The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-qTOF-MS Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Design
2.3. Bacterial Culture
2.4. Lethal Dose (LD50) Bacterial Challenge
2.5. Sample Collection
2.6. Metabolite Extraction
2.7. LC–MS Sample Analysis Using LC-qTOF-MS
2.8. Mass Spectrometry Data Processing and Data Analysis
2.9. Metabolite Identification
2.10. Metabolic Biosynthetic Pathway Mapping
3. Results
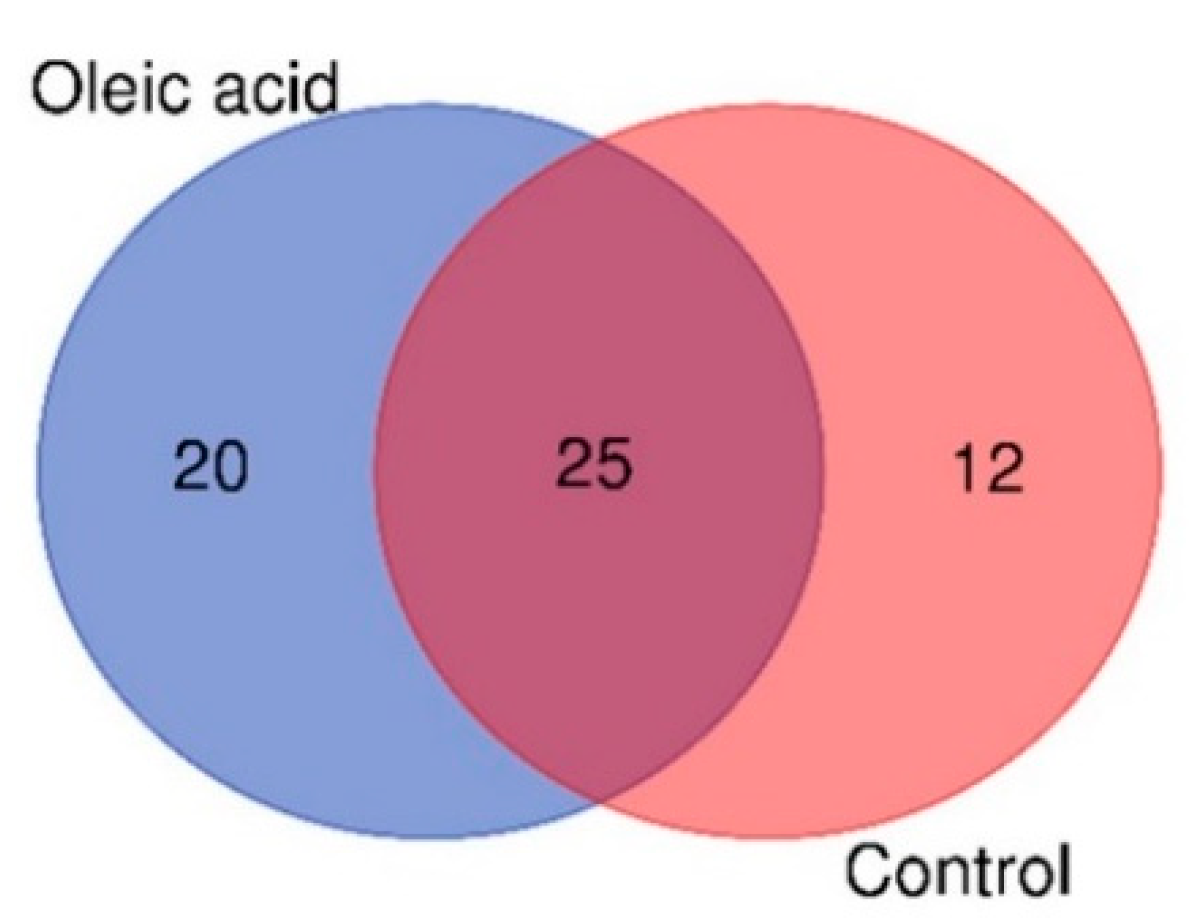
3.1. Metabolite Profile of the Liver of Surviving Infected Grouper Fed Control and Oleic Acid Diets
3.2. Metabolite Comparison between Control and Oleic Acid Diets
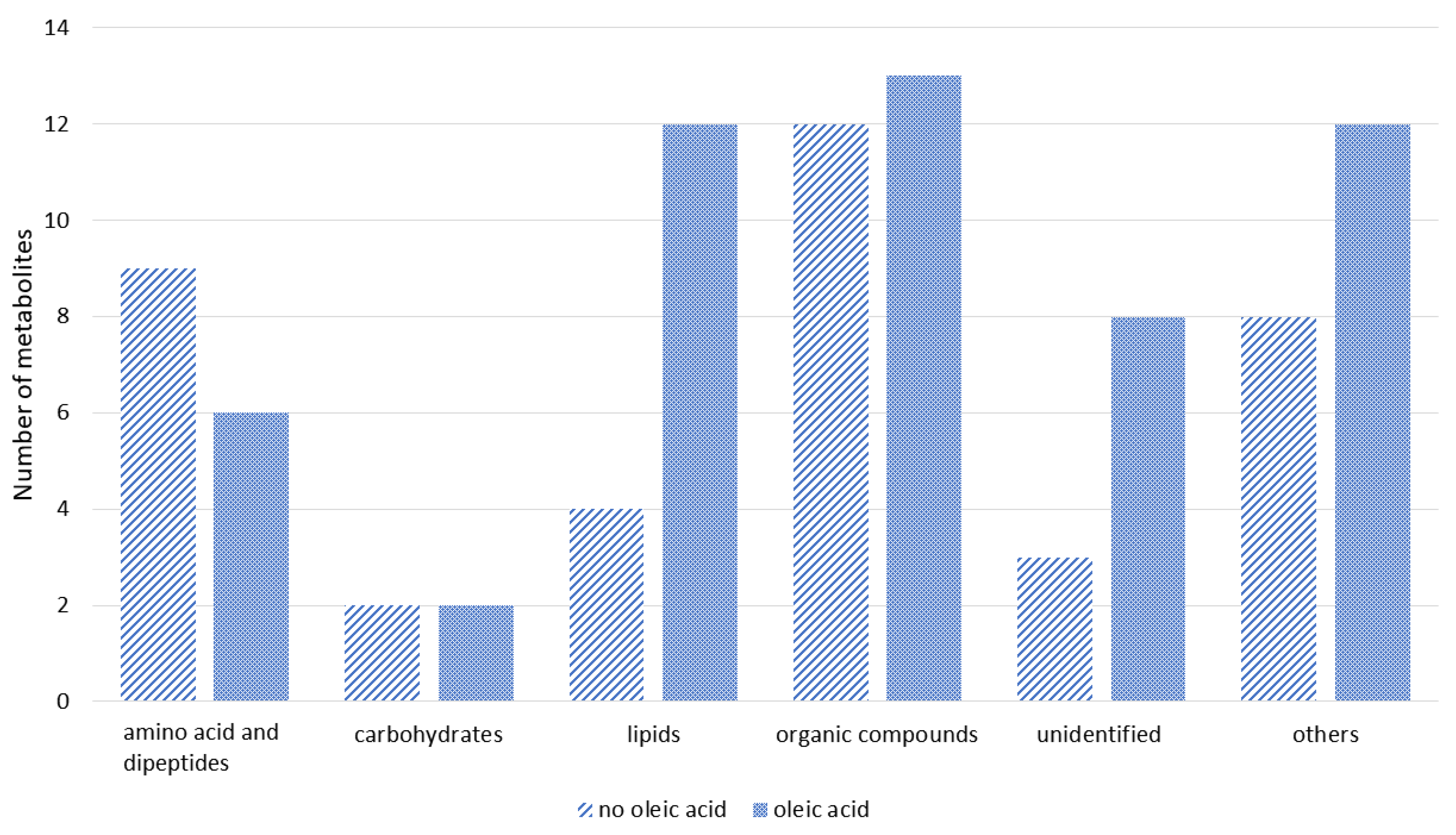
3.3. Identification and Classification of Metabolites
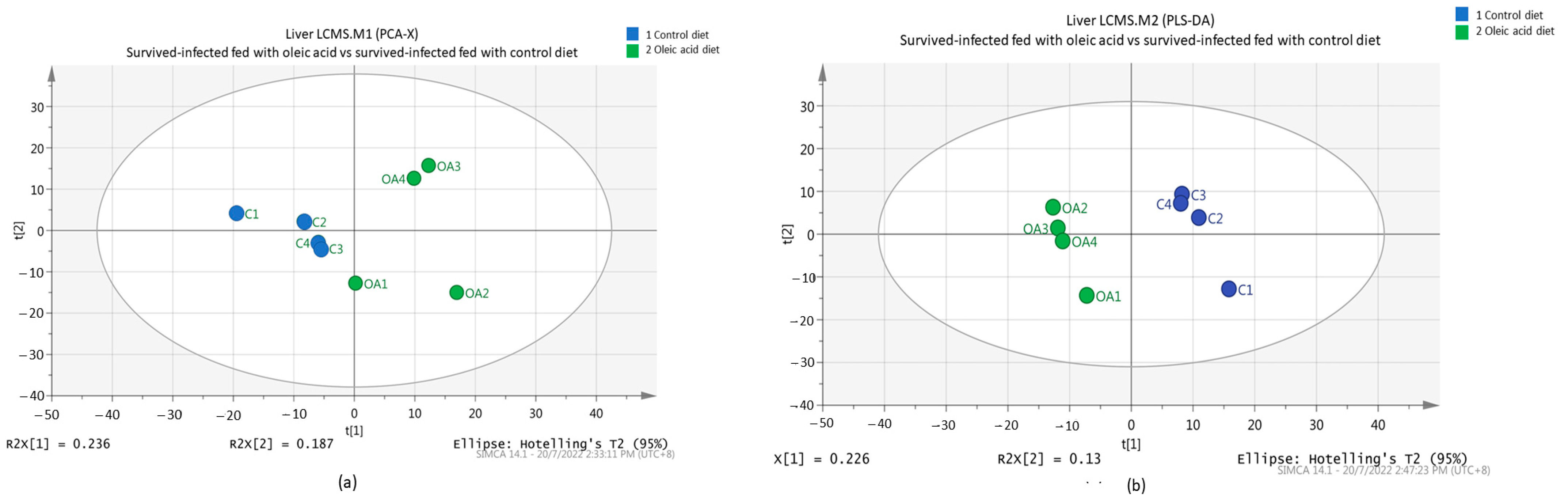
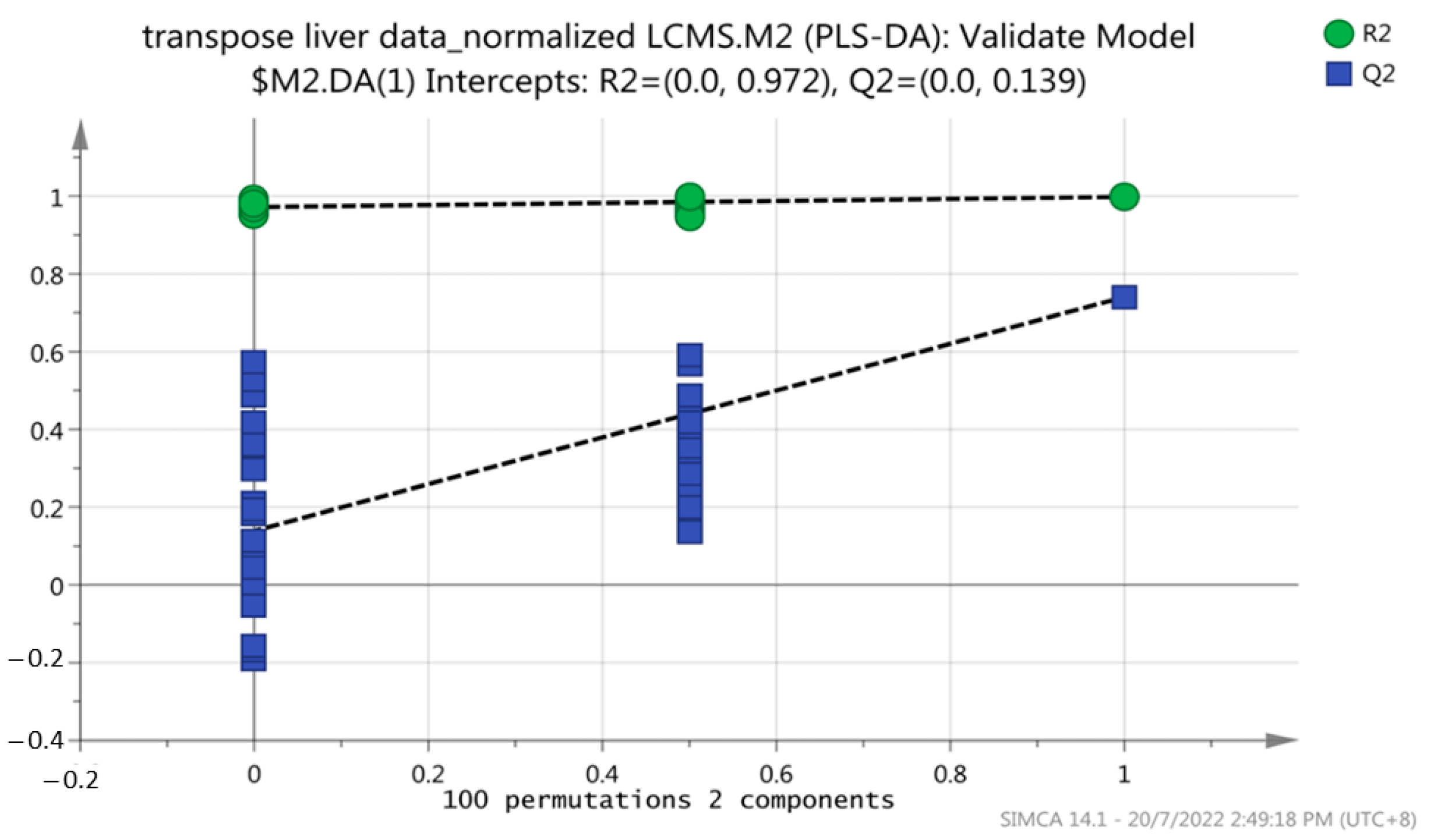
3.4. Metabolic Pattern Analysis Using Multivariate Statistical Analysis (MVA) between Grouper Fed Oleic Acid and Control Diets
3.5. PLS-DA Loading Plot and Variable Importance on Projection (VIP) Score Plot
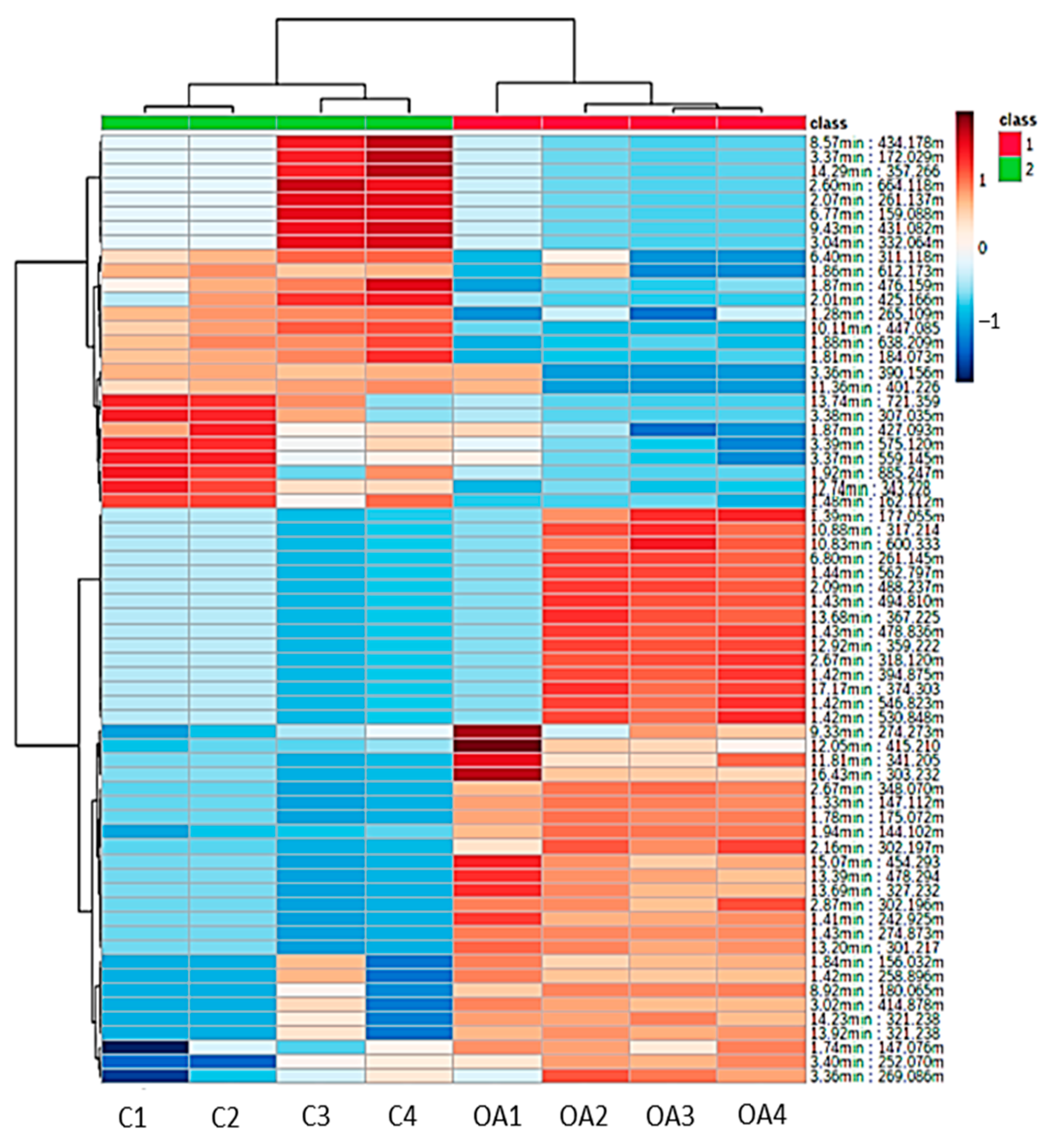
3.6. Hierarchical Clustering Analysis of Metabolites (Heatmap)
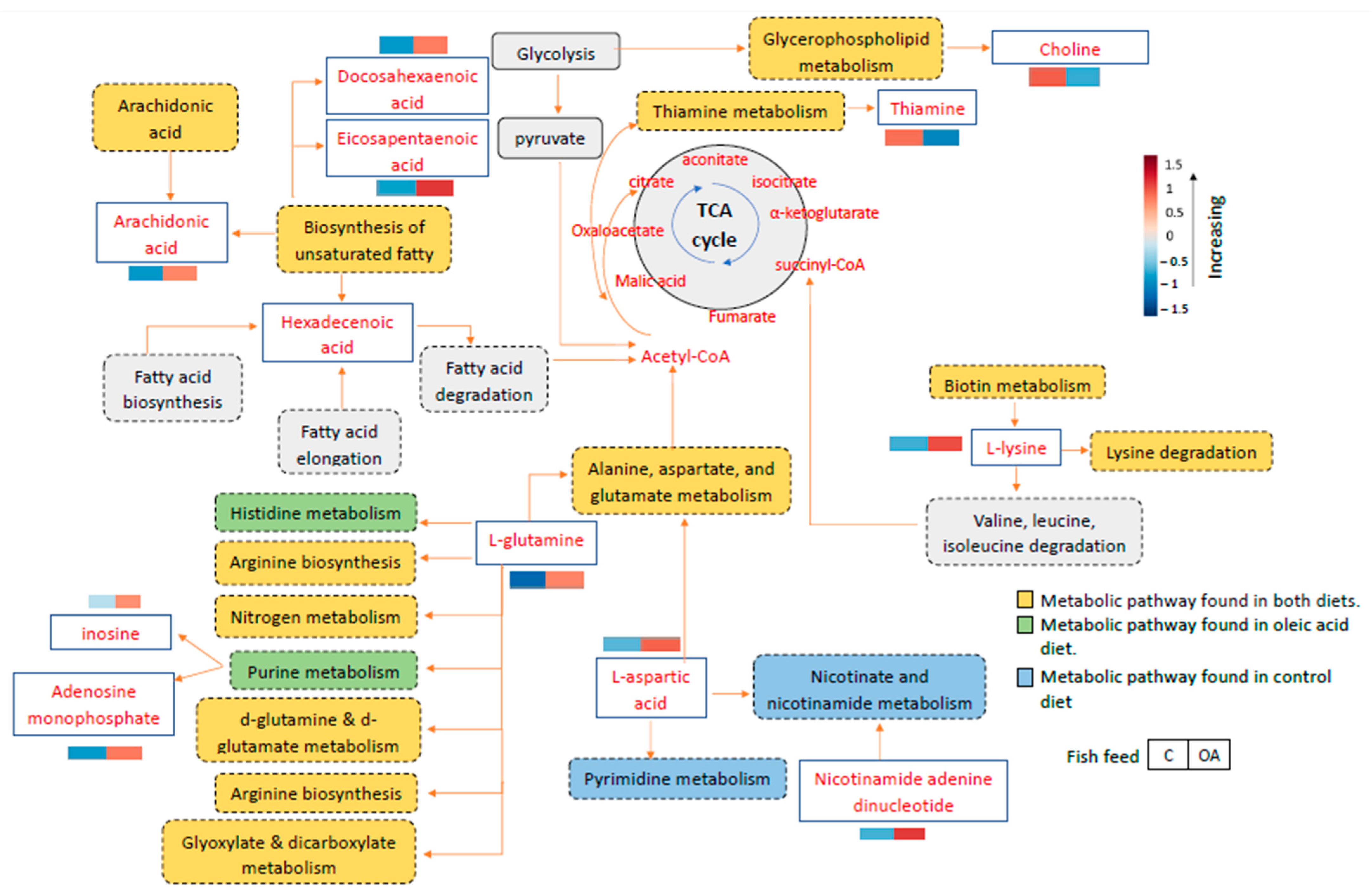
3.7. Mapping of Metabolic Pathway Enrichment of Grouper Immunity Infected with Vibriosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PEMSEA. Partnerships in Environmental Management for the Seas of East Asia; PEMSEA: Kalakhang Maynila, Philippines, 2017. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO Statistic; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Chong, R.; Bousfield, B.; Brown, R. Fish disease management. Vet. Bull. Agric. Fish. Conserv. Dep. Newsl. 2011, 1, 8. [Google Scholar]
- Abd El-Galil, M.A.A.; Mohamed, M.H. First Isolation of Vibrio alginolyticus from Ornamental Bird Wrasse Fish (Gomphosus caeruleus) of the Red Sea in Egypt. J. Fish. Aquat. Sci. 2012, 7, 461–467. [Google Scholar] [CrossRef][Green Version]
- Liao, J.D.; Leano, I.C. The Aquaculture of Groupers; Asian Fisheries Society: Selangor, Malaysia, 2008. [Google Scholar]
- Amalina, N.Z.; Santha, S.; Zulperi, D.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Ina-Salwany, M.Y. Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp. isolated from cultured groupers in Peninsular Malaysia. BMC Microbiol. 2019, 19, 251. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Chen, H.; Liu, S.; Guo, Z.; Cheng, C.; Ma, H.; Feng, J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci. Rep. 2020, 10, 14329. [Google Scholar] [CrossRef]
- Achmad, M.J.; Isnansetyo, A.; Andriani, R.; Samman, A.; Marus, I. The analysis of challenges test of catfish (Clarias sp.) with fatty acid compounds from starfish (Acantaster planci). IOP Conf. Ser. Earth Environ. Sci. 2020, 584, 012014. [Google Scholar] [CrossRef]
- Achmad, M.J.; Isnansetyo, A.; Hasanah, N.; Ustadi, U.U. Macrophage Immunomodulatory Activity of Unsaturated Fatty Acid Isolated from the Crown-of-thorns Star Fish (Acanthaster planci). Pharmacogn. J. 2018, 10, 951–957. [Google Scholar] [CrossRef]
- Schmitt, P.; Wacyk, J.; Morales-Lange, B.; Rojas, V.; Guzmán, F.; Dixon, B.; Mercado, L. Immunomodulatory effect of cathelicidins in response to a β-glucan in intestinal epithelial cells from rainbow trout. Dev. Comp. Immunol. 2015, 51, 160–169. [Google Scholar] [CrossRef]
- Sherif, A.H.; Mahfouz, M.E. Immune status of Oreochromis niloticus experimentally infected with Aeromonas hydrophila following feeding with 1, 3 β-glucan and levamisole immunostimulants. Aquaculture 2019, 509, 40–46. [Google Scholar] [CrossRef]
- Guluarte, C.; Reyes-Becerril, M.; Gonzalez-Silvera, D.; Cuesta, A.; Angulo, C.; Esteban, M.Á. Probiotic properties and fatty acid composition of the yeast Kluyveromyces lactis M3. In vivo immunomodulatory activities in gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2019, 94, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Natnan, M.E.; Low, C.-F.; Chong, C.-M.; Bunawan, H.; Baharum, S.N. Integration of Omics Tools for Understanding the Fish Immune Response Due to Microbial Challenge. Front. Mar. Sci. 2021, 8, 668771. [Google Scholar] [CrossRef]
- Du, C.; Yang, M.; Li, M.-Y.; Yang, J.; Peng, B.; Li, H.; Peng, X. Metabolic Mechanism for l-Leucine-Induced Metabolome To Eliminate Streptococcus iniae. J. Proteome Res. 2017, 16, 1880–1889. [Google Scholar] [CrossRef]
- Nurdalila, A.A.; Natnan, M.E.; Baharum, S.N. The effects of amino acids and fatty acids on the disease resistance of Epinephelus fuscoguttatus in response to Vibrio vulnificus infection. 3 Biotech 2020, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Padra, J.T.; Sundh, H.; Sundell, K.; Venkatakrishnan, V.; Jin, C.; Samuelsson, T.; Karlsson, N.G.; Lindén, S.K. Aeromonas salmonicida Growth in Response to Atlantic Salmon Mucins Differs between Epithelial Sites, Is Governed by Sialylated and N-Acetylhexosamine-Containing O -Glycans, and Is Affected by Ca2+. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Jiang, M.; Gong, Q.; Lai, S.; Cheng, Z.; Chen, Z.; Zheng, J.; Peng, B. Phenylalanine enhances innate immune response to clear ceftazidime-resistant Vibrio alginolyticus in Danio rerio. Fish Shellfish Immunol. 2019, 84, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Jouneau, L.; Tacchi, L.; Macqueen, D.J.; Alzaid, A.; Secombes, C.J.; Martin, S.A.M.; Boudinot, P. Disparate developmental patterns of immune responses to bacterial and viral infections in fish. Sci. Rep. 2015, 5, 15458. [Google Scholar] [CrossRef]
- Low, C.-F.; Rozaini, M.Z.H.; Musa, N.; Syarul Nataqain, B. Current knowledge of metabolomic approach in infectious fish disease studies. J. Fish Dis. 2017, 40, 1267–1277. [Google Scholar] [CrossRef]
- Natnan, M.E.; Low, C.F.; Chong, C.M.; Daud, N.I.N.A.A.; Om, A.D.; Baharum, S.N. Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology 2022, 11, 1288. [Google Scholar] [CrossRef]
- Nurdalila, A.A.; Mayalvanan, Y.; Baharum, S.N. Metabolite profiling of Epinephelus fuscoguttatus infected with vibriosis reveals Omega 9 as potential metabolite biomarker. Fish Physiol. Biochem. 2019, 45, 1203–1215. [Google Scholar] [CrossRef]
- Mayalvanan, Y. Metabolomics Study of the Response of Epinephelus Fuscoguttatus towards Vibriosis. Master’s Dissertation, Universiti Kebangsaan Malaysia, Selangor, Malaysia, 2019. [Google Scholar]
- Wu, H.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef]
- Ahmad, R.; Lim, C.K.; Marzuki, N.F.; Goh, Y.-K.; Azizan, K.A.; Goh, Y.K.; Goh, K.J.; Ramzi, A.B.; Baharum, S.N. Metabolic Profile of Scytalidium parasiticum-Ganoderma boninense Co-Cultures Revealed the Alkaloids, Flavonoids and Fatty Acids that Contribute to Anti-Ganoderma Activity. Molecules 2020, 25, 5965. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-G.; Won, S.-R.; Rhee, H.-I. Oleic Acid and Inhibition of Glucosyltransferase. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1375–1383. [Google Scholar]
- Firdaus, R.F.; Lim, L.S.; Kawamura, G.; Shapawi, R. Assessment on the acceptability of hybrid grouper, Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂ to soybeanmeal-based diets. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2016, 9, 284–290. [Google Scholar]
- Mohd Faudzi, N.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2018, 49, 431–441. [Google Scholar] [CrossRef]
- Adekoya, A.; Porcadilla, M.; Varga, D.; Kucska, B. Replacing fish meal with alternative protein sources in common carp’s feed. Acta Agrar. Kaposváriensis 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Ishak, W.M.W.; Katas, H.; Yuen, N.P.; Abdullah, M.A.; Zulfakar, M.H. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv. Transl. Res. 2019, 9, 418–433. [Google Scholar] [CrossRef]
- Fadjar, M.; Andajani, S.; Zaelani, K. Squid (Loligo edulis) ink raw extract as an anti– vibriosis substance in grouper (Epinephelus fuscoguttatus) juvenile culture infected by Vibrio alginolyticus. AACL Bioflux 2016, 9, 422–428. [Google Scholar]
- Reddy, K.V.K.; Naidu, K.A. Oleic acid, hydroxytyrosol and n -3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int. Immunopharmacol. 2016, 35, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Aggarwal, A. M2 macrophages and their role in rheumatic diseases. Rheumatol. Int. 2019, 39, 769–780. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, J.; Li, D.; Zhou, D.; Zhang, Y.; Wang, J.; Hu, B.; Ju, A.; Ye, Z. Nontargeted metabolomics approach for the differentiation of cultivation ages of mountain cultivated ginseng leaves using UHPLC/QTOF-MS. J. Pharm. Biomed. Anal. 2017, 141, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Baharum, S.N.; Mayalvanan, Y.; Natnan, M.E.; Azizan, K.A.; Bunawan, H.; Him, N.R.N.; Low, C.-F.; Chong, C.-M. LC–qTOF-MS analysis of fish immune organs reveals the distribution of amino acids in response to metabolic adaptation of the survival phenotype in grouper against Vibrio infection. 3 Biotech 2022, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.C.; Wade, N.M.; de Mello, P.H.; Rodrigues-Filho, J.d.A.; Garcia, C.E.O.; de Campos, M.F.; Botwright, N.A.; Hashimoto, D.T.; Moreira, R.G. Characterization of lipid metabolism genes and the influence of fatty acid supplementation in the hepatic lipid metabolism of dusky grouper (Epinephelus marginatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 219–220, 1–9. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Zheng, S.; Wu, G. Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids 2017, 49, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef]
- Iser, I.C.; Bracco, P.A.; Gonçalves, C.E.I.; Zanin, R.F.; Nardi, N.B.; Lenz, G.; Battastini, A.M.O.; Wink, M.R. Mesenchymal Stem Cells From Different Murine Tissues Have Differential Capacity to Metabolize Extracellular Nucleotides. J. Cell Biochem. 2014, 115, 1673–1682. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, D.; Jiang, M.; Zheng, J.; Peng, B. L-aspartic acid promotes fish survival against Vibrio alginolyticus infection through nitric oxide-induced phagocytosis. Fish Shellfish Immunol. 2020, 97, 359–366. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Kuang, S.; Tang, L.; Zhou, X. Lysine deficiency impaired growth performance and immune response and aggravated inflammatory response of the skin, spleen and head kidney in grown-up grass carp (Ctenopharyngodon idella). Anim. Nutr. 2021, 7, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, W.; Chi, S.; Mai, K.; Song, F.; Wang, L. Effects of dietary lysine on regulating GH-IGF system, intermediate metabolism and immune response in largemouth bass (Micropterus salmoides). Aquac. Rep. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Dietary lysine modulates growth performance, haemato-biochemical indices, non-specific immune response, intestinal enzymatic activities and antioxidant properties of rainbow trout, Oncorhynchus mykiss fingerlings. Aquac. Nutr. 2021, 27, 124–139. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, Z.; Li, H.; Zhang, T.; Yang, M.; Peng, X.; Peng, B. Succinate and inosine coordinate innate immune response to bacterial infection. PLoS Pathog. 2022, 18, e1010796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, M.; Peng, B.; Peng, X.; Li, H. Reduced ROS-mediated antibiotic resistance and its reverting by glucose in Vibrio alginolyticus. Environ. Microbiol. 2020, 22, 4367–4380. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.C.; Skrzynska, A.K.; Marques, V.H.; Tinajero, A.; Del Rio-Zaragoza, O.B.; Viana, M.T.; Mata-Sotres, J.A. Dietary Arachidonic Acid (20:4n-6) Levels and Its Effect on Growth Performance, Fatty Acid Profile, Gene Expression for Lipid Metabolism, and Health Status of Juvenile California Yellowtail (Seriola dorsalis). Fishes 2022, 7, 185. [Google Scholar] [CrossRef]
- Reddy, K.V.K.; Naidu, K.A. Maternal and neonatal dietary intake of balanced n-6/n-3 fatty acids modulates experimental colitis in young adult rats. Eur. J. Nutr. 2016, 55, 1875–1890. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef]
- Araújo, B.C.; Flores-Galvez, K.; Honji, R.M.; Barbosa, V.M.; Viana, M.T.; Tinajero, A.; Mata-Sotres, J.A. Arachidonic acid effects on the overall performance, fatty acid profile, hepatopancreas morphology and lipid-relevant genes in Litopenaeus vannamei juveniles. Aquaculture 2020, 523, 735207. [Google Scholar] [CrossRef]
- Salini, M.J.; Wade, N.M.; Araújo, B.C.; Turchini, G.M.; Glencross, B.D. Eicosapentaenoic Acid, Arachidonic Acid and Eicosanoid Metabolism in Juvenile Barramundi Lates calcarifer. Lipids 2016, 51, 973–988. [Google Scholar] [CrossRef]
- Allen, F.; Greiner, R.; Wishart, D. Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 2015, 11, 98–110. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Alfaro, A.C.; Young, T.; Ravi, S.; Merien, F. Metabolomics Study of Immune Responses of New Zealand GreenshellTM Mussels (Perna canaliculus) Infected with Pathogenic Vibrio sp. Mar. Biotechnol. 2018, 20, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Ballantyne, R.; Huang, P.-L.; Ding, S.; Hong, M.-C.; Lin, T.-Y.; Wu, F.-C.; Xu, Z.-Y.; Chiu, K.; Chen, B.; et al. Sarcodia suae modulates the immunity and disease resistance of white shrimp Litopenaeus vannamei against Vibrio alginolyticus via the purine metabolism and phenylalanine metabolism. Fish Shellfish Immunol. 2022, 127, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Maha, I.F.; Xie, X.; Zhou, S.; Yu, Y.; Liu, X.; Zahid, A.; Lei, Y.; Ma, R.; Yin, F.; Qian, D. Skin metabolome reveals immune responses in yellow drum Nibea albiflora to Cryptocaryon irritans infection. Fish Shellfish Immunol. 2019, 94, 661–674. [Google Scholar] [CrossRef]

| Experimental Diets | ||
|---|---|---|
| Ingredients | Control | Oleic Acid |
| Fish meal | 11.5 | 11.5 |
| Soybean meal | 50.5 | 50.5 |
| Corn flour | 24.0 | 24.0 |
| Vegetable oil | 7.5 | 7.5 |
| Mineral mix a | 2.0 | 2.0 |
| Vitamin mix b | 2.0 | 2.0 |
| α-Cellulose c | 2.5 | 0.5 |
| Oleic acid d (immunostimulant) | 0.0 | 2.0 a |
| Proximate composition e | ||
| Crude carbohydrate | 45.7 | 43.7 |
| Crude protein | 31.3 | 31.4 |
| Crude lipid | 9.4 | 11.4 |
| Crude ash | 6.9 | 6.7 |
| Moisture | 6.7 | 6.8 |
| Energy (kcl/100 g) | 392 | 403 |
| Control Diet | Oleic Acid Diet | |
|---|---|---|
| Saturated fatty acids | ||
| C4:0 | 0.19 | 0.65 |
| C8:0 | 0.58 | 0.81 |
| C10:0 | 0.51 | 0.76 |
| C11:0 | 0.54 | 1.00 |
| C12:0 | 3.41 | 15.11 |
| C13:0 | 0.00 | 1.06 |
| C14:0 | 19.45 | 30.39 |
| C15:0 | 5.55 | 7.61 |
| C16:0 | 947.56 | 877.36 |
| C17:0 | 9.14 | 10.34 |
| C18:0 | 204.02 | 221.62 |
| C20:0 | 49.43 | 48.15 |
| C22:0 | 21.27 | 23.25 |
| C23:0 | 14.70 | 23.58 |
| C24:0 | 32.13 | 83.99 |
| Total | 1308.47 | 1345.68 |
| Monounsaturated fatty acids | ||
| C14:1 | 1.39 | 2.77 |
| C15:1 | 0.97 | 1.69 |
| C16:1 | 32.01 | 40.31 |
| C17:1 | 7.67 | 13.04 |
| C18:1n9 cis | 3144.73 | 4536.41 |
| C20:1n9 | 46.22 | 57.29 |
| C22:1n9 | 2.32 | 6.63 |
| C24:1 | 13.83 | 135.28 |
| Total | 3249.15 | 4793.43 |
| Polyunsaturated fatty acids | ||
| C18:2n6 cis | 4633.96 | 5001.67 |
| C18:3n6 | 10.60 | 7.23 |
| C18:3n3 | 116.47 | 134.44 |
| C20:2 | 3.34 | 4.96 |
| C20:3n3 | 8.41 | 16.99 |
| C20:4n6 | 4.77 | 5.67 |
| C20:5n3 | 15.11 | 23.45 |
| C22:2 | 10.82 | 7.62 |
| C22:6n3 | 38.89 | 58.86 |
| Total | 4842.38 | 5260.89 |
| No | rT (min) | m/z Value | p-Value | VIP Score | Possible Identity Metabolites | Molecular Formula | Classification | Metabolite Found in C or OA Diets |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.94 | 144.102 | 2.91 × 10−6 | 2.12187 | L-2-Amino-3-methylenehexanoic acid | C7H13NO2 | Organic compound | C and OA |
| 2 | 1.88 | 638.209 | 5.01 × 10−6 | 2.11906 | Glu Tyr Cys Met Ala | C25H37N5O9S2 | Peptides | C and OA |
| 3 | 12.74 | 343.228 | 3.09 × 10−6 | 2.11784 | Docosahexaenoic Acid | C22H30O3 | Fatty acid | OA |
| 4 | 1.78 | 175.072 | 3.30 × 10−6 | 2.11728 | Formimino-L-glutamic acid | C6H10N2O4 | Organic compound | OA |
| 5 | 1.43 | 274.873 | 3.66 × 10−6 | 2.11725 | gamma-Pentachlorocyclohexene | C6H5Cl5 | Organic compound | OA |
| 6 | 2.67 | 348.070 | 5.74 × 10−6 | 2.11203 | Adenosine monophosphate | C10H14N5O7P | Nucleotide | OA |
| 7 | 13.20 | 301.217 | 8.67 × 10−6 | 2.10981 | Retinoic acid | C20H28O2 | Organic compound | OA |
| 8 | 1.81 | 184.073 | 2.36 × 10−5 | 2.09841 | Phosphocholine | C5H14NO4P | Organic compound | C and OA |
| 9 | 10.11 | 447.085 | 2.04 × 10−5 | 2.09709 | Mollicellin E | C22H19ClO8 | Organic acid | C |
| 10 | 1.41 | 242.925 | 3.01 × 10−5 | 2.09427 | 2-Iodophenol | C6H5IO | Hydrocarbon | OA |
| 11 | 13.39 | 478.294 | 5.97 × 10−5 | 2.08286 | Lysophosphatidylethanolamine(18:2) | C23H44NO7P | Glycerophospholipid | OA |
| 12 | 13.69 | 327.232 | 6.64 × 10−5 | 2.08066 | 4,5-dehydro Docosahexaenoic Acid | C22H30O2 | Fatty acid | OA |
| 13 | 15.07 | 454.293 | 0.00010 | 2.07141 | Glycerophospho-N-Palmitoyl Ethanolamine | C21H44NO7P | Glycerophospholipids | OA |
| 14 | 1.33 | 147.112 | 0.00051 | 2.03694 | L-Lysine | C6H14N2O2 | Amino acid | C and OA |
| 15 | 1.48 | 162.112 | 0.00113 | 2.00266 | L-Carnitine | C7H15NO3 | Amino acid | C and OA |
| 16 | 1.28 | 265.109 | 0.00084 | 1.99468 | Thiamine | C12H16N4OS | Pyrimidine | C and OA |
| 17 | 11.81 | 341.205 | 0.00107 | 1.98498 | Leukotriene A4 | C20H30O3 | Fatty acid | OA |
| 18 | 8.92 | 180.065 | 0.00150 | 1.97575 | Hippuric acid | C9H9NO3 | Organic compound | C and OA |
| 19 | 16.43 | 303.232 | 0.00150 | 1.96799 | Eicosapentaenoic Acid | C20H30O2 | Fatty acid | OA |
| 20 | 1.87 | 476.159 | 0.00184 | 1.9441 | L-Isobutanol | C21H27NO10 | Organic compound | C and OA |
| 21 | 14.23 | 321.238 | 0.00488 | 1.89812 | 18-hydroxy-arachidonic acid | C20H32O3 | Fatty acid | C and OA |
| 22 | 6.40 | 311.118 | 0.00394 | 1.88328 | 1-Pentadecanecarboxylic acid | C15H18O7 | Fatty acid | C and OA |
| 23 | 13.92 | 321.238 | 0.00684 | 1.86482 | 18-hydroxy-arachidonic acid | C20H32O3 | Fatty acid | C and OA |
| 24 | 3.02 | 414.878 | 0.01087 | 1.82293 | Bromophos-ethyl | C10H12BrCl2O3 | Organic acid | C and OA |
| 25 | 12.05 | 415.210 | 0.01074 | 1.80898 | Lys His Met | C17H30N6O4S1 | Peptides | C and OA |
| 26 | 2.01 | 425.166 | 0.01268 | 1.75605 | Glu Asn Tyr | C18H24N4O8 | Peptides | C |
| 27 | 1.84 | 156.032 | 0.02399 | 1.716 | 4-Nitrocatechol | C6H5NO4 | Phenol | C and OA |
| 28 | 1.74 | 147.076 | 0.01974 | 1.70444 | L-Glutamine | C5H10N2O3 | Amino acid | C and OA |
| 29 | 1.86 | 612.173 | 0.01889 | 1.70281 | Cyanidin 3-glucogalactoside | C27H31O16 | Organic compound | C and OA |
| 30 | 1.92 | 885.247 | 0.02990 | 1.67622 | Salviamalvin | C42H44O21 | polyketides | C |
| Metabolic Pathway | Total | p-Value | −log 10 (p) | FDR | Impact Value |
|---|---|---|---|---|---|
| Biosynthesis of unsaturated fatty acids | 35 | 0.00024881 | 3.6041 | 0.0209 | 0 |
| Purine metabolism | 66 | 0.023424 | 1.6303 | 0.754 | 0.05452 |
| Alanine, aspartate and glutamate metabolism | 27 | 0.026928 | 1.5698 | 0.754 | 0.13296 |
| D-Glutamine and D-glutamate metabolism | 6 | 0.057147 | 1.243 | 0.79645 | 0 |
| Nitrogen metabolism | 6 | 0.057147 | 1.243 | 0.79645 | 0 |
| Pyrimidine metabolism | 41 | 0.058204 | 1.235 | 0.79645 | 0.02446 |
| Thiamine metabolism | 7 | 0.066371 | 1.178 | 0.79645 | 0 |
| Aminoacyl-tRNA biosynthesis | 48 | 0.077021 | 1.1134 | 0.80872 | 0 |
| Biotin metabolism | 10 | 0.093544 | 1.029 | 0.87307 | 0 |
| Arginine biosynthesis | 14 | 0.12863 | 0.89065 | 0.96043 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natnan, M.E.; Low, C.-F.; Chong, C.-M.; Rungrassamee, W.; Baharum, S.N. The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-qTOF-MS Approach. J. Mar. Sci. Eng. 2023, 11, 1563. https://doi.org/10.3390/jmse11081563
Natnan ME, Low C-F, Chong C-M, Rungrassamee W, Baharum SN. The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-qTOF-MS Approach. Journal of Marine Science and Engineering. 2023; 11(8):1563. https://doi.org/10.3390/jmse11081563
Chicago/Turabian StyleNatnan, Maya Erna, Chen-Fei Low, Chou-Min Chong, Wanilada Rungrassamee, and Syarul Nataqain Baharum. 2023. "The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-qTOF-MS Approach" Journal of Marine Science and Engineering 11, no. 8: 1563. https://doi.org/10.3390/jmse11081563
APA StyleNatnan, M. E., Low, C.-F., Chong, C.-M., Rungrassamee, W., & Baharum, S. N. (2023). The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-qTOF-MS Approach. Journal of Marine Science and Engineering, 11(8), 1563. https://doi.org/10.3390/jmse11081563







