Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece
Abstract
:1. Introduction
2. Materials and Methods
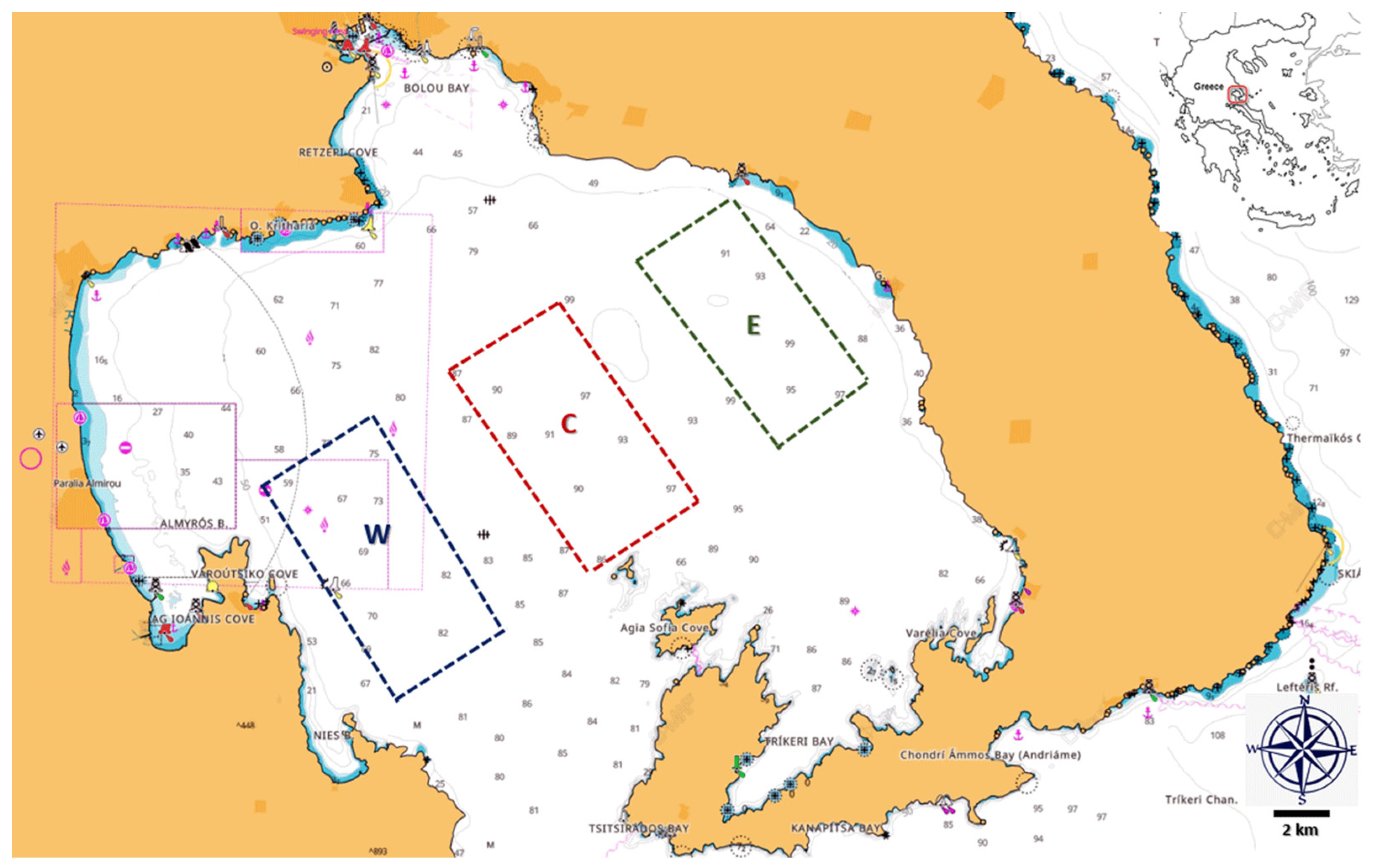
2.1. Sampling
2.2. Univariate Statistical Analysis
2.3. Multivariate Statistical Analysis
2.4. Ecological Assessment
2.5. Species Association and Niche Overlap
3. Results
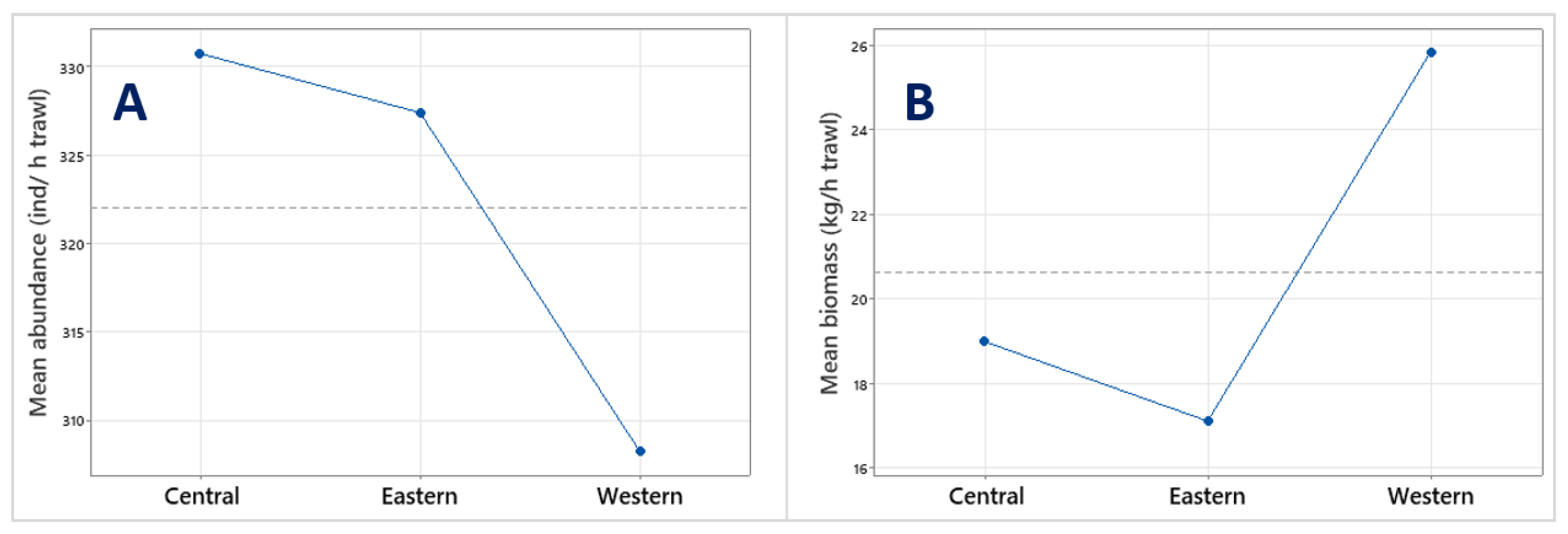
3.1. Abundance and Biomass of Major Taxa
3.2. Niche Overlap
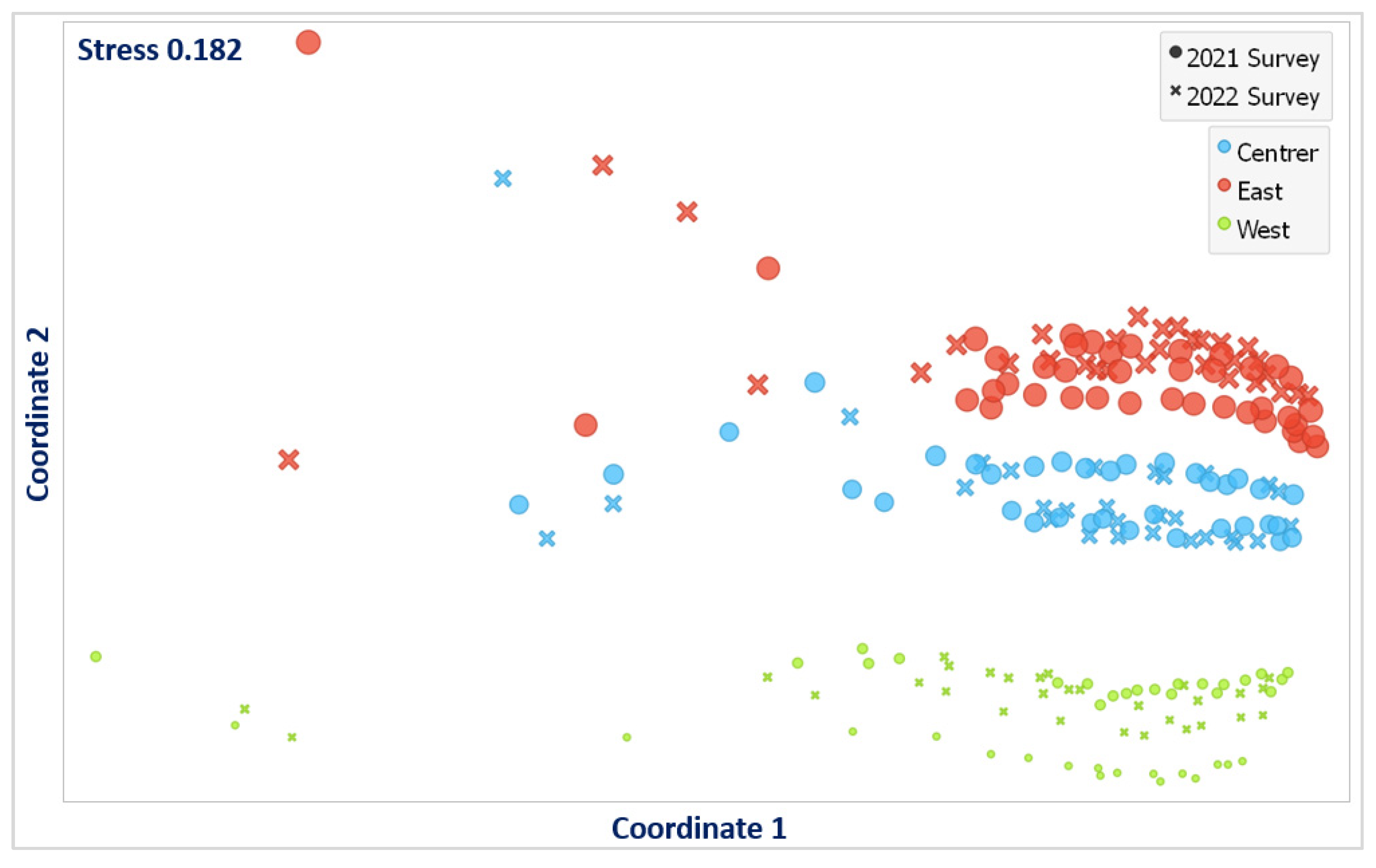
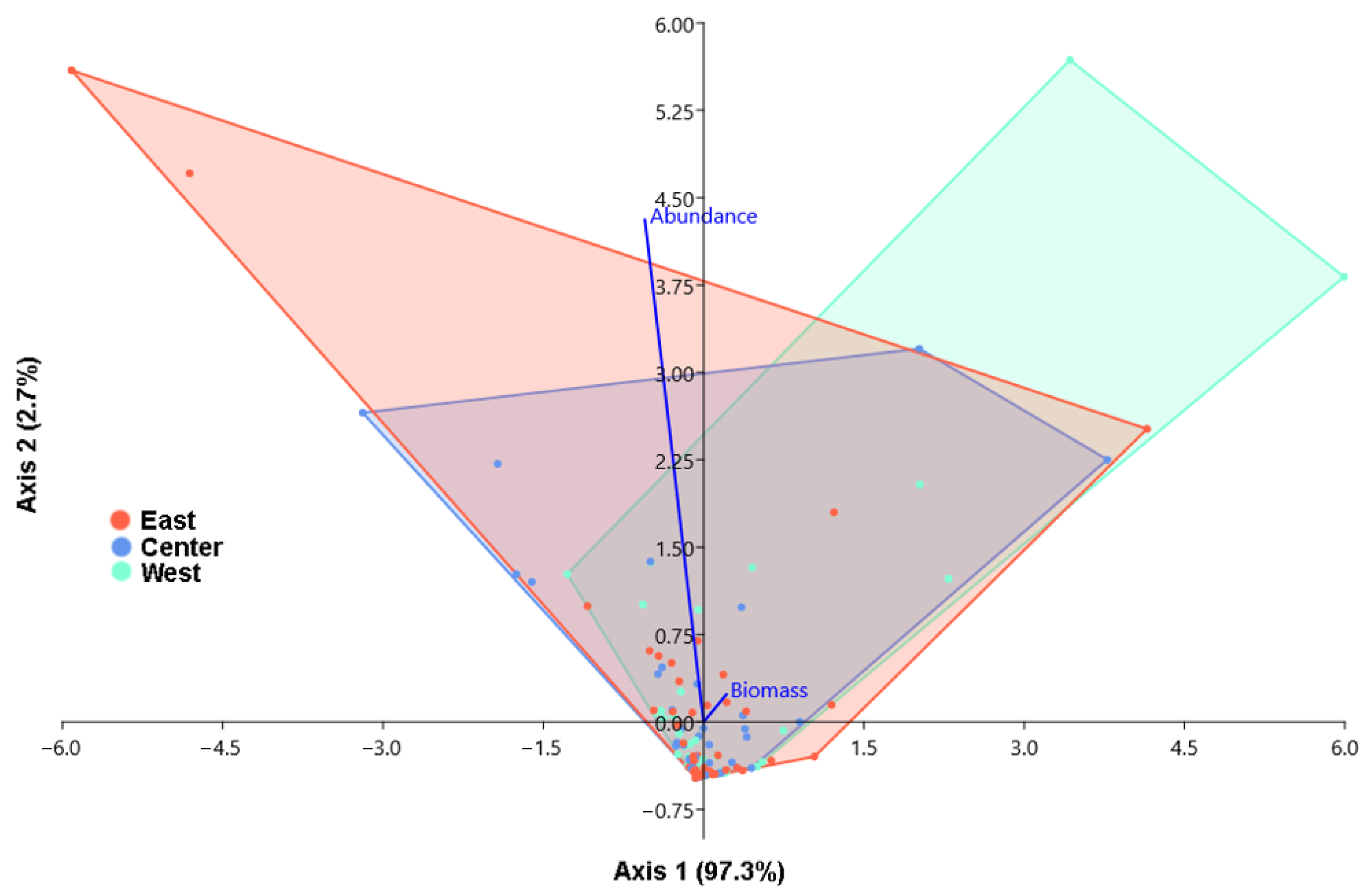
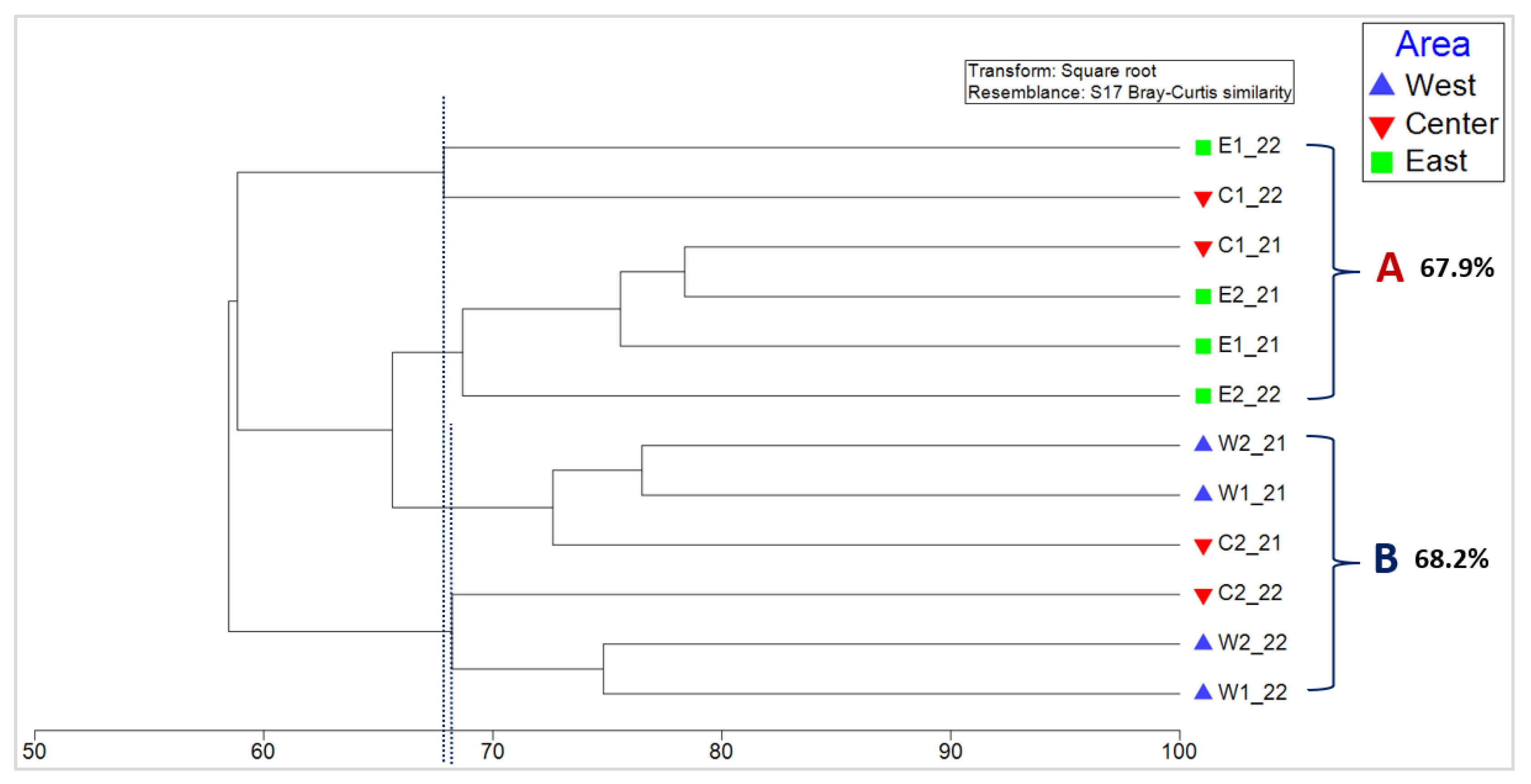
3.3. Analysis of Similarities
3.4. Diversity Indices
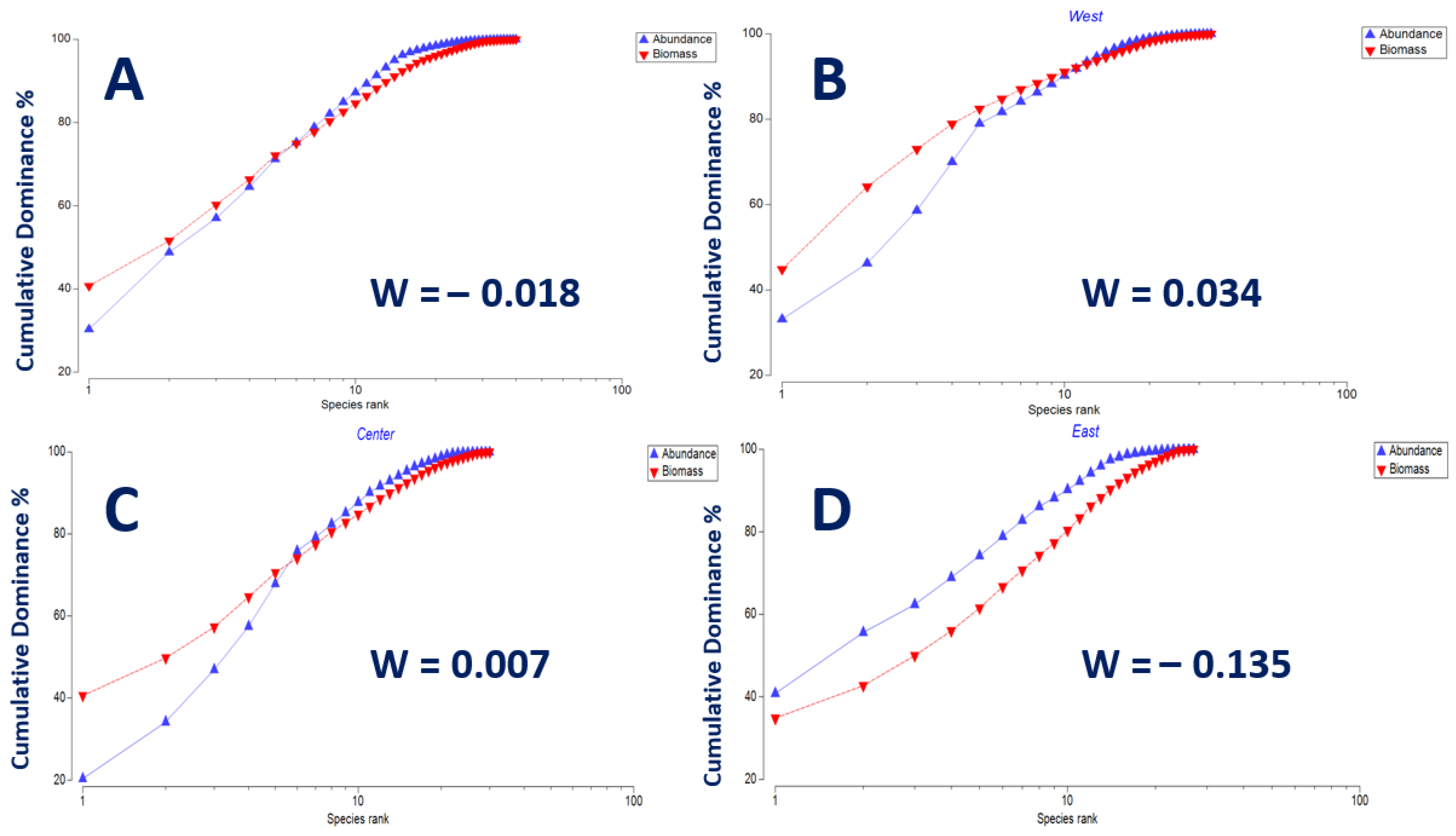
3.5. Ecological Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beaumont, N.J.; Austen, M.C.; Mangi, S.C.; Townsend, M. Economic Valuation for the Conservation of Marine Biodiversity. Mar. Pollut. Bull. 2008, 56, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, P.; Law, R.J. Contaminants and Their Effects on Estuarine and Coastal Organisms in the United Kingdom in the Late Twentieth Century. Environ. Pollut. 2002, 120, 739–757. [Google Scholar] [CrossRef]
- Lotze, H.K.; Reise, K.; Worm, B.; van Beusekom, J.; Busch, M.; Ehlers, A.; Heinrich, D.; Hoffmann, R.C.; Holm, P.; Jensen, C. Human Transformations of the Wadden Sea Ecosystem through Time: A Synthesis. Helgol. Mar. Res. 2005, 59, 84–95. [Google Scholar] [CrossRef]
- McCauley, D.J.; Pinsky, M.L.; Palumbi, S.R.; Estes, J.A.; Joyce, F.H.; Warner, R.R. Marine Defaunation: Animal Loss in the Global Ocean. Science 2015, 347, 1255641. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and Rebuilding of European Fisheries. Mar. Policy 2018, 93, 159–170. [Google Scholar] [CrossRef]
- Prato, G.; Guidetti, P.; Bartolini, F.; Mangialajo, L.; Francour, P. The Importance of High-Level Predators in Marine Protected Area Management: Consequences of Their Decline and Their Potential Recovery in the Mediterranean Context. Adv. Oceanogr. Limnol. 2013, 4, 176–193. [Google Scholar] [CrossRef]
- Statistics—ELSTAT. Available online: https://www.statistics.gr/en/statistics/-/publication/SPA03/ (accessed on 10 February 2023).
- Directorate General of Fisheries, Hellenic Ministry of Rural Development. Annual Fleet Report 2022. Available online: http://www.alieia.minagric.gr/node/8 (accessed on 10 July 2023).
- Moutopoulos, D.K.; Koutsikopoulos, C. Fishing Strange Data in National Fisheries Statistics of Greece. Mar. Policy 2014, 48, 114–122. [Google Scholar] [CrossRef]
- Stathopoulou, E.; Apostologamvrou, C.; Vafidis, D.; Mente, E.; Berillis, P.; Katselis, G. Sex Differentiation in the Histology of the Digestive Gland of Nephrops Norvegicus from Pagasitikos Gulf during Spring. Aquat. Living Resour. 2017, 30, 28. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Tyler, P.A., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 34, pp. 201–352. [Google Scholar] [CrossRef]
- Rogers, S.I.; Maxwell, D.; Rijnsdorp, A.D.; Damm, U.; Vanhee, W. Fishing Effects in Northeast Atlantic Shelf Seas: Patterns in Fishing Effort, Diversity and Community Structure. IV. Can Comparisons of Species Diversity Be Used to Assess Human Impacts on Demersal Fish Faunas? Fish. Res. 1999, 40, 135–152. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field Data Analysis and Application of a Complex Water Column Biogeochemical Model in Different Areas of a Semi-Enclosed Basin: Towards the Development of an Ecosystem Management Tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Perissoratis, C.; Zacharaki, P.; Andrianopoulos, A. Sedimentology of Surface Sediments of Pagasitikos Gulf and Trikeri Strait w. Aegean Sea, Greece. Thalassographica 1990, 13, 35–45. [Google Scholar]
- Petihakis, G.; Triantafyllou, G.; Korres, G.; Tsiaras, K.; Theodorou, A. Ecosystem Modelling: Towards the Development of a Management Tool for a Marine Coastal System Part-II, Ecosystem Processes and Biogeochemical Fluxes. J. Mar. Syst. 2012, 94, S49–S64. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Korres, G.; Pollani, A.; Theodorou, A. Ecosystem Modeling: Towards the Development of a Management Tool for a Marine Coastal System. Part I: General Circulation, Hydrological and Dynamical Structure. J. Mar. Syst. 2012, 94, S34–S48. [Google Scholar] [CrossRef]
- Maravelias, C.D. Factors Affecting Purse Seine Catches: An Observer-Based Analysis. Mediterr. Mar. Sci. 2006, 7, 27–40. [Google Scholar]
- Caragitsou, E.; Siapatis, A.; Anastasopoulou, A. Seasonal Structure of Fish Larvae Assemblages in the Pagasitikos Gulf (Greece). Rapp. Comm. Int. Mer Mediterr. 2001, 36, 250. [Google Scholar]
- Dimarchopoulou, D.; Keramidas, I.; Tsagarakis, K.; Tsikliras, A.C. Ecosystem Models and Effort Simulations of an Untrawled Gulf in the Central Aegean Sea. Front. Mar. Sci. 2019, 6, 648. [Google Scholar] [CrossRef]
- Papaconstantinou, C.; Zenetos, A.; Vassilopoulou, V.; Tserpes, G. SoHelFI. In State of Hellenic Fisheries; HCMR: Gournes, Greece, 2007; p. 466. [Google Scholar]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Krishnamoorthy, K.; Lu, F.; Mathew, T. A Parametric Bootstrap Approach for ANOVA with Unequal Variances: Fixed and Random Models. Comput. Stat. Data Anal. 2007, 51, 5731–5742. [Google Scholar] [CrossRef]
- Şahin, M.; Aybek, E. Jamovi: An Easy to Use Statistical Software for the Social Scientists. Int. J. Assess. Tools Educ. 2019, 6, 670–692. [Google Scholar] [CrossRef]
- Rutherford, A. Introducing ANOVA and ANCOVA: A GLM Approach; SAGE: Thousand Oaks, CA, USA, 2001; ISBN 9780761951612. [Google Scholar]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy. The Principles and Practice of Numerical Classification; W.H. Freeman and Company: San Francisco, CA, USA, 1973. [Google Scholar]
- Clarke, K.R.; Gorley, R.; Sommerfield, P.J.; Warwick, R.M. Change in Marine Communities—Statistical Analysis; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Scholkopft, B.; Mullert, K.-R. Fisher Discriminant Analysis with Kernels. In Proceedings of the Neural Networks for Signal Processing IX Society Workshop (Cat. No.98TH8468), Madison, WI, USA, 25 August 1999. [Google Scholar]
- Clarke, K.R. Non-parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; Urbana University: Urbana, OH, USA, 1949. [Google Scholar]
- Margalef, D.R. Information Theory in Ecology. Gen. Syst. Yearb. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. Mathematical Ecology; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Warwick, R.M. A New Method for Detecting Pollution Effects on Marine Macrobenthic Communities. Mar. Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Feinsinger, P.; Spears, E.E.; Poole, R.W. A Simple Measure of Niche Breadth. Ecology 1981, 62, 27–32. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Reynolds, J.F. Statistical Ecology: A Primer in Methods and Computing; John Wiley & Sons: Hoboken, NJ, USA, 1988; Volume 1, ISBN 0471832359. [Google Scholar]
- MacArthur, R.; Levins, R. The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Petraitis, P.S. Likelihood Measures of Niche Breadth and Overlap. Ecology 1979, 60, 703–710. [Google Scholar] [CrossRef]
- Pianka, E.R. The Structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Pianka, E.R. Organization in Natural Assemblages of Desert Lizards and Tropical Fishes. Ecol. Monogr. 1990, 60, 27–55. [Google Scholar] [CrossRef]
- Morisita, M. Measuring of Interspecific Association and Similarity between Assemblages. Mem. Fac. Sci. Kyushu Univ. Ser. E Biol. 1959, 3, 65–80. [Google Scholar]
- Garratt, M.W.; Steinhorst, R.K. Testing for Significance of Morisita’s, Horn’s and Related Measures of Overlap. Am. Midl. Nat. 1976, 96, 245–251. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Zhang, J.L.; Ma, K.P. Spaa: An R Package for Computing Species Association and Niche Overlap. Res. Prog. Biodivers. Conserv. China 2014, 10, 165–174. [Google Scholar]
- Lambshead, P.J.D.; Platt, H.M.; Shawj, K.M. The Detection of Differences among Assemblages of Marine Benthic Species Based on an Assessment of Dominance and Diversity. J. Nat. Hist. 1983, 17, 859–874. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Relearning the ABC: Taxonomic Changes and Abundance/Biomass Relationships in Disturbed Benthic Communities. Mar. Biol. 1994, 118, 739–744. [Google Scholar] [CrossRef]
- Warwick, R.M.; Pearson, T.H. Ruswahyuni Detection of Pollution Effects on Marine Macrobenthos: Further Evaluation of the Species Abundance/Biomass Method. Mar. Biol. 1987, 95, 193–200. [Google Scholar] [CrossRef]
- Agard, J.B.R.; Gobin, J.; Warwick, R.M. Analysis of Marine Macrobenthic Community Structure in Relation to Pollution, Natural Oil Seepage and Seasonal Disturbance in a Tropical Environment (Trinidad, West Indies). Mar. Ecol. Prog. Ser. 1993, 92, 233–243. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Shurin, J.B.; Borer, E.T.; Seabloom, E.W.; Anderson, K.; Blanchette, C.A.; Broitman, B.; Cooper, S.D.; Halpern, B.S. A Cross-ecosystem Comparison of the Strength of Trophic Cascades. Ecol. Lett. 2002, 5, 785–791. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Gunderson, D.R. Surveys of Fisheries Resources; John Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
- Schwarz, C.J.; Seber, G.A.F. Estimating Animal Abundance: Review III. Stat. Sci. 1999, 14, 427–456. [Google Scholar] [CrossRef]
- Nicholson, M.D.; Jennings, S. Testing Candidate Indicators to Support Ecosystem-Based Management: The Power of Monitoring Surveys to Detect Temporal Trends in Fish Community Metrics. ICES J. Mar. Sci. 2004, 61, 35–42. [Google Scholar] [CrossRef]
- Smith, C.J.; Papadopoulou, K.N.; Diliberto, S. Impact of Otter Trawling on an Eastern Mediterranean Commercial Trawl Fishing Ground. ICES J. Mar. Sci. 2000, 57, 1340–1351. [Google Scholar] [CrossRef]
- Rose, G.A.; Leggett, W.C. Interactive Effects of Geophysically-Forced Sea Temperatures and Prey Abundance on Mesoscale Coastal Distributions of a Marine Predator, Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 1989, 46, 1904–1913. [Google Scholar] [CrossRef]
- Swain, D.P. Age- and Density-Dependent Bathymetric Pattern of Atlantic Cod (Gadus morhua) in the Southern Gulf of St Lawrence. Can. J. Fish. Aquat. Sci. 1993, 50, 1255–1264. [Google Scholar] [CrossRef]
- MacCall, A.D. Dynamic Geography of Marine Fish Populations; Washington Sea Grant Program: Seattle, WA, USA, 1990. [Google Scholar]
- Swain, D.P.; Wade, E.J. Density-Dependent Geographic Distribution of Atlantic Cod (Gadus morhua) in the Southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 1993, 50, 725–733. [Google Scholar] [CrossRef]
- Daan, N. Introduction: Changes in the North Sea Ecosystem and Their Causes: Århus 1975 Revisted. ICES J. Mar. Sci. 1996, 53, 879–883. [Google Scholar] [CrossRef]
- Lolas, A.; Vafidis, D. Population Dynamics, Fishery, and Exploitation Status of Norway Lobster (Nephrops norvegicus) in Eastern Mediterranean. Water 2021, 13, 289. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Hatziioannou, M.; Malandrakis, E.E.; Tsigenopoulos, C.S.; Karapanagiotidis, I.T.; Mente, E.; Vafidis, D.; Exadactylos, A. Heterozygosity Fitness Correlations and Generation Interval of the Norway Lobster in the Aegean Sea, Eastern Mediterranean. J. Biol. Res. 2019, 26, 14. [Google Scholar] [CrossRef]
- Smith, C.J.; Papadopoulou, K.N. Burrow Density and Stock Size Fluctuations of Nephrops Norvegicus in a Semi-Enclosed Bay. ICES J. Mar. Sci. 2003, 60, 798–805. [Google Scholar] [CrossRef]
- Adams, S.M. Biological Indicators of Aquatic Ecosystem Stress; American Fisheries Society: Bethesda, MD, USA, 2002. [Google Scholar]
- Noss, R.F.; Dinerstein, E.; Gilbert, B.; Gilpin, M.; Miller, B.J.; Terborgh, J.; Trombuk, S. Continental Conservation: Scientific Foundations of Regional Reserve Networks; Island Press: Washington, DC, USA, 1999. [Google Scholar]
- Beukema, J.J. An Evaluation of the ABC-Method (Abundance/Biomass Comparison) as Applied to Macrozoobenthic Communities Living on Tidal Flats in the Dutch Wadden Sea. Mar. Biol. 1988, 99, 425–433. [Google Scholar] [CrossRef]
- Clarke, K.R. Comparisons of Dominance Curves. J. Exp. Mar. Biol. Ecol. 1990, 138, 143–157. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. A Comparison of Some Methods for Analysing Changes in Benthic Community Structure. J. Mar. Biol. Assoc. U. K. 1991, 71, 225–244. [Google Scholar] [CrossRef]
- Warwick, R.M. Environmental Impact Studies on Marine Communities: Pragmatical Considerations. Aust. J. Ecol. 1993, 18, 63–80. [Google Scholar] [CrossRef]
- Klaoudatos, D.; Tziantziou, L.; Lolas, A.; Neofitou, N.; Vafidis, D. Population Characteristics of the Upper Infralittoral Sea Urchin Arbacia Lixula (Linnaeus, 1758) in Eastern Mediterranean (Central Greece): An Indicator Species for Coastal Water Quality. J. Mar. Sci. Eng. 2022, 10, 395. [Google Scholar] [CrossRef]
- Conides, A.; Klaoudatos, D.; Kalamaras, M.; Neophytou, N.; Exadaktylos, A.; Papaconstantinou, C.; Klaoudatos, S. Capture Fisheries and Aquaculture Exploitation in the Aegean Sea Archipelago; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–26. [Google Scholar]

| Species | Sampling Area | |||
|---|---|---|---|---|
| Abundance | Phylum | West | Center | East |
| Pagellus bogaraveo | O | 40.25 | 35.77 | 15.92 |
| Parapenaeus longirostris | Cr | 15.37 | 50.65 | |
| Trachurus mediterraneus | O | 16.60 | 14.21 | 8.10 |
| Mullus barbatus | O | 15.38 | 11.87 | 6.82 |
| Pagellus erythrinus | O | 15.28 | 4.54 | |
| Illex coindetii | Ce | 4.14 | 5.75 | |
| Cumulative abundance (%) | 87.51 | 85.90 | 87.24 | |
| Biomass | ||||
| Pagellus bogaraveo | O | 53.85 | 56.48 | 37.26 |
| Pagellus erythrinus | O | 21.07 | 7.95 | |
| Merluccius merluccius | O | 7.06 | 5.69 | |
| Mullus barbatus | O | 9.06 | 8.82 | 9.79 |
| Trachurus mediterraneus | O | 6.28 | 6.90 | 6.81 |
| Parapenaeus longirostris | Cr | 9.13 | ||
| Illex coindetii | Ce | 9.04 | ||
| Trigla lucerna | O | 4.08 | ||
| Arnoglossus laterna | O | 3.09 | ||
| Trisopterus minutus capelanus | O | 2.72 | ||
| Cumulative biomass (%) | 90.26 | 87.21 | 87.61 | |
| Biomass | Abundance | ||||||
|---|---|---|---|---|---|---|---|
| Center | East | Center | East | ||||
| Levins | West | 0.083 | 0.071 | Levins | West | 0.639 | 0.453 |
| Center | 0.862 | Center | 0.992 | ||||
| Schoener | West | 0.786 | 0.692 | Schoener | West | 0.644 | 0.493 |
| Center | 0.793 | Center | 0.634 | ||||
| Petraitis | West | 0.835 | 0.747 | Petraitis | West | 0.747 | 0.785 |
| Center | 0.884 | Center | 0.795 | ||||
| Pianka | West | 0.954 | 0.919 | Pianka | West | 0.786 | 0.407 |
| Center | 0.978 | Center | 0.724 | ||||
| Czech | West | 0.786 | 0.692 | Czech | West | 0.645 | 0.493 |
| Center | 0.793 | Center | 0.634 | ||||
| Morisita | West | 0.946 | 0.889 | Morisita | West | 0.769 | 0.404 |
| Center | 0.97 | Center | 0.689 |
| West Group | East Group | Average Dissimilarity: 55.42 | ||
|---|---|---|---|---|
| Species | Average Abundance | Average Abundance | Contribution % | Cumulative Contribution% |
| Parapenaeus longirostris | 89.69 | 2001.32 | 31.59 | 31.59 |
| Pagellus bogaraveo | 1560.55 | 914.17 | 11.03 | 42.62 |
| Trigla lucerna | 570.97 | 327.56 | 8.26 | 50.87 |
| Engraulis encrasicolus | 27.78 | 448.72 | 7.66 | 58.53 |
| Pagellus erythrinus | 513.09 | 115.24 | 6.76 | 65.29 |
| Trachurus mediterraneus | 633.99 | 438.68 | 4.22 | 69.52 |
| Mullus barbatus | 555.58 | 384.93 | 3.94 | 73.45 |
| West Group | East Group | Average Dissimilarity: 43.01 | ||
|---|---|---|---|---|
| Species | Average Biomass | Average Biomass | Contribution % | Cumulative Contribution% |
| Pagellus bogaraveo | 177.17 | 109.71 | 25.24 | 25.24 |
| Pagellus erythrinus | 63.48 | 13.38 | 16.12 | 41.37 |
| Mullus barbatus | 36.52 | 24.37 | 5.67 | 47.04 |
| Merluccius merluccius | 15.34 | 25.35 | 5.21 | 52.25 |
| Parapenaeus longirostris | 0.72 | 15.42 | 4.95 | 57.20 |
| Trachurus mediterraneus | 25.64 | 17.41 | 3.86 | 61.06 |
| Raja montagui | 5.84 | 8.22 | 3.30 | 64.36 |
| Trigla lucerna | 11.48 | 8.49 | 3.23 | 67.6 |
| Illex coindetii | 5.13 | 14.70 | 3.13 | 70.73 |
| Group | Species Number | Numerical Abundance | Species Richness Margalef (d) | Evenness (J) | Shannon Diversity Index (H′) | Simpson 1-Lambda’ |
|---|---|---|---|---|---|---|
| A (East) | 33 | 3636 | 3.293 | 0.7253 | 2.418 | 0.877 |
| B (West) | 41 | 3003 | 4.496 | 0.7054 | 2.547 | 0.879 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaoudatos, D.; Vardali, S.; Apostologamvrou, C.; Lolas, A.; Neofitou, N.; Conides, A.; Gkafas, G.A.; Sarantopoulou, J.; Kolindrini, D.; Roditi, K.; et al. Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece. J. Mar. Sci. Eng. 2023, 11, 1668. https://doi.org/10.3390/jmse11091668
Klaoudatos D, Vardali S, Apostologamvrou C, Lolas A, Neofitou N, Conides A, Gkafas GA, Sarantopoulou J, Kolindrini D, Roditi K, et al. Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece. Journal of Marine Science and Engineering. 2023; 11(9):1668. https://doi.org/10.3390/jmse11091668
Chicago/Turabian StyleKlaoudatos, Dimitris, Sofia Vardali, Chrisoula Apostologamvrou, Alexios Lolas, Nikolaos Neofitou, Alexios Conides, Georgios A. Gkafas, Joanne Sarantopoulou, Dorothea Kolindrini, Kyriakoula Roditi, and et al. 2023. "Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece" Journal of Marine Science and Engineering 11, no. 9: 1668. https://doi.org/10.3390/jmse11091668
APA StyleKlaoudatos, D., Vardali, S., Apostologamvrou, C., Lolas, A., Neofitou, N., Conides, A., Gkafas, G. A., Sarantopoulou, J., Kolindrini, D., Roditi, K., Exadactylos, A., & Vafidis, D. (2023). Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece. Journal of Marine Science and Engineering, 11(9), 1668. https://doi.org/10.3390/jmse11091668












