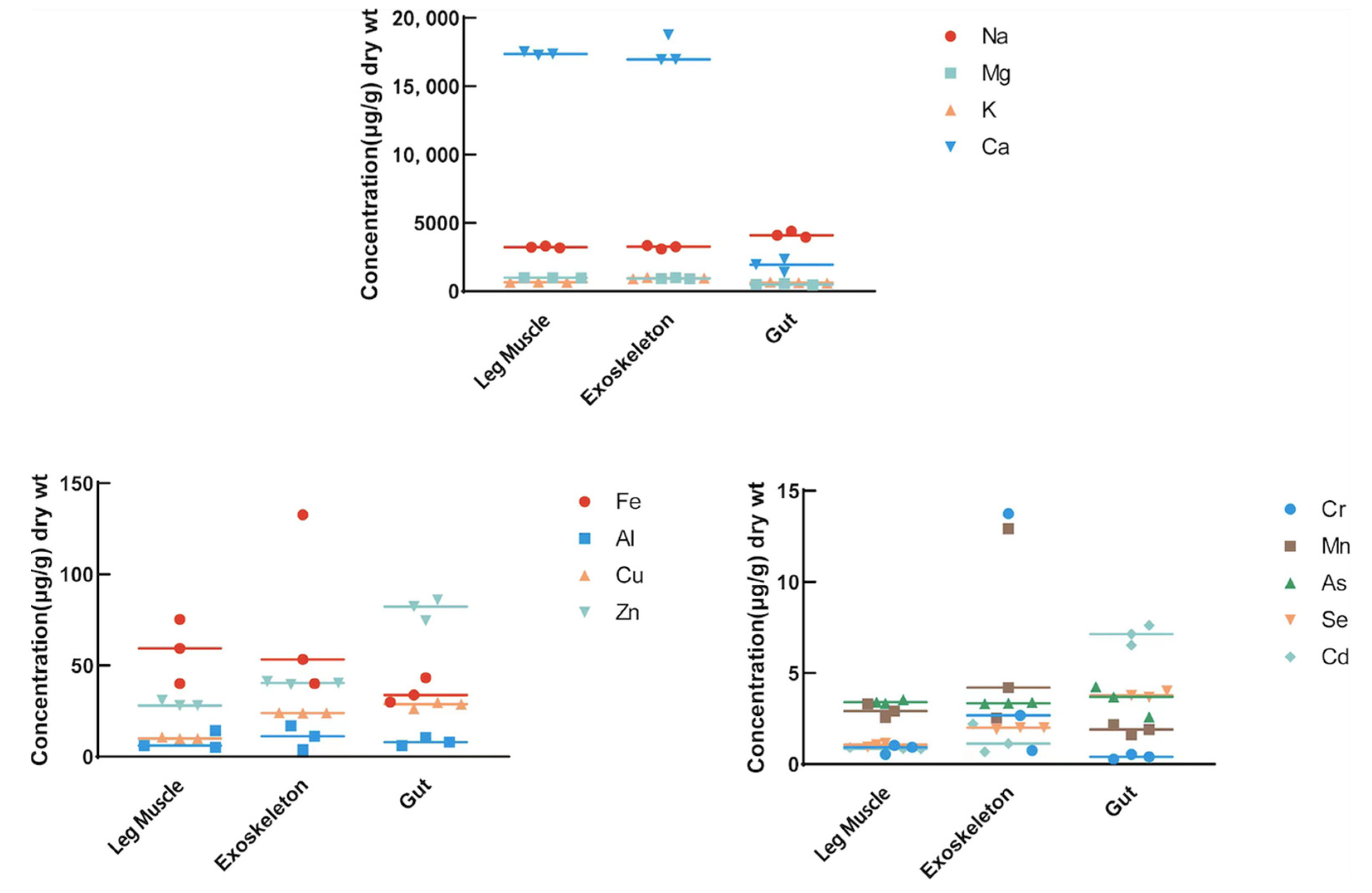
3.2. Metal Analyses in Different Tissues of the Two Amphipods
4. Discussion
| Species | Site (Depth/m) | Trace Element Concentrations (µg/g Dry Weight) | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Al | Cr | Mn | Cu | Zn | As | Se | Cd | |||
| E. gryllus | Mariana Trench (6040 m) | 49.101 (8.192) | 16.174 (3.498) | 8.390 (2.005) | 2.792 (0.236) | 6.392 (0.672) | 8.646 (1.093) | 1.173 (0.075) | 0.222 (0.055) | 1.693 (0.452) | Our study |
| P. plebs | Ross Sea (600 m) | 58.290 (14.412) | 8.464 (4.166) | 0.840 (0.217) | 2.930 (0.309) | 10.114 (0.382) | 29.008 (1.360) | 3.428 (0.084) | 1.050 (0.081) | 0.871 (0.027) | Our study |
| H. gigas | Izu-Bonin Trench (8172 m) | 415.50 (50.6) | - | - | 51.04 (6.39) | 32.67 (4.97) | 265.48 (35.66) | - | - | 4.84 (2.44) | [40] |
| H. gigas | Izu-Bonin Trench (9316 m) | 592.81 (132.29) | - | - | 85.34 (10.54) | 28.98 (2.84) | 172 (9.15) | - | - | 6.06 (0.51) | [40] |
| H. dubia | Kermadec Trench (6999 m) | 73.41 (14.16) | - | - | 3.58 (0.33) | 43.31 (4.85) | 111.81 (6.63) | - | - | 23.35 (1.01) | [40] |
| H. dubia | Kermadec Trench (8148 m) | 48.17 (14.25) | - | - | 8.32 (1.92) | 22.77 (6.78) | 136.02 (21.92) | - | - | 7.32 (2.1) | [40] |
| H. dubia | Kermadec Trench (9053 m) | 304.25 (103) | - | - | 24.51 (1.65) | 44.94 (0.94) | 224.71 (6.27) | - | - | 8.63 (0.08) | [40] |
| H. dubia | Kermadec Trench (9908 m) | 94.44 (22.3) | - | - | 12.03 (1.05) | 31.95 (1.54) | 170.69 (3.63) | - | - | 5.45 (0.76) | [40] |
| E. gryllus | Kermadec Trench (3268 m) | 105.62 | - | - | 2.99 | 17.9 | 187.33 | - | - | 1.44 | [40] |
| E. gryllus | Kermadec Trench (4519 m) | 243.84 (23.97) | - | - | 14.84 (2.92) | 21.5 (3.42) | 198.2 (37.68) | - | - | 3.52 (0.24) | [40] |
| E. gryllus | Kermadec Trench (5242 m) | 91.75 (19.09) | - | - | 2.35 (0.36) | 10.34 (0.74) | 192.86 (14.19) | - | - | 3.01 (0.61) | [40] |
| E. gryllus | Peru-Chile Trench (4602 m) | 167.3 (19.61) | - | - | 7.18 (0.65) | 24.73 (10.29) | 188.43 (3.59) | - | - | 15.56 (15.55) | [40] |
| E. gryllus | Peru-Chile Trench (5329 m) | 146.92 (57.43) | - | - | 5.69 (0.14) | 15.21 (1.63) | 230.2 (17.8) | - | - | 6.19 (3.13) | [40] |
| E. gryllus | Peru-Chile Trench (6173 m) | 230.11 (156.75) | - | - | 7.32 (2.38) | 22.23 (4.13) | 120.27 (17.13) | - | - | 18.25 (3.84) | [40] |
| A. gigantea | New Britain Trench (8824 m) | - | - | 0.23 (0.030) | 0.776 (0.535) | - | - | - | 0.38 (0.034) | 0.361 (0.880) | [48] |
| H. gigas | Mariana Trench (10,839 m) | - | - | 3.39 (1.490) | 1.558 (0.532) | - | - | - | 0.34 (0.042) | 0.370 (0.172) | [48] |
| S. schellenbergi | Marceau Trench (6690 m) | - | - | 2.08 (1.107) | 2.177 (1.155) | - | - | - | 0.36 (0.081) | 0.685 (0.228) | [48] |
| P. kerathurus | Senegalese coastal (shallow) | 6.83 (4.13) | - | 0.20 (0.10) | 0.19 (0.06) | 18.5 (7.5) | 44.0 (4.4) | 7.52 (3.59) | 0.66 (0.23) | 0.05 (0.05) | [75] |
| M. rosenbergii | South Vietnam (shallow) | - | - | 0.20 (0.01) | 2.68 (0.57) | 31.4 (9.1) | 55.9 (2.1) | 0.51 (0.56) | 0.86 (0.05) | 0.048 (0.044) | [76] |
| P. semisulcatus | İskenderun Bay (shallow) | - | - | 0.215 (0.020) | 0.382 (0.018) | - | - | 29.254 (0.473) | - | 0.008 (0.001) | [77] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Gao, Y.; Gong, L.; Li, M.; Pang, K.L.; Luo, Z.H. Fungal diversity in the deep-sea hadal sediments of the Yap Trench by cultivation and high throughput sequencing methods based on ITS rRNA gene. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 145, 125–136. [Google Scholar] [CrossRef]
- Nunoura, T.; Takaki, Y.; Hirai, M.; Shimamura, S.; Makabe, A. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, E1230–E1236. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, S.; Wang, Y.; Suo, Y.; Dai, L.; Geli, L. Causes of earthquake spatial distribution beneath the Izu-Bonin-Mariana Arc. J. Asian Earth Sci. 2018, 151, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Jamieson, A.; Piertney, S. Phylogenetic relationships among hadal amphipods of the Superfamily Lysianassoidea: Implications for taxonomy and biogeography. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 105, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Hiraoka, S.; Hirai, M.; Matsui, Y.; Makabe, A.; Minegishi, H.; Tsuda, M.; Rastelli, E.; Danovaro, R.; Corinaldesi, C.; Kitahashi, T.; et al. Microbial community and geochemical analyses of trans-trench sediments for understanding the roles of hadal environments. ISME. J. 2020, 14, 740–756. [Google Scholar] [CrossRef] [Green Version]
- Bao, R.; Hirai, M.; Matsui, Y. Tectonically-triggered sediment and carbon export to the Hadal zone. Nat. Commun. 2018, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Glud, R.N.; Wenzhöfer, F.; Middelboe, M.; Oguri, K.; Turnewitsch, R.; Canfield, D. High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth. Nat. Geosci. 2013, 6, 284–288. [Google Scholar] [CrossRef]
- Chan, J.; Pan, B.; Geng, D.; Zhang, Q.; Zhang, S.; Guo, J.; Xu, Q. Genetic diversity and population structure analysis of three deep-sea amphipod species from geographically isolated hadal trenches in the Pacific Ocean. Biochem. Genet. 2020, 58, 157–170. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Wei, Y.; Fang, J. The hadal biosphere: Recent insights and new directions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 155, 11–18. [Google Scholar] [CrossRef]
- Chan, J.; Geng, D.; Pan, B.; Zhang, Q.; Xu, Q. Metagenomic Insights Into the Structure and Function of Intestinal Microbiota of the Hadal Amphipods. Front. Microbiol. 2021, 12, 668989. [Google Scholar] [CrossRef]
- Li, Y.; Kong, X.; Chen, J.; Liu, H.; Zhang, H. Characteristics of the copper, zinc superoxide dismutase of a hadal sea cucumber (Paelopatides sp.) from the mariana trench. Mar. Drugs 2018, 16, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, A.J. Hadal trenches: The ecology of the deepest places on Earth. Trends Ecol. Evol. 2010, 25, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, Y.; Yang, Y.; Gan, X.; Liu, G. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nat. Ecol. Evol. 2019, 3, 823–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankenship, L.E.; Yayanos, A.A.; Cadien, D.B.; Levin, L.A. Vertical zonation patterns of scavenging amphipods from the Hadal zone of the Tonga and Kermadec Trenches. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 48–61. [Google Scholar] [CrossRef]
- Li, A.; Han, X.B.; Zhang, M.X.; Zhou, Y.; Chen, M.; Yao, Q.; Zhu, H.H. Culture-dependent and-independent analyses reveal the diversity, structure, and assembly mechanism of benthic bacterial community in the Ross Sea, Antarctica. Front. Microbiol. 2019, 10, 2523. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Sporta Caputi, S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic food web architecture under varying dynamics of sea ice cover. Sci. Rep. 2019, 9, 12454. [Google Scholar] [CrossRef] [Green Version]
- Norkko, A.; Thrush, S.F.; Cummings, V.J.; Gibbs, M.M.; Andrew, N.L.; Norkko, J.; Schwarz, A.-M. Trophic structure of coastal Antarctic food webs associated with changes in sea ice and food supply. Ecology 2007, 88, 2810–2820. [Google Scholar] [CrossRef]
- Clarke, A. Antarctic marine benthic diversity: Patterns and processes. J. Exp. Mar. Biol. Ecol. 2008, 366, 48–55. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, C.-H.C.; Zhang, J. Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc. Natl. Acad. Sci. USA 2008, 105, 12944–12949. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Li, W. The genomic basis for colonizing the freezing Southern Ocean revealed by Antarctic toothfish and Patagonian robalo genomes. GigaScience 2019, 8, giz016. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Cai, C.; Hu, X. Evolutionary suppression of erythropoiesis via the modulation of TGF-β signalling in an Antarctic icefish. Mol. Ecol. 2015, 24, 4664–4678. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, A.J.; Malkocs, T.; Piertney, S.B.; Fujii, T.; Zhang, Z. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat. Ecol. Evol. 2017, 1, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.K.; Mishra, V.K.; Kim, K.H.; Hong, S.; Lee, K. Aerosol composition and its sources at the King Sejong Station, Antarctic peninsula. Atmos. Environ. 2004, 38, 4069–4084. [Google Scholar] [CrossRef]
- Fujii, T.; Kilgallen, N.M.; Rowden, A.A.; Jamieson, A.J. Deep-sea amphipod community structure across abyssal to hadal depths in the Peru-Chile and Kermadec trenches. Mar. Ecol. Prog. Ser. 2013, 492, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zeng, C.; Yan, G.; He, L. Characterization of the mitochondrial genome of an ancient amphipod Halice sp. MT-2017 (Pardaliscidae) from 10,908 m in the Mariana Trench. Sci. Rep. 2019, 9, 2610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basher, Z.; Bowden, D.A.; Costello, M.J. Diversity and distribution of deep-sea shrimps in the Ross Sea region of Antarctica. PLoS ONE 2014, 9, e103195. [Google Scholar] [CrossRef] [Green Version]
- Conradi, M.; López-González, P.; García-Gómez, C. The amphipod community as a bioindicator in Algeciras Bay (southern Iberian Peninsula) based on a spatio temporal distribution. Mar. Ecol. 1997, 18, 97–111. [Google Scholar] [CrossRef]
- Rehm, P.; Thatje, S.; Mühlenhardt-Siegel, U.; Brandt, A. Composition and distribution of the peracarid crustacean fauna along a latitudinal transect off Victoria Land (Ross Sea, Antarctica) with special emphasis on the Cumacea. Polar Biol. 2007, 30, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Nyssen, F.; Brey, T.; Dauby, P.; Graeve, M. Trophic position of Antarctic amphipods—Enhanced analysis by a 2-dimensional biomarker assay. Mar. Ecol. Prog. Ser. 2005, 300, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Havermans, C.; Sonet, G.; d’Udekem d’Acoz, C.; Nagy, Z. Genetic and morphological divergences in the cosmopolitan deep-sea amphipod Eurythenes gryllus reveal a diverse abyss and a bipolar species. PLoS ONE 2013, 8, e74218. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.W.; Coleman, C.O.; Yoon, S.M. Pseudorchomene boreoplebs, a new lysianassid amphipod from Korean waters (Crustacea, Amphipoda, Lysianassoidea). Zoosyst. Evol. 2017, 93, 343. [Google Scholar] [CrossRef] [Green Version]
- Dauby, P.; Nyssen, F.; De Broyer, C. Amphipods as food sources for higher trophic levels in the Southern Ocean: A synthesis. Antarctic Biology in a Global Context; Backhuis: Leiden, The Netherlands, 2003; pp. 129–134. [Google Scholar]
- Zhang, W.; Watanabe, H.K.; Ding, W.; Lan, Y. Gut microbial divergence between two populations of the hadal amphipod Hirondellea gigas. Appl. Environ. Microbiol. 2019, 85, e02032-18. [Google Scholar] [CrossRef] [Green Version]
- Asante, K.A.; Agusa, T.; Kubota, R.; Mochizuki, H.; Ramu, K. Trace elements and stable isotope ratios (δ13C and δ15N) in fish from deep-waters of the Sulu Sea and the Celebes Sea. Mar. Pollut. Bull. 2010, 60, 1560–1570. [Google Scholar] [CrossRef]
- Amiard, J.C.; Amiard-Triquet, C.; Berthet, B.; Metayer, C. Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms. J. Exp. Mar. Biol. Ecol. 1987, 106, 73–89. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L. Health and environmental effects of heavy metals. J. King Saud Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Varol, M.; Kaya, G.K.; Alp, A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: Risk-based consumption advisories. Sci. Total Environ. 2017, 599, 1288–1296. [Google Scholar] [CrossRef]
- Garcia-Cegarra, A.M.; Padilha, J.A.; Braz, B.F.; Ricciardi, R. Concentration of trace elements in long-finned pilot whales stranded in northern Patagonia, Chile. Mar. Pollut. Bull. 2020, 151, 110822. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.D.; Cuomo, N.J.; Jamieson, A.J. Geographic and bathymetric comparisons of trace metal concentrations (Cd, Cu, Fe, Mn, and Zn) in deep-sea lysianassoid amphipods from abyssal and hadal depths across the Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 138, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Lipy, E.P.; Hakim, M.; Mohanta, L.C.; Islam, D.; Lyzu, C. Assessment of heavy metal concentration in water, sediment and common fish species of Dhaleshwari River in Bangladesh and their health implications. Biol. Trace Elem. Res. 2021, 199, 4295–4307. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, S.; Mohamed, A.S.; Mohamed, H.A.; Fahmy, W.S. The Effect of Zinc Concentration on Physiological, Immunological, and Histological Changes in Crayfish (Procambarus clarkii) as Bio-indicator for Environment Quality Criteria. Biol. Trace Elem. Res. 2022, 200, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jazdzewski, K.; Sket, B. Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Marsden, I.; Rainbow, P. Does the accumulation of trace metals in crustaceans affect their ecology—The amphipod example? J. Exp. Mar. Biol. Ecol. 2004, 300, 373–408. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Emson, R.H.; Smith, B.D.; Moore, P.G.; Mladenov, P.V. Talitrid amphipods as biomonitors of trace metals near Dunedin, New Zealand. N. Z. J. Mar. Freshw. Res. 1993, 27, 201–207. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Jiang, S.; Pan, B.; Chan, J.; Xu, Q. The Adaptive Evolution and Gigantism Mechanisms of the Hadal “Supergiant” Amphipod Alicella gigantea. Front. Mar. Sci. 2021, 8, 743663. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, S.; Li, W.; Pan, B.; Xu, Q. Trimethylamine N-Oxide (TMAO) and Trimethylamine (TMA) Determinations of Two Hadal Amphipods. J. Mar. Sci. Eng. 2022, 10, 454. [Google Scholar] [CrossRef]
- Zhu, L.; Geng, D.; Pan, B.; Li, W.; Jiang, S.; Xu, Q. Trace Elemental Analysis of the Exoskeleton, Leg Muscle, and Gut of Three Hadal Amphipods. Biol. Trace Elem. Res. 2022, 200, 1395–1407. [Google Scholar] [CrossRef]
- Kise, H.; Iguchi, A.; Ikegami, T.; Onishi, Y.; Goto, K.; Tanaka, Y.; Washburn, T.W.; Nishijima, M.; Kunishima, T.; Okamoto, N.; et al. Genetic population structures of common scavenging species near hydrothermal vents in the Okinawa Trough. Sci. Rep. 2023, 13, 2348. [Google Scholar] [CrossRef]
- Irnius, A.; Speiciene, D.; Tautkus, S.; Kareiva, A. Distribution of sodium, potassium, magnesium and calcium in blood plasma. Mendeleev Commun. 2007, 4, 216–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Cogswell, M.E.; Gillespie, C.; Fang, J.; Loustalot, F. Association between usual sodium and potassium intake and blood pressure and hypertension among US adults: NHANES 2005–2010. PLoS ONE 2013, 8, e75289. [Google Scholar]
- Whelton, P.K. Sodium and potassium intake in US adults. Circulation. 2018, 137, 247–249. [Google Scholar] [CrossRef]
- Luo, M.; Glud, R.N.; Pan, B.; Wenzhöfer, F.; Xu, Y.; Lin, G.; Chen, D. Benthic carbon mineralization in hadal trenches: Insights from in situ determination of benthic oxygen consumption. Geophys. Res. Lett. 2018, 45, 2752–2760. [Google Scholar] [CrossRef] [Green Version]
- Stewart, H.A.; Jamieson, A.J. Habitat heterogeneity of hadal trenches: Considerations and implications for future studies. Prog. Oceanogr. 2018, 161, 47–65. [Google Scholar] [CrossRef]
- Keil, S.; De Broyer, C.; Zauke, G.P. Significance and interspecific variability of accumulated trace metal concentrations in Antarctic benthic crustaceans. Int. Rev. Hydrobiol. 2008, 93, 106–126. [Google Scholar] [CrossRef]
- Rainbow, P.S. Heavy Metals in Aquatic Invertebrates. In Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 405–426. [Google Scholar]
- Roberts, D.A. Causes and ecological effects of resuspended contaminated sediments (RCS) in marine environments. Environ. Int. 2012, 40, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Planchon, F.A.; Boutron, C.F.; Barbante, C.; Cozzi, G.; Gaspari, V. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth Planet. Sci. Lett. 2002, 200, 207–222. [Google Scholar] [CrossRef]
- Do Hur, S.; Cunde, X.; Hong, S.; Barbante, C.; Gabrielli, P. Seasonal patterns of heavy metal deposition to the snow on Lambert Glacier basin, East Antarctica. Atmos. Environ. 2007, 41, 8567–8578. [Google Scholar] [CrossRef]
- Wardell, L.; Kyle, P.; Counce, D. Volcanic emissions of metals and halogens from White Island (New Zealand) and Erebus volcano (Antarctica) determined with chemical traps. J. Volcanol. Geotherm. Res. 2008, 177, 734–742. [Google Scholar] [CrossRef]
- Arístegui, J. Microbial oceanography of the dark ocean’s pelagic realm. Limnol. Oceanogr. 2009, 54, 1501–1529. [Google Scholar] [CrossRef] [Green Version]
- Bargagli, R. Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 2008, 400, 212–226. [Google Scholar] [CrossRef]
- Beyer, A.; Matthies, M. Long-range transport potential of semivolatile organic chemicals in coupled air-water systems. Environ. Sci. Pollut. Res. 2001, 8, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001, 222, 149–158. [Google Scholar] [CrossRef]
- Cui, J.; Yu, Z.; Mi, M.; He, L.; Sha, Z.; Yao, P.; Fang, J.; Sun, W. Occurrence of halogenated organic pollutants in hadal trenches of the western Pacific Ocean. Environ. Sci. Technol. 2020, 54, 15821–15828. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Shimoshige, H.; Nakajima, Y.; Arai, W.; Takami, H. An aluminum shield enables the amphipod Hirondellea gigas to inhabit deep-sea environments. PLoS ONE 2019, 14, e0206710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlin, O.H.; Salvador, G.L.; Vitturi, L.M.; Pistolato, M.; Rampazzo, G. Preliminary results on trace element geochemistry of sediments from the Ross Sea, Antarctica. Bolletino Oceanol. Teor. Apl. 1989, 7, 97–108. [Google Scholar]
- Ferguson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK; New York, NY, USA, 1990; pp. 1–412. [Google Scholar]
- Grotti, M.; Soggia, F.; Lagomarsino, C.; Dalla Riva, S.; Goessler, W.; Francesconi, K.A. Natural variability and distribution of trace elements in marine organisms from Antarctic coastal environments. Antarct. Sci. 2008, 20, 39–52. [Google Scholar] [CrossRef]
- Bargagli, R.; Nelli, L.; Ancora, S.; Focardi, S. Elevated cadmium accumulation in marine organisms from Terra Nova Bay (Antarctica). Polar Biol. 1996, 16, 513–520. [Google Scholar] [CrossRef]
- Bennett, S.A.; Achterberg, E.P.; Connelly, D.P.; Statham, P.J.; Fones, G.R.; German, C.R. The distribution and stabilisation of dissolved Fe in deep-sea hydrothermal plumes. Earth Planet. Sci. Lett. 2008, 270, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Resing, J.A.; Sedwick, P.N.; German, C.R.; Jenkins, W.J.; Moffett, J.W.; Sohst, B.M.; Tagliabue, A. Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean. Nature 2015, 523, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Xiao, W.; Liu, Z.; Pan, B.; Xu, Y. Diet change of hadal amphipods revealed by fatty acid profile: A close relationship with surface ocean. Mar. Environ. Res. 2018, 142, 250–256. [Google Scholar] [CrossRef]
- Gallo, N.D.; Cameron, J.; Hardy, K.; Fryer, P.; Bartlett, D.H.; Levin, L.A. Submersible-and lander-observed community patterns in the Mariana and New Britain trenches: Influence of productivity and depth on epibenthic and scavenging communities. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 99, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Diop, M.; Howsam, M.; Diop, C.; Goossens, J.F.; Diouf, A.; Amara, R. Assessment of trace element contamination and bioaccumulation in algae (Ulva lactuca), mussels (Perna perna), shrimp (Penaeus kerathurus), and fish (Mugil cephalus, Saratherondon melanotheron) along the Senegalese coast. Mar. Pollut. Bull. 2016, 103, 339–343. [Google Scholar] [CrossRef]
- Nghuyen, P.C.T.; Nghuyen, N.H.; Tokutaka, I.; Bui, C.T.; Shinsuke, T.; Ichiro, T. Bioaccumulation and distribution of trace elements in tissues of giant river prawn Macrobrachium rosenbergii (Decapoda: Palaemonidae) from South Vietnam. Fish. Sci. 2008, 74, 109–119. [Google Scholar]
- Kaya, G.; Turkoglu, S. Bioaccumulation of Heavy Metals in Various Tissues of Some Fish Species and Green Tiger Shrimp (Penaeus semisulcatus) from A degrees skenderun Bay, Turkey, and Risk Assessment for Human Health. Biol. Trace Elem. Res. 2017, 180, 314–326. [Google Scholar] [CrossRef] [PubMed]
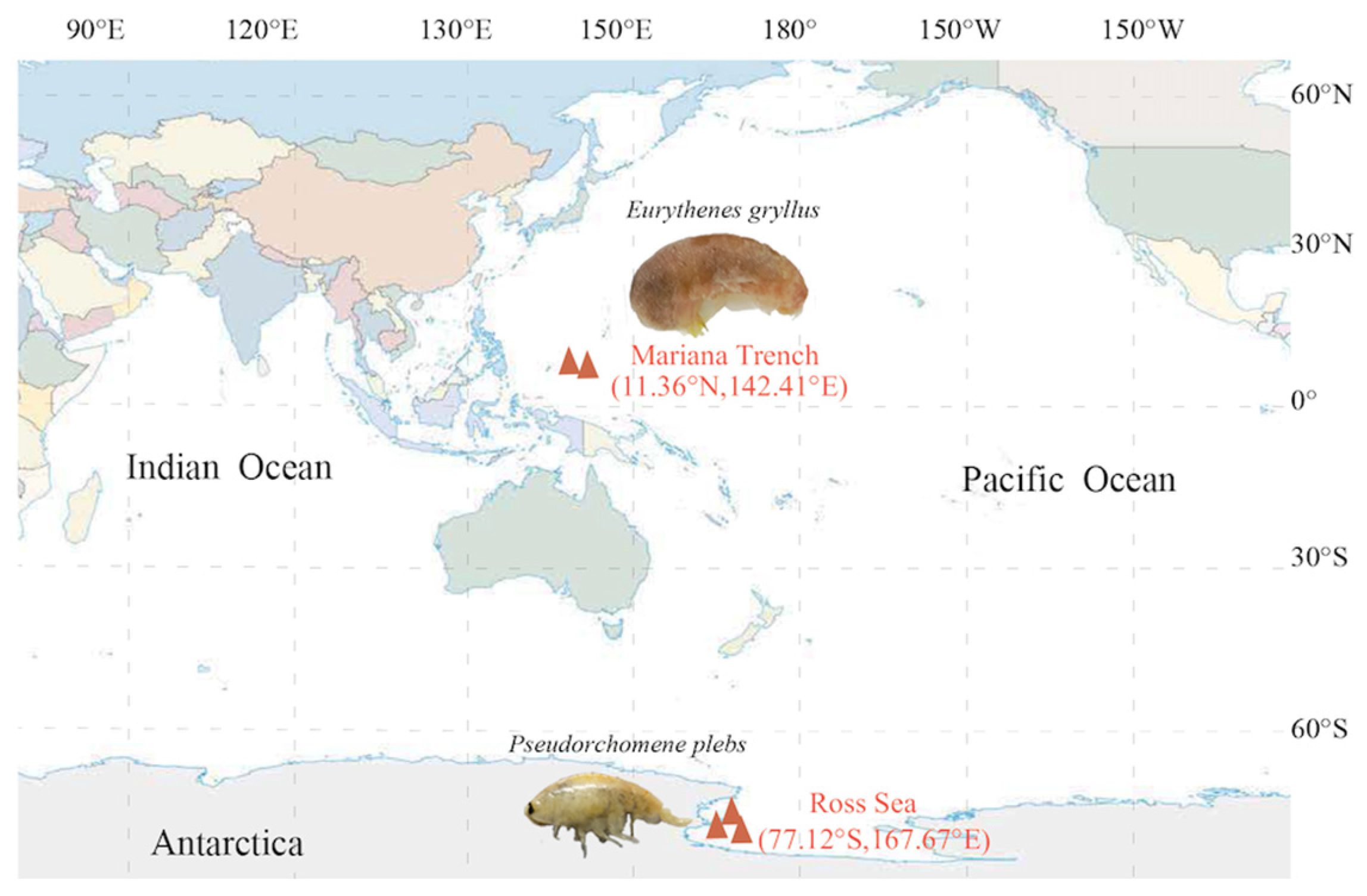

| Species | Sample Location | Latitude Longitude | Depth (m) | n | Length Range (mm) | Length (mm) Mean ± SD | Weight Range (g) | Weight (g) Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| E. gryllus | Mariana Trench | 11.36° N, 142.41° E | 6040 | 5 | 35.3–53.24 | 45.5 ± 1.63 | 1.28–2.14 | 1.6 ± 0.33 |
| P. plebs | Ross Sea | 77.12° S, 167.67° W | 600 | 30 | 11.8–21.37 | 16.4 ± 2.67 | 0.068–0.121 | 0.1 ± 0.01 |
| Eurythenes gryllus | Pseudorchomene plebs | p-Value (a) | p-Value (b) | p-Value (c) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Leg Muscle | Exoskeleton | Gut | Leg Muscle | Exoskeleton | Gut | ||||
| Na | 2809.317 ± 322.903 | 2622.396 ± 18.804 | 3095.552 ± 106.222 | 3237.988 ± 54.205 | 3227.960 ± 98.507 | 4146.693 ± 182.949 | 0.137 | 0.001 ** | 0.002 ** |
| Mg | 514.496 ± 45.754 | 494.970 ± 38.734 | 422.659 ± 15.395 | 981.832 ± 10.003 | 940.265 ± 33.982 | 506.747 ± 38.917 | 0.0001 ** | 0.0002 ** | 0.047 * |
| K | 274.930 ± 33.966 | 293.297 ± 5.988 | 262.844 ± 24.871 | 670.033 ± 7.129 | 961.889 ± 38.762 | 625.340 ± 32.183 | 8.705 × 10−05 ** | 1.756 × 10−05 ** | 0.0002 ** |
| Ca | 4588.906 ± 398.423 | 4643.466 ± 23.52 | 417.545 ± 92.189 | 17,382.824 ± 102.483 | 17,556.783 ± 854.971 | 1893.008 ± 386.347 | 1.598 × 10−06 ** | 6.615 × 10−05 ** | 0.006 ** |
| Fe | 49.101 ± 8.192 | 65.077 ± 17.900 | 25.157 ± 7.981 | 58.290 ± 14.412 | 75.350 ± 40.915 | 35.694 ± 5.645 | 0.477 | 0.761 | 0.202 |
| Al | 16.174 ± 3.498 | 26.873 ± 7.765 | 13.785 ± 6.854 | 8.464 ± 4.166 | 10.650 ± 5.349 | 8.154 ± 1.785 | 0.116 | 0.072 | 0.324 |
| Cr | 8.390 ± 2.005 | 10.376 ± 4.303 | 0.480 ± 0.444 | 0.840 ± 0.217 | 5.723 ± 5.723 | 0.408 ± 0.104 | 0.006 ** | 0.41 | 0.836 |
| Mn | 2.792 ± 0.236 | 7.466 ± 2.527 | 0.959 ± 0.172 | 2.930 ± 0.309 | 6.553 ± 4.551 | 1.899 ± 0.221 | 0.642 | 0.816 | 0.008 ** |
| Cu | 6.392 ± 0.672 | 6.142 ± 0.325 | 2.261 ± 0.131 | 10.114 ± 0.382 | 23.946 ± 0.139 | 28.292 ± 1.411 | 0.002 ** | 2.331 × 10−07 ** | 1.306 × 10−05 ** |
| Zn | 8.646 ± 1.093 | 14.103 ± 1.790 | 9.148 ± 0.962 | 29.008 ± 1.360 | 40.460 ± 0.721 | 81.005 ± 4.766 | 7.888 × 10−05 ** | 4.230 × 10−05 ** | 3.098 × 10−05 ** |
| As | 1.173 ± 0.075 | 1.031 ± 0.045 | 1.169 ± 0.049 | 3.428 ± 0.084 | 3.349 ± 0.031 | 3.514 ± 0.694 | 9.446 × 10−06 ** | 4.801 × 10−07 ** | 0.008 ** |
| Se | 0.222 ± 0.055 | 0.231 ± 0.067 | 0.497 ± 0.020 | 1.050 ± 0.081 | 1.974 ± 0.044 | 3.825 ± 0.142 | 0.0002 ** | 6.823 × 10−06 ** | 5.181 × 10−06 ** |
| Cd | 1.693 ± 0.452 | 1.635 ± 0.183 | 0.708 ± 0.059 | 0.871 ± 0.027 | 1.342 ± 0.638 | 7.097 ± 0.445 | 0.062 | 0.566 | 3.591 × 10−05 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhu, L.; Jiang, S.; Zhai, W.; Pan, B.; Wu, Z.; Xu, Q. Metal Accumulations in Two Extreme-Environment Amphipods, Hadal Eurythenes gryllus and Antarctic Pseudorchomene plebs. J. Mar. Sci. Eng. 2023, 11, 1515. https://doi.org/10.3390/jmse11081515
Huang S, Zhu L, Jiang S, Zhai W, Pan B, Wu Z, Xu Q. Metal Accumulations in Two Extreme-Environment Amphipods, Hadal Eurythenes gryllus and Antarctic Pseudorchomene plebs. Journal of Marine Science and Engineering. 2023; 11(8):1515. https://doi.org/10.3390/jmse11081515
Chicago/Turabian StyleHuang, Shaojun, Lingyue Zhu, Shouwen Jiang, Wanying Zhai, Binbin Pan, Zhichao Wu, and Qianghua Xu. 2023. "Metal Accumulations in Two Extreme-Environment Amphipods, Hadal Eurythenes gryllus and Antarctic Pseudorchomene plebs" Journal of Marine Science and Engineering 11, no. 8: 1515. https://doi.org/10.3390/jmse11081515





