A Possible Synergistic Approach: Case Study of Saccharina latissima Extract and Nitrifying Bacteria in Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Harvesting and Processing
2.2. Extract Preparation
2.3. Experimental Conditions
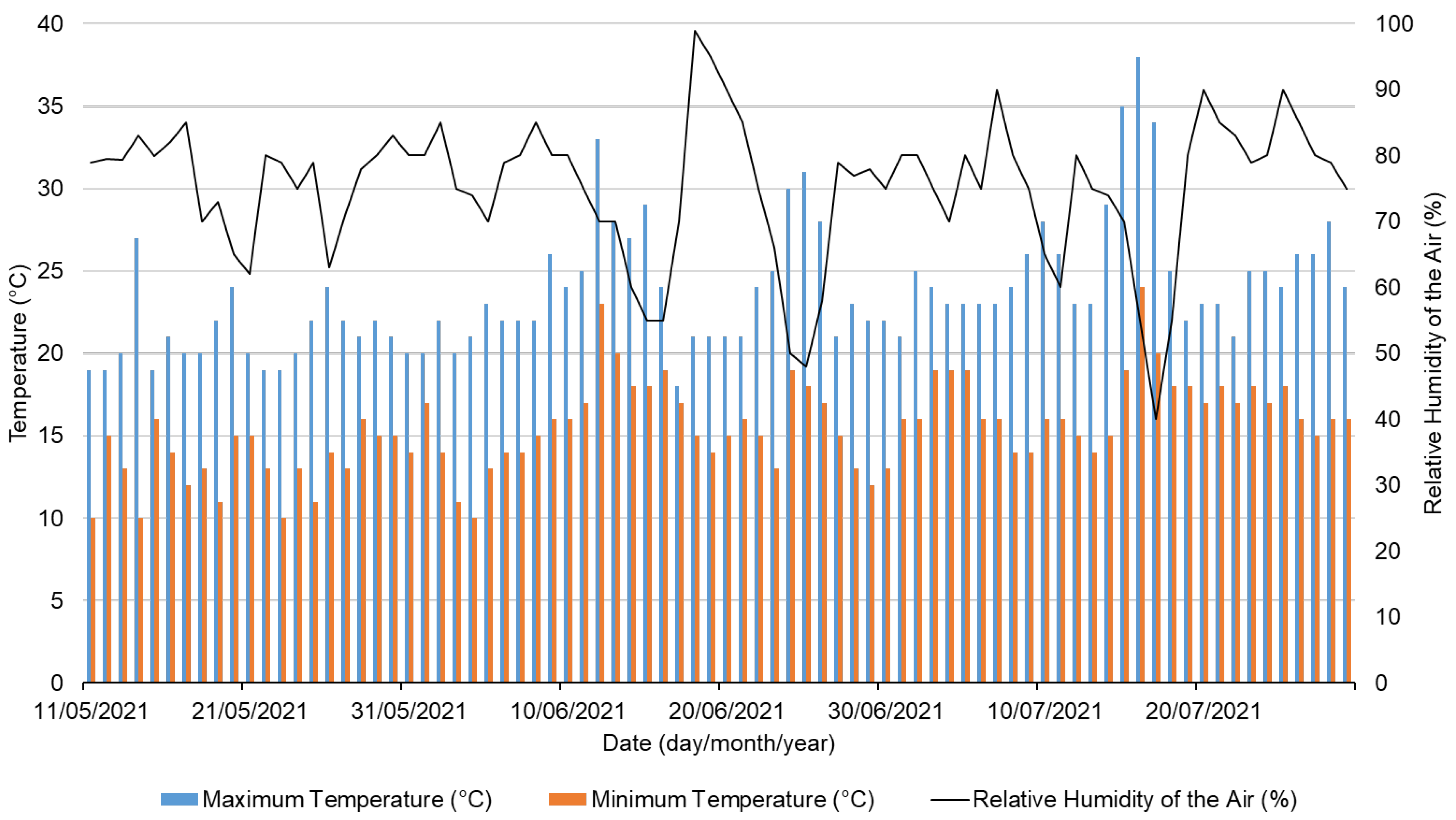
2.4. Abiotic Parameters during the Experiment
2.5. Algal Biomass and Extract Characterization
2.5.1. pH, Electrical Conductivity, and TDS Extract Analysis
2.5.2. Moisture and Ash Content
2.5.3. Crude Lipids
2.5.4. Total Protein
2.5.5. Crude Fiber and Total Carbohydrates/Nitrogen-Free Extractives
2.5.6. Energy
2.5.7. Mineral and Trace Element Characterization
2.5.8. Total Phenolic Compounds Quantification
2.5.9. Alginate Extraction
2.6. Biometric and Biochemical Lettuce’s Characterization
2.6.1. Growth Parameters, Moisture and Ashes Content
2.6.2. Total Nitrogen/Protein
2.6.3. Mineral and Trace element Characterization
2.7. Substrate Characterization
2.8. Statistical Analysis
3. Results
3.1. Algal Biomass and Extract Characterization
3.2. Extracts’ Physical-Chemical Characterization
3.3. Substrate Characterization
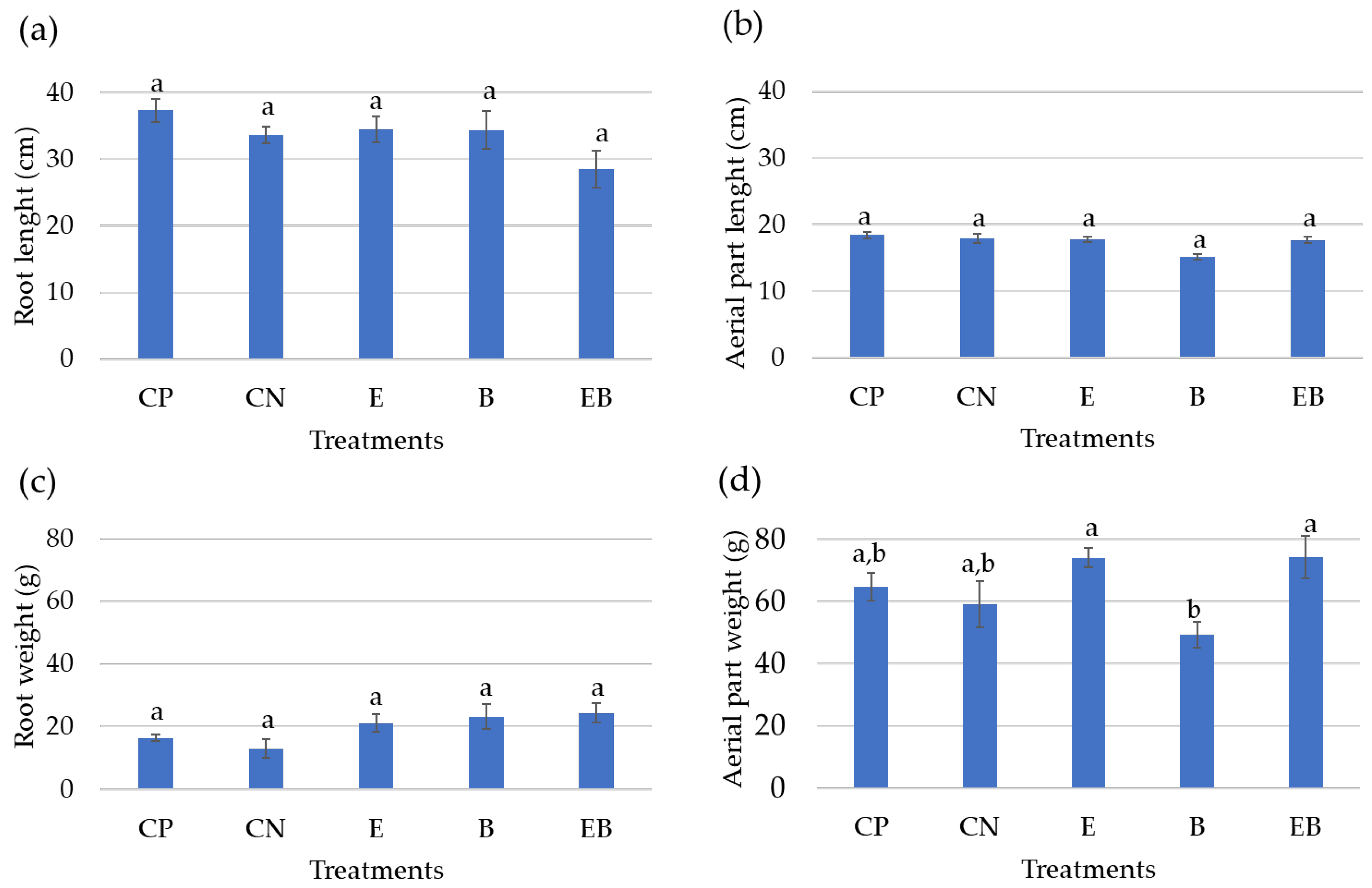
3.4. Biometric Lettuce’s Characterization
3.5. Biochemical Lettuce’s Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the economic and nutritional power of vegetables. Glob. Food Sec. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture–Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.; Snapp, S. What was sustainable intensification? Views from experts. Land Use Policy 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Ammaturo, C.; Pacheco, D.; Cotas, J.; Formisano, L.; Ciriello, M.; Pereira, L.; Bahcevandziev, K. Use of Chlorella vulgaris and Ulva lactuca as Biostimulant on Lettuce. Appl. Sci. 2023, 13, 9046. [Google Scholar] [CrossRef]
- Sousa, T.; Nunes, J.P.; Lopes, J.; Cotas, J.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweed as Plant Biostimulants. In Seaweed Biotechnology; Apple Academic Press: Boca Raton, FL, USA, 2022; pp. 183–200. [Google Scholar]
- Melo, P.C.; Sousa, T.; Teixeira, R.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds and Their Derivates as a Multirole Tool in Agriculture. In Seaweed Biotechnology; Apple Academic Press: Boca Raton, FL, USA, 2022; pp. 201–227. [Google Scholar]
- Mzibra, A.; Aasfar, A.; El Arroussi, H.; Khouloud, M.; Dhiba, D.; Kadmiri, I.M.; Bamouh, A. Polysaccharides extracted from Moroccan seaweed: A promising source of tomato plant growth promoters. J. Appl. Phycol. 2018, 30, 2953–2962. [Google Scholar] [CrossRef]
- Demir, N.; Dural, B.; Yildirim, K. Effect of Seaweed Suspensions on Seed Germination of Tomato, Pepper and Aubergine. J. Biol. Sci. 2006, 6, 1130–1133. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol. 2019, 31, 2025–2037. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Hernández Carmona, G. Seaweed as potential plant growth stimulants for agriculture in Mexico. Hidrobiológica 2018, 28, 129–140. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Sousa, T.; Cotas, J.; Bahcevandziev, K.; Pereira, L. Effects of “sargaço” extraction residues on seed germination. Millenium 2020, 2, 29–37. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Dmytryk, A.; Schroeder, G.; Chojnacka, K. The Application of Homogenate and Filtrate from Baltic Seaweeds in Seedling Growth Tests. Appl. Sci. 2017, 7, 230. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt Stress Effects on Avocado (Persea americana Mill.) Plants with and without Seaweed Extract (Ascophyllum nodosum) Application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef]
- Bharath, B.; Nirmalraj, S.; Mahendrakumar, M.; Perinbam, K. Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo. Asian Pacific J. Reprod. 2018, 7, 27. [Google Scholar] [CrossRef]
- van den Burg, S.; Selnes, T.; Alves, L.; Giesbers, E.; Daniel, A. Prospects for upgrading by the European kelp sector. J. Appl. Phycol. 2021, 33, 557–566. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Zhang, X.; Boderskov, T.; Bruhn, A.; Thomsen, M. Blue growth and bioextraction potentials of Danish Saccharina latissima aquaculture—A model of eco-industrial production systems mitigating marine eutrophication and climate change. Algal Res. 2022, 64, 102686. [Google Scholar] [CrossRef]
- Marinho, G.S.; Holdt, S.L.; Birkeland, M.J.; Angelidaki, I. Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. J. Appl. Phycol. 2015, 27, 1963–1973. [Google Scholar] [CrossRef]
- Fossberg, J.; Forbord, S.; Broch, O.J.; Malzahn, A.M.; Jansen, H.; Handå, A.; Førde, H.; Bergvik, M.; Fleddum, A.L.; Skjermo, J.; et al. The Potential for Upscaling Kelp (Saccharina latissima) Cultivation in Salmon-Driven Integrated Multi-Trophic Aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 418. [Google Scholar] [CrossRef]
- Araújo, G.S.; Morais, T.; Cotas, J.; García-Poza, S.; Silva, J.W.A.; Gonçalves, A.M.M.; Pereira, L. A Road to the Sustainable Seaweed Aquaculture. In Sustainable Global Resources of Seaweeds Volume 1; Springer International Publishing: Cham, Switzerland, 2022; pp. 63–73. [Google Scholar]
- Monteiro, P.; Cotas, J.; Pacheco, D.; Figueirinha, A.; da Silva, G.J.; Pereira, L.; Gonçalves, A.M.M. Seaweed as Food: How to Guarantee Their Quality? In Sustainable Global Resources of Seaweeds Volume 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 309–321. [Google Scholar]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Miranda, G.; Rocha, C.P.; Pato, R.L.; Cotas, J.; Gonçalves, A.M.M.; Santos, S.M.D.; Bahcevandziev, K.; Pereira, L. Portuguese Kelps: Feedstock Assessment for the Food Industry. Appl. Sci. 2021, 11, 10681. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Gonçalves, A.M.M.; Dias, J.M.; Pereira, L. Seaweeds Used in Wastewater Treatment: Steps to Industrial Commercialization. In Phycology-Based Approaches for Wastewater Treatment and Resource Recovery; Verma, P., Shah, M.P., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 247–262. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Murugan, A.; Rubavathi, A.; Visal, K.; Neginah, V. Efficacy of Methylobacterium oryzae Supplemented Sargassum wightii Seaweed Liquid Fertilizer on Chilly and Tomato Plant Growth. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kennedy, I. Non-symbiotic bacterial diazotrophs in crop-farming systems: Can their potential for plant growth promotion be better exploited? Soil Biol. Biochem. 2004, 36, 1229–1244. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A survey of Methylobacterium species and strains reveals widespread production and varying profiles of cytokinin phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Corteva. BlueN. Available online: https://www.corteva.pt/produtos-e-solucoes/protecao-de-cultivos/bluen.html#t3 (accessed on 21 July 2021).
- Pereira, L. MACOI. Available online: http://www.flordeutopia.pt/macoi/ (accessed on 21 July 2021).
- Pereira, L. Guia Ilustrado das Macroalgas: Conhecer e Reconhecer Algumas Espécies da Flora Portuguesa; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2009; ISBN 9789892603971. [Google Scholar]
- Currey, C.J. Lettuce and leafy greens 101: A production guide. Available online: https://www.producegrower.com/article/lettuce-and-leafy-greens-101-a-production-guide/ (accessed on 21 July 2021).
- Cunniff, P. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- FAO. Methods of food analysis. In Food Energy—Methods of Analysis and Conversion Factors; FAO: Rome, Italy, 2003; pp. 7–17. [Google Scholar]
- Lucas, M.D.; Sequeira, E.M. Determinação do Cu, Zn, Mn, Fe, Ca, Mg, K, e Na totais das plantas por espectrofotometria de absorção atómica e fotometria de chama. Pedologia 1976, 11, 163–169. [Google Scholar]
- Ribas, M.C.; Veiga, M.E.; Curto, A.; Oliveira, E.; Barbeitos, M.M.; Ferreira, M.; Pacheco, C.; Peralta, M.F.; Duarte, M. Métodos de análise de material vegetal e terras. Pedologia 1988, 11, 163–169. [Google Scholar]
- Laboratório Químico Agrícola Rebelo da Silva. Sector de Fertilidade do Solo; DGSA-Ministério da Agricultura: Lisbon, Portugal, 1977. [Google Scholar]
- Póvoas, I.; Barral, M.F. Métodos de Análise de Solos; Instituto de Investigação Científica Tropical: Lisbon, Portuga, 1992. [Google Scholar]
- Chapman, H.D. Soluble salts. In Methods of Soil Analyses Part 2 Chemical and Microbiological Properties; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clarck, F., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 933–951. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis Chemical and Microbiological Properies. Part 2; Black, A., Evans, D.D., White, J.L., Ensmingert, L.E., Clark, F.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1979; pp. 1149–1176. [Google Scholar]
- Sleutel, S.; De Neve, S.; Singier, B.; Hofman, G. Quantification of Organic Carbon in Soils: A Comparison of Methodologies and Assessment of the Carbon Content of Organic Matter. Commun. Soil Sci. Plant Anal. 2007, 38, 2647–2657. [Google Scholar] [CrossRef]
- Verardo, D.J.; Froelich, P.N.; McIntyre, A. Determination of organic carbon and nitrogen in marine sediments using the Carlo Erba NA-1500 analyzer. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1990, 37, 157–165. [Google Scholar] [CrossRef]
- Bikker, P.; Stokvis, L.; van Krimpen, M.M.; van Wikselaar, P.G.; Cone, J.W. Evaluation of seaweeds from marine waters in Northwestern Europe for application in animal nutrition. Anim. Feed Sci. Technol. 2020, 263, 114460. [Google Scholar] [CrossRef]
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Sterner, M.; Edlund, U. Multicomponent fractionation of Saccharina latissima brown algae using chelating salt solutions. J. Appl. Phycol. 2016, 28, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of alginate-extracted seaweed biomass for the development of cellulose-based packaging films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okçu, G.; Atak, M.; Çıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Belmehdi, O.; Bouyahya, A.; Skali Senhaji, N.; Abrini, J. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–160. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Carlson, W.H. pH Affects Seed Germination of Eight Bedding Plant Species. HortScience 1990, 25, 762–764. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of Salinity on Seed Germination and Early Seedling Stage. In Abiotic Stress in Plants; Fahad, S., Saud, S., Wu, C., Chen, Y., Wang, D., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Schneider, J.; Thiesen, L.; Engroff, T.; Holz, E.; Altíssimo, B. Growth analysis of lettuce under different substrate compositions. Adv. Hortic. Sci. 2018, 32, 221–227. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of crisphead ‘iceberg’ and romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Koudela, M.; Petříková, K. Nutrients content and yield in selected cultivars of leaf lettuce (Lactuca sativa L. var. crispa). Hortic. Sci. 2008, 35, 99–106. [Google Scholar] [CrossRef]
- Apdl-Administração dos Portos do Douro, Leixões e Viana do Castelo. Dragagem do Canal de Acesso aos Estaleiros Navais de Viana do Castelo; Apdl-Administração dos Portos do Douro, Leixões e Viana do Castelo: Lisboa, Portugal, 2017. [Google Scholar]
- Gusmão, A.G. Caraterização Química e Biológica de Lamas de Estações de Tratamento de Águas Residuais (ETAR´s); Escola Superior Agrária de Bragança: Bragança, Portugal, 2023. [Google Scholar]
- Rocha, A.C.S.; Teixeira, C.; Almeida, C.M.R.; Basto, M.C.P.; Reis-Henriques, M.A.; Guimarães, L.; Ferreira, M. Assessing contamination from maritime trade and transportation on Iberian waters: Impact on Platichthys flesus. Environ. Sustain. Indic. 2021, 9, 100098. [Google Scholar] [CrossRef]
- Ferreira, L.M.A. PRojeto de Unidade de Produção de Bivalves no Estuário do rio Lima Viana do Castelo. Direção Geral de Recursos Naturais Segurança e Serviços Maritimos: Viana do Castelo, Portugal, 2018. [Google Scholar]
- Samanta, P.; Jang, S.; Shin, S.; Kim, J.K. Effects of pH on growth and biochemical responses in Agarophyton vermiculophyllum under different temperature conditions. J. Appl. Phycol. 2020, 32, 499–509. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Yong, M.; Wang, S.; Cheng, F.; Li, Q.; Hu, J. Effects of alginic acid on radish growth and osmotic adjustment substance content under cadmium stress. IOP Conf. Ser. Earth Environ. Sci. 2020, 480, 012006. [Google Scholar] [CrossRef]
- USDA. Technical Evaluation Report of Alginic Acid Handling/ Processin; USDA: Washington DC, USA, 2015.
- Sangu, T.A.C. Potencial da Ulva rigida na Alimentação Humana e Animal. Bachelor’s Thesis, Escaola Agrária de Coimbra, Coimbra, Portugal, 2020. [Google Scholar]
- Rusu, T.; Moraru, P.I.; Mintas, O.S. Influence of environmental and nutritional factors on the development of lettuce (Lactuca sativa L.) microgreens grown in a hydroponic system: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12427. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of Pre-Harvest Supplemental UV-A/Blue and Red/Blue LED Lighting on Lettuce Growth and Nutritional Quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Battafarano, F.; da Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED Enhances Plant Performance and Both Carotenoids and Nitrates Profiles in Lettuce. Plant Foods Hum. Nutr. 2021, 76, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Huett, D. Growth, nutrient uptake and tipburn severity of hydroponic lettuce in response to electrical conductivity and K:Ca ratio in solution. Aust. J. Agric. Res. 1994, 45, 251. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Weerasinghe, P.A.; Weerakkody, W.A.P. Effect of eletric conductivity [EC] of the nutrient solution on nutrient uptake, growth and yield of leaf lettuce (Lactuca sativa L.) in stationary culture. Trop. Agric. Res. 2006, 18, 13–21. [Google Scholar]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in Macronutrient Content, Phytochemical Constitution and In Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.; Santamaria, P. Microgreens Production with Low Potassium Content for Patients with Impaired Kidney Function. Nutrients 2018, 10, 675. [Google Scholar] [CrossRef]
- Roosta, H.R. Interaction between water alkalinity and nutrient solution ph on the vegetative growth, chlorophyll fluorescence and leaf magnesium, iron, manganese, and zinc concentrations in lettuce. J. Plant Nutr. 2011, 34, 717–731. [Google Scholar] [CrossRef]
- Sefer, B.; Guuml lsuuml, S.M. The effects of drip line depths and irrigation levels on yield, quality and water use characteristics of lettuce under greenhouse condition. Afr. J. Biotechnol. 2011, 10, 3370–3379. [Google Scholar] [CrossRef]
- Pacheco, D. Seaweeds as Plant Health Promoters. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2022; p. 121.
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 07. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- White, P.J.; George, T.S.; Gregory, P.J.; Bengough, A.G.; Hallett, P.D.; McKenzie, B.M. Matching roots to their environment. Ann. Bot. 2013, 112, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Gallardo-Valdéz, M.; Pérez-Decelis, V.A.; Magdaleno-Armas, L.; Ochoa, I.; Lynch, J.P. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res. 2011, 121, 350–362. [Google Scholar] [CrossRef]
- Fitton, H.; Meyers, S.; Brooks, L.; Mulder, A.; Rolfe, M.; Baker, D.; Robinson, S. Effects of fucoidan from Fucus vesiculosus in reducing symptoms of osteoarthritis: A randomized placebo-controlled trial. Biol. Targets Ther. 2016, 2016, 81–88. [Google Scholar] [CrossRef]
- Klevay, L.M. Coronary heart disease: The zinc/copper hypothesis. Am. J. Clin. Nutr. 1975, 28, 764–774. [Google Scholar] [CrossRef]
- Pathak, P.; Kapil, U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J. Pediatr. 2004, 71, 1003–1005. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Prasad, D.M.R.; Bono, A. Mineral Content of Some Seaweeds from Sabah’s South China Sea. Asian J. Sci. Res. 2008, 1, 166–170. [Google Scholar] [CrossRef]
- Manganese-Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/#en1 (accessed on 21 July 2021).
- Marriott, B.P.; Birt, D.F.; Stallings, V.A.; Yates, A. Present Knowledge in Nutrition. Volume 1, Basic Nutrition and Metabolism; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Chen, P. Manganese metabolism in humans. Front. Biosci. 2018, 23, 4665. [Google Scholar] [CrossRef]
- PlantVillage Lettuce. Available online: https://plantvillage.psu.edu/topics/lettuce/infos (accessed on 21 July 2021).
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Symborg. BlueN. Available online: https://symborg.com/es/biofertilizantes/bluen/ (accessed on 21 July 2021).
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.M. The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Schauer, S.; Kutschera, U. A novel growth-promoting microbe, Methylobacterium funariae sp. nov., isolated from the leaf surface of a common moss. Plant Signal. Behav. 2011, 6, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, M.-Y.; Khan, N.; Tan, L.-L.; Yang, S. Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941. [Google Scholar] [CrossRef]
- Floro, R.D.; Lee, J.; Bogosian, G.; Bryant, D. Compositions and Methods for Improving Lettuce Production. Patent AU 2014360414 B2, 26 July 2016. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Reed, R.H.; Davison, I.R.; Chudek, J.A.; Foster, R. The osmotic role of mannitol in the Phaeophyta: An appraisal. Phycologia 1985, 24, 35–47. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Novembre, A.D.C.; Chamma, H.M.C.P. Seaweed extract improves the vigor and provides the rapid emergence of dry bean seeds. Am. J. Agric. Environ. Sci. 2013, 13, 1104–1107. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Osman, H.S. Effect of exogenous application of boric acid and seaweed extract on growth, biochemical content and yield of eggplant. J. Hortic. Sci. Ornam. Plants 2014, 6, 133–143. [Google Scholar] [CrossRef]
- Crouch, I.J.; Beckett, R.P.; van Staden, J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J. Appl. Phycol. 1990, 2, 269–272. [Google Scholar] [CrossRef]
- Möller, M.; Smith, M.L. The significance of the mineral component of seaweed suspensions on lettuce (Lactuca sativa L.) seedling growth. J. Plant Physiol. 1998, 153, 658–663. [Google Scholar] [CrossRef]
- Sousa, T. Uso Do “Sargaço” Como Bioestimulante e Biofertilizante Natural. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2020. [Google Scholar]
- Anderson, G. The Australian & New Zealand Grapegrower and Winemaker. Winetitles media: Broadview, Australia, 2009; pp. 17–22. [Google Scholar]
- Blunden, G.; Morse, P.F.; Mathe, I.; Hohmann, J.; Critchleye, A.T.; Morrell, S. Betaine yields from marine algal species utilized in the preparation of seaweed extracts used in agriculture. Nat. Prod. Commun. 2010, 5, 581–585. [Google Scholar] [CrossRef]
- Blunden, G.; Jenkins, T.; Liu, Y.-W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Deshmukh, R.; Bélanger, R.R. Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2016, 30, 1277–1285. [Google Scholar] [CrossRef]
- Hegazy, E.A.; Abdel-Rehim, H.; Diaa, D.A.; El-Barbary, A. Controlling of Degradation Effects in Radiation Processing of Polymers; International Atomic Energy Agency: Austria, Vienna, 2009. [Google Scholar]
- Chen, S.-K.; Edwards, C.A.; Subler, S. The influence of two agricultural biostimulants on nitrogen transformations, microbial activity, and plant growth in soil microcosms. Soil Biol. Biochem. 2003, 35, 9–19. [Google Scholar] [CrossRef]
- Lattner, D.; Flemming, H.-C.; Mayer, C. 13C-NMR study of the interaction of bacterial alginate with bivalent cations. Int. J. Biol. Macromol. 2003, 33, 81–88. [Google Scholar] [CrossRef]
- Verkleij, F.N. Seaweed Extracts in Agriculture and Horticulture: A Review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Zhang, X.; Thomsen, M. Biomolecular Composition and Revenue Explained by Interactions between Extrinsic Factors and Endogenous Rhythms of Saccharina latissima. Mar. Drugs 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Kopta, T.; Pavlíková, M.; Sękara, A.; Pokluda, R.; Maršálek, B. Effect of Bacterial-algal Biostimulant on the Yield and Internal Quality of Lettuce (Lactuca sativa L.) Produced for Spring and Summer Crop. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef]


| ID | Designation | Treatment |
|---|---|---|
| CP | Positive control | 12 mL of Profertil 1.5% (v/v) |
| CN | Negative control | No treatment |
| E | Algal extract | 12 mL of S. latissima aqueous extract 1.2% (v/v) |
| EB | Algal extract + BlueN | 12 mL of S. latissima aqueous extract (1.2% v/v) + 30 mL of BlueN 0.03% (m/v) |
| B | BlueN | 30 mL of BlueN 0.03% (m/v) |
| g 100 g−1 of Dry Seaweed | Concentration | Literature Median Values | Reference |
|---|---|---|---|
| Ash | 17.81 ± 0.05 | 24.30–27.30 | [52,53,54] |
| Crude lipids | 1.52 ± 0.11 | 0.10–5.50 | |
| Fiber | 6.39 ± 0.13 | 6.20–7.10 | |
| Protein | 13.63 ± 0.02 | 7.40–11.70 | |
| Total Carbohydrates | 60.64 ± 0.09 | 60.30–66.80 | [53,54] |
| Energy (Kcal 100 g−1) | 311 ± 1.22 | NA | |
| Nitrogen | 2.31 ± 0.04 | 1.63 | |
| Phosphorus | 0.11 ± 0.00 | NA | |
| Calcium | 0.44 ± 0.01 | 0.92 | |
| Magnesium | 0.28 ± 0.00 | 0.61 | |
| Potassium | 0.25 ± 0.01 | 3.87 | |
| Sodium | 1.25 ± 0.02 | 3.05 | |
| Iron | 0.06 ± 0.13 | 0.19 | |
| Copper | 0.01 ± 0.42 | 0.01 | |
| Zinc | 0.01 ± 0.12 | 0.01 | |
| Manganese | 0.01 ± 0.90 | 0.00056 | |
| Total Phenolic Content (g GAE 100 g−1) | 4.91 × 10−3 ± 1.08 × 10−4 | 1.11 × 10−4 | [54] |
| Alginate (%) | 24.90 ± 0.01 | 15–20% | [55,56,57] |
| Extract | pH | Electrical Conductivity (μS/cm) | Total Dissolved Solids (mg/L) |
|---|---|---|---|
| Profertil (positive control) 1.5% | 6.91 ± 0.01 | 117 ± 5 | 59 ± 2 |
| BlueN 0.03% | 6.70 ± 0.01 | 103 ± 5 | 54 ± 2 |
| Algal extract 1.2% | 6.93 ± 0.01 | 331 ± 5 | 165 ± 2 |
| Tap water | 7.45 ± 0.01 | 106 ± 5 | 54 ± 2 |
| Distilled water | 7.00 ± 0.01 | 1.90 ± 5 | 1 ± 2 |
| Physical–Chemical Parameters | SI | CN | CP | E | EB | B |
|---|---|---|---|---|---|---|
| Material <2 mm (%, w/w) | 79.48 | 71.00 | 70.2 | 73.07 | 71.89 | 73.03 |
| pH | 6.60 ± 0.01 | 6.52 ± 0.02 a | 6.55 ± 0.01 a | 6.39 ± 0.01 a | 6.43 ± 0.01 a | 6.42 ± 0.01 a |
| EC (mS/cm) | 0.20 ± 0.01 | 0.37 ± 0.03 a | 0.30 ± 0.01 a | 0.45 ± 0.01 a | 0.42 ± 0.01 a | 0.52 ± 0.01 a |
| Treatment | Ratio Root Length: Aerial-Part Diameter | Ratio Root Weight: Aerial-Part Weight |
|---|---|---|
| CP | 2.04 | 0.27 |
| CN | 1.89 | 0.21 |
| E | 1.95 | 0.28 |
| EB | 1.59 | 0.37 |
| B | 2.07 | 0.47 |
| Treatment | CP | CN | E | EB | B | Values Reported in the Literature | Ref. |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 6.30 ± 0.18 a | 6.52 ± 0.93 a | 5.46 ± 0.61 a | 5.90 ± 0.09 a | 5.08 ± 0.47 a | NA | - |
| Ashes (%) | 84.62 ± 0.34 a | 83.80 ± 0.11 a | 83.94 ± 0.75 a | 84.79 ± 0.53 a | 85.30 ± 1.8 a | NA | - |
| N (%) | 1.44 ± 0.05 a | 1.77 ± 0.12 a | 1.73 ± 0.20 a | 1.65 ± 0.08 a | 1.50 ± 0.15 a | NA | - |
| P (%) | 0.39 ± 0.01 a | 0.38 ± 0.02 a | 0.39 ± 0.02 a | 0.41 ± 0.02 a | 0.38 ± 0.01 a | 0.24 | [63] |
| Ca (%) | 1.13 ± 0.12 a | 1.25 ± 0.06 a | 1.18 ± 0.13 a | 1.09 ± 0.09 a | 1.12 ± 0.11 a | 0.04–0.81 | [63,64] |
| Mg (%) | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.02 a | 0.17 ± 0.01 a | 0.18 ± 0.02 a | 0.01–0.50 | |
| K (%) | 4.61 ± 0.51 a | 5.09 ± 0.29 a | 5.03 ± 0.21 a | 4.97 ± 0.12 a | 4.91 ± 0.45 a | 0.36–1.89 | |
| Na (%) | 0.24 ± 0.02 a | 0.24 ± 0.03 a | 0.20 ± 0.01 a | 0.28 ± 0.03 a | 0.25 ± 0.04 a | 0.01–0.05 | |
| Cu (mg/kg) | 5.68 ± 0.95 a | 4.05 ± 0.51 a | 4.96 ± 0.62 a | 6.49 ± 1.06 a | 4.82 ± 0.90 a | NA | [63] |
| Zn (mg/kg) | 51.72 ± 0.18 a | 53.88 ± 0.44 a | 58.45 ± 7.83 a | 58.58 ± 4.97 a | 47.70 ± 4.59 a | 22.5 | |
| Fe (mg/kg) | 1035.86 ± 226.55 a | 1451.66 ± 558.50 a | 1322.73 ± 54.37 a | 1385.31 ± 438.49 a | 1209.82 ± 108.89 a | 26.8 | |
| Mn (mg/kg) | 61.31 ± 3.81 a | 57.48 ± 3.96 a | 80.28 ± 9.92 b | 62.72 ± 13.17 a | 62.23 ± 21.59 a | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, D.; Cotas, J.; Pereira, L.; Bahcevandziev, K. A Possible Synergistic Approach: Case Study of Saccharina latissima Extract and Nitrifying Bacteria in Lettuce. J. Mar. Sci. Eng. 2023, 11, 1645. https://doi.org/10.3390/jmse11091645
Pacheco D, Cotas J, Pereira L, Bahcevandziev K. A Possible Synergistic Approach: Case Study of Saccharina latissima Extract and Nitrifying Bacteria in Lettuce. Journal of Marine Science and Engineering. 2023; 11(9):1645. https://doi.org/10.3390/jmse11091645
Chicago/Turabian StylePacheco, Diana, João Cotas, Leonel Pereira, and Kiril Bahcevandziev. 2023. "A Possible Synergistic Approach: Case Study of Saccharina latissima Extract and Nitrifying Bacteria in Lettuce" Journal of Marine Science and Engineering 11, no. 9: 1645. https://doi.org/10.3390/jmse11091645
APA StylePacheco, D., Cotas, J., Pereira, L., & Bahcevandziev, K. (2023). A Possible Synergistic Approach: Case Study of Saccharina latissima Extract and Nitrifying Bacteria in Lettuce. Journal of Marine Science and Engineering, 11(9), 1645. https://doi.org/10.3390/jmse11091645









