Regional Algorithm of Quantitative Assessment of Cyanobacteria Blooms in the Eastern Part of the Gulf of Finland Using Satellite Ocean Color Data
Abstract
:1. Introduction
2. Materials and Methods
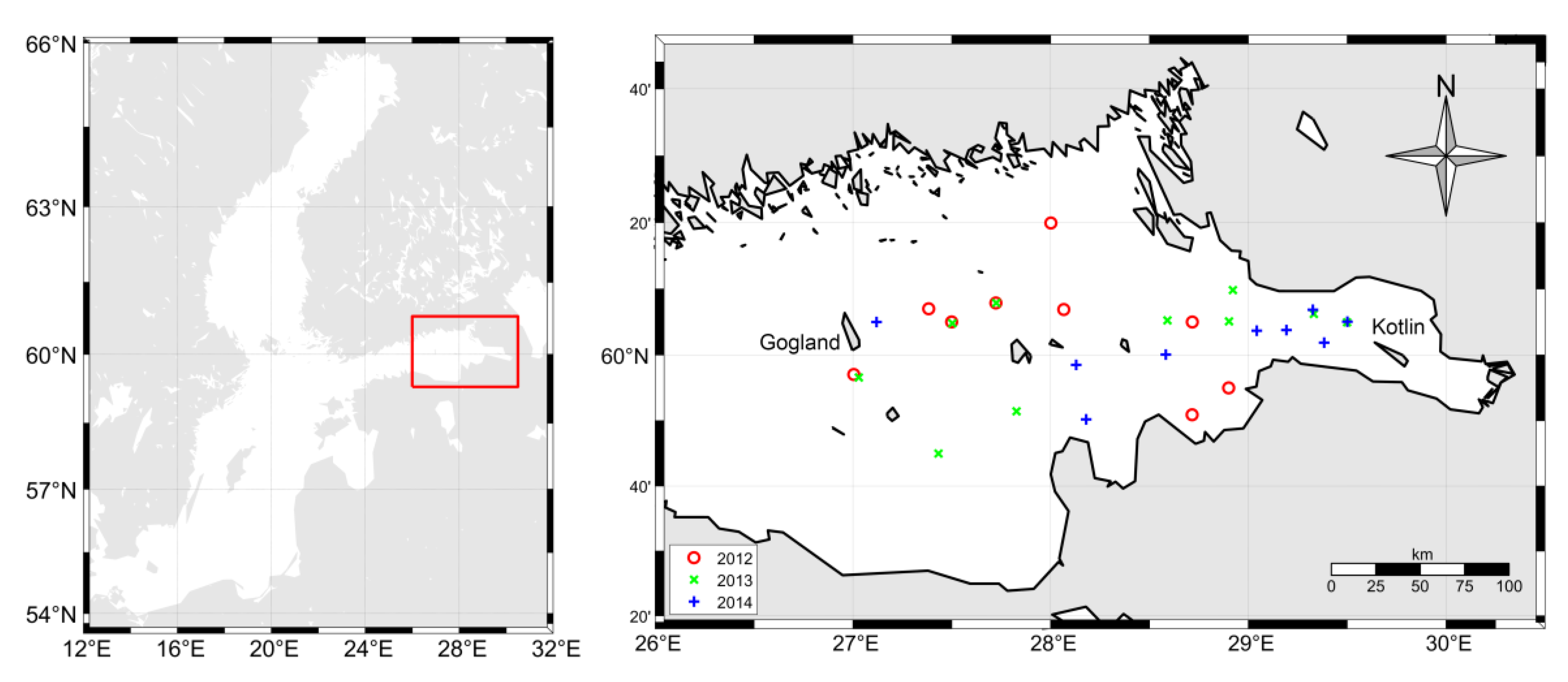
2.1. In Situ Data
2.2. Satellite Data
2.3. Accuracy Assessment Metrics
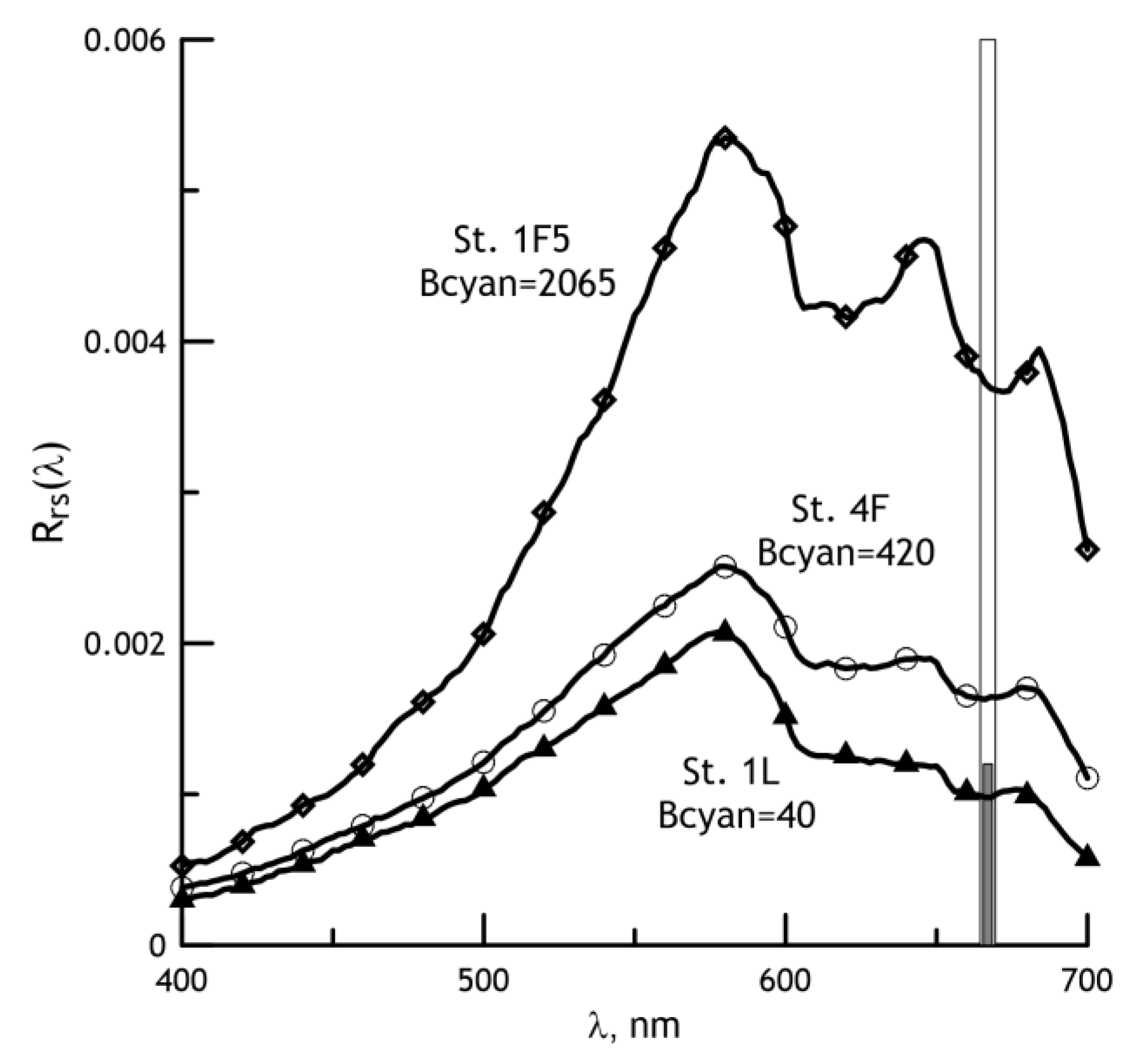
2.4. Development of a Regional Algorithm for Estimating the Cyanobacteria Biomass
3. Results
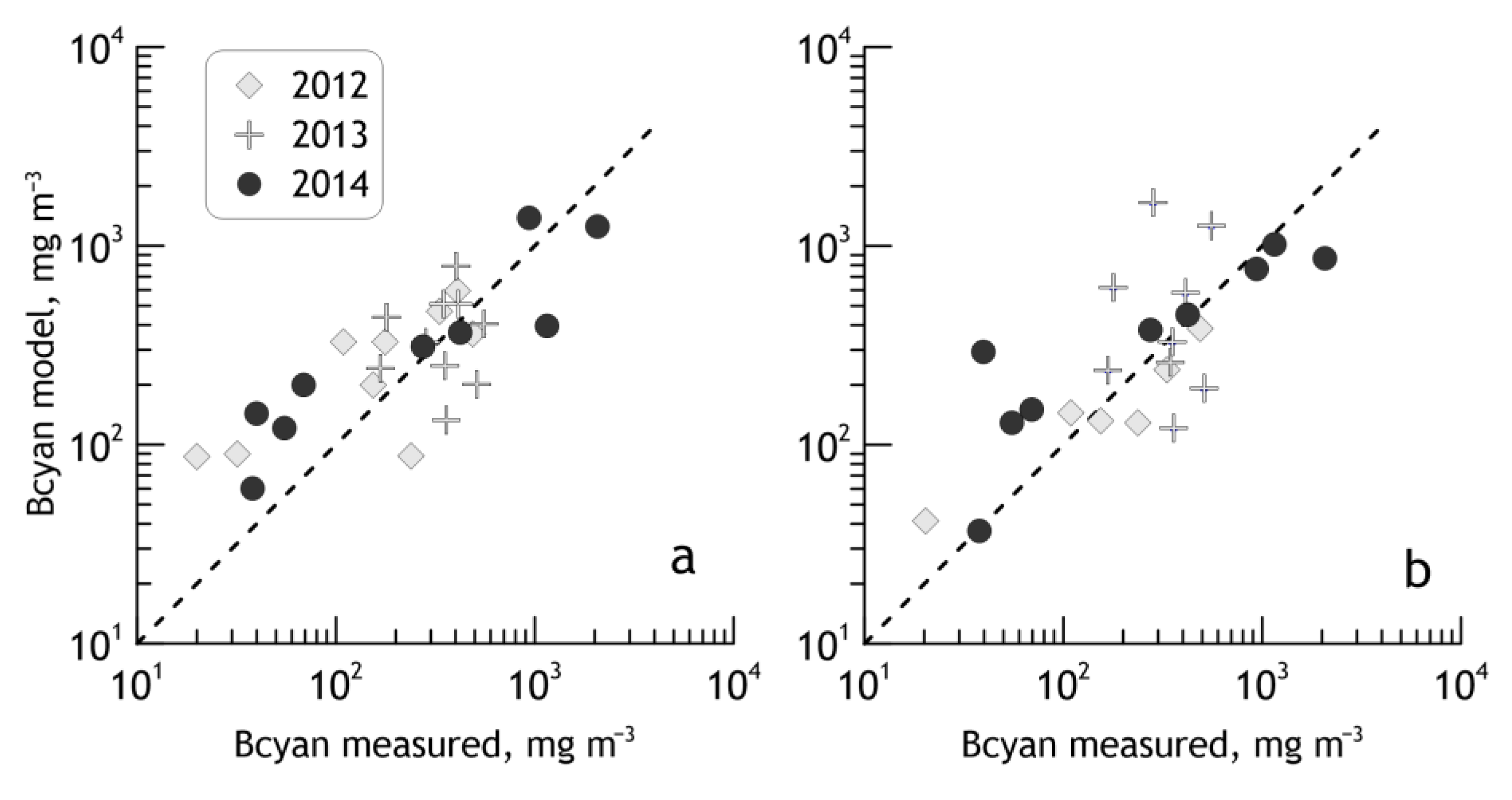
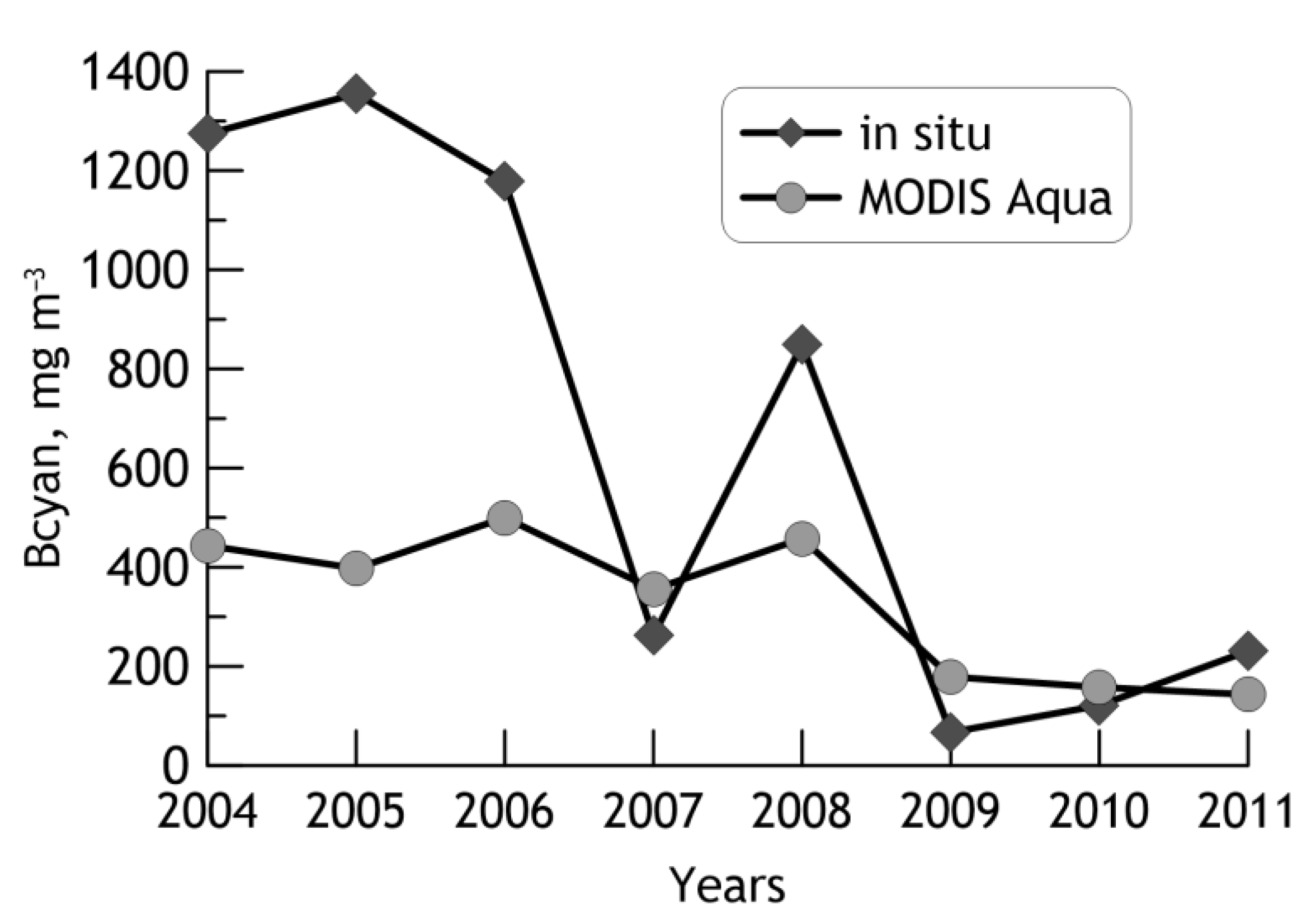
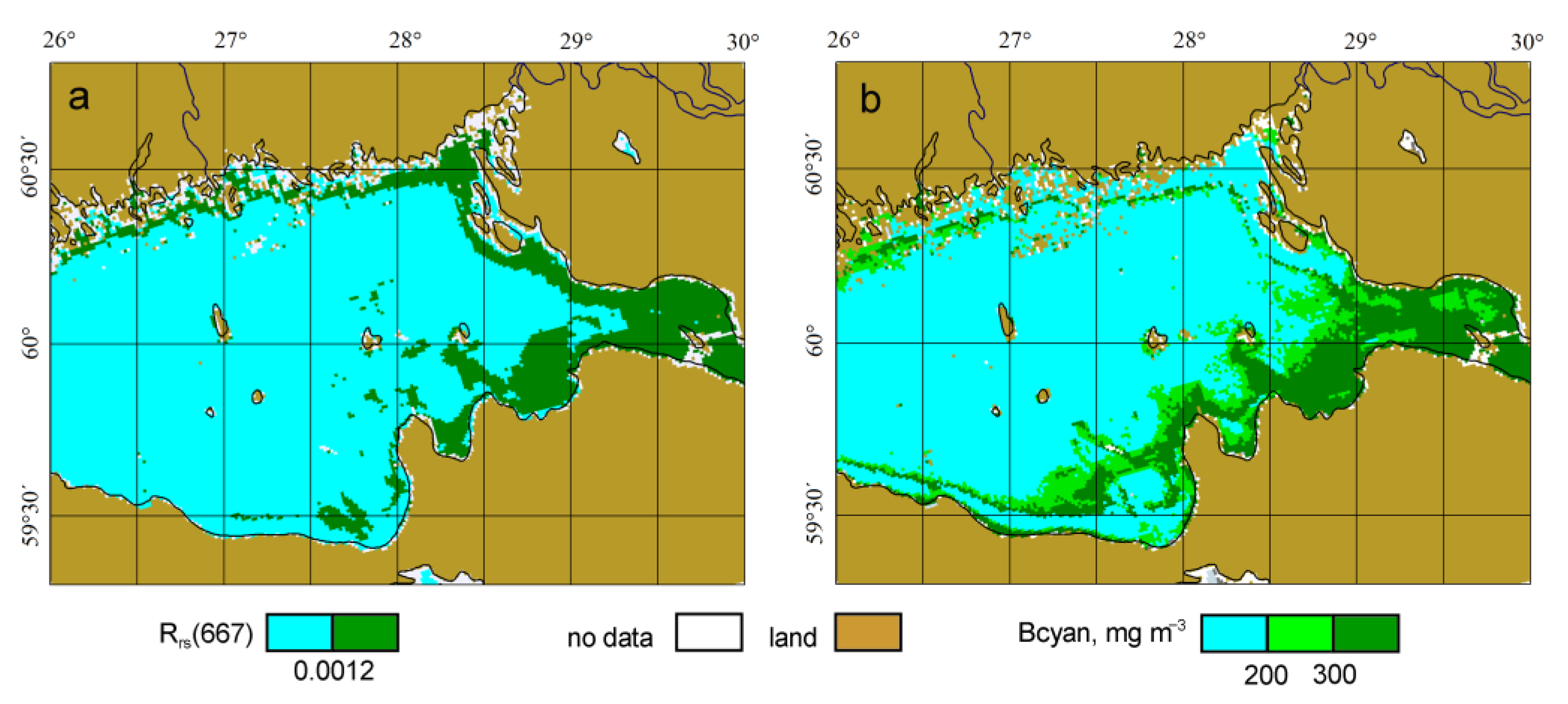
3.1. Validation of the Algorithm with Satellite Data
3.2. Threshold for Determining the Cyanobacteria Bloom
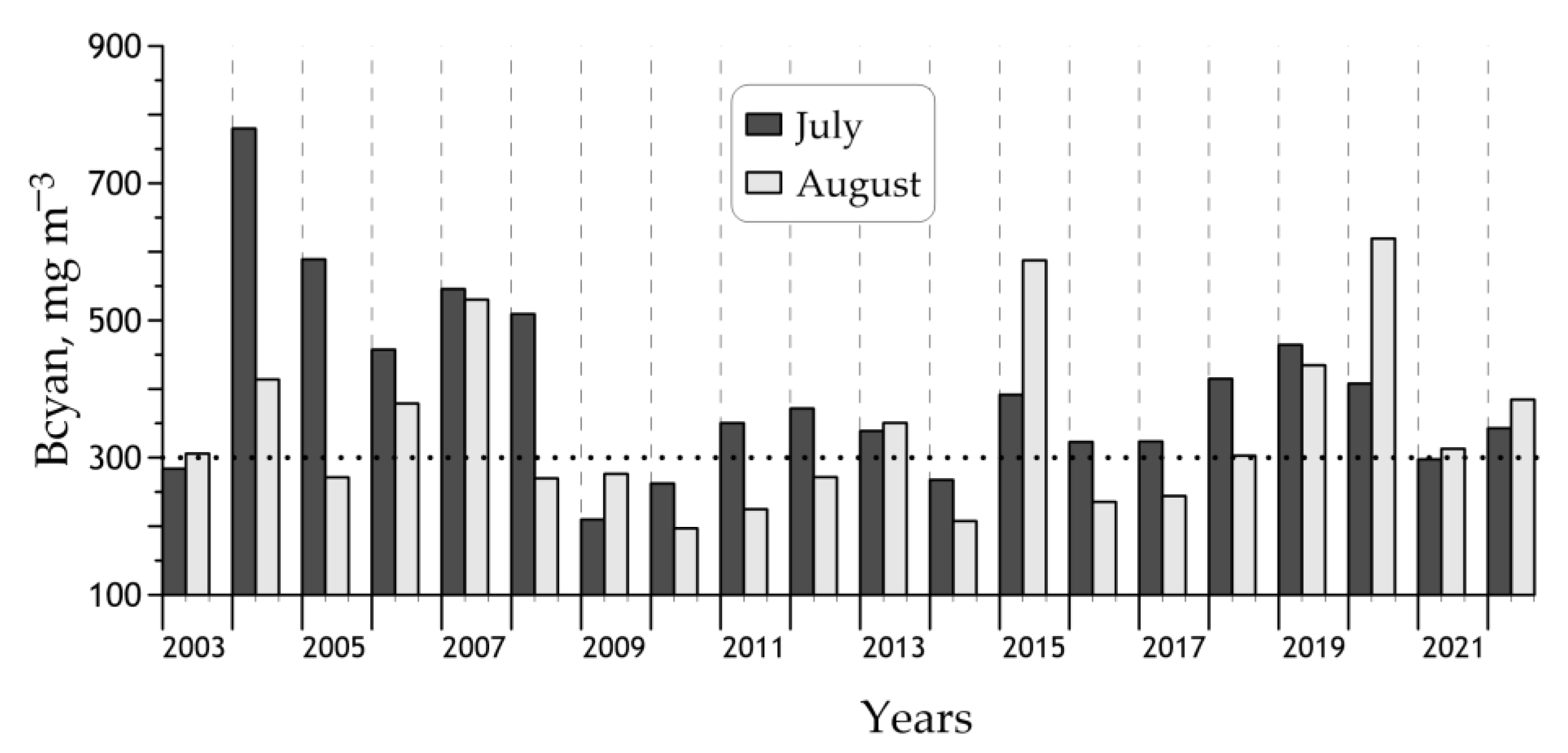
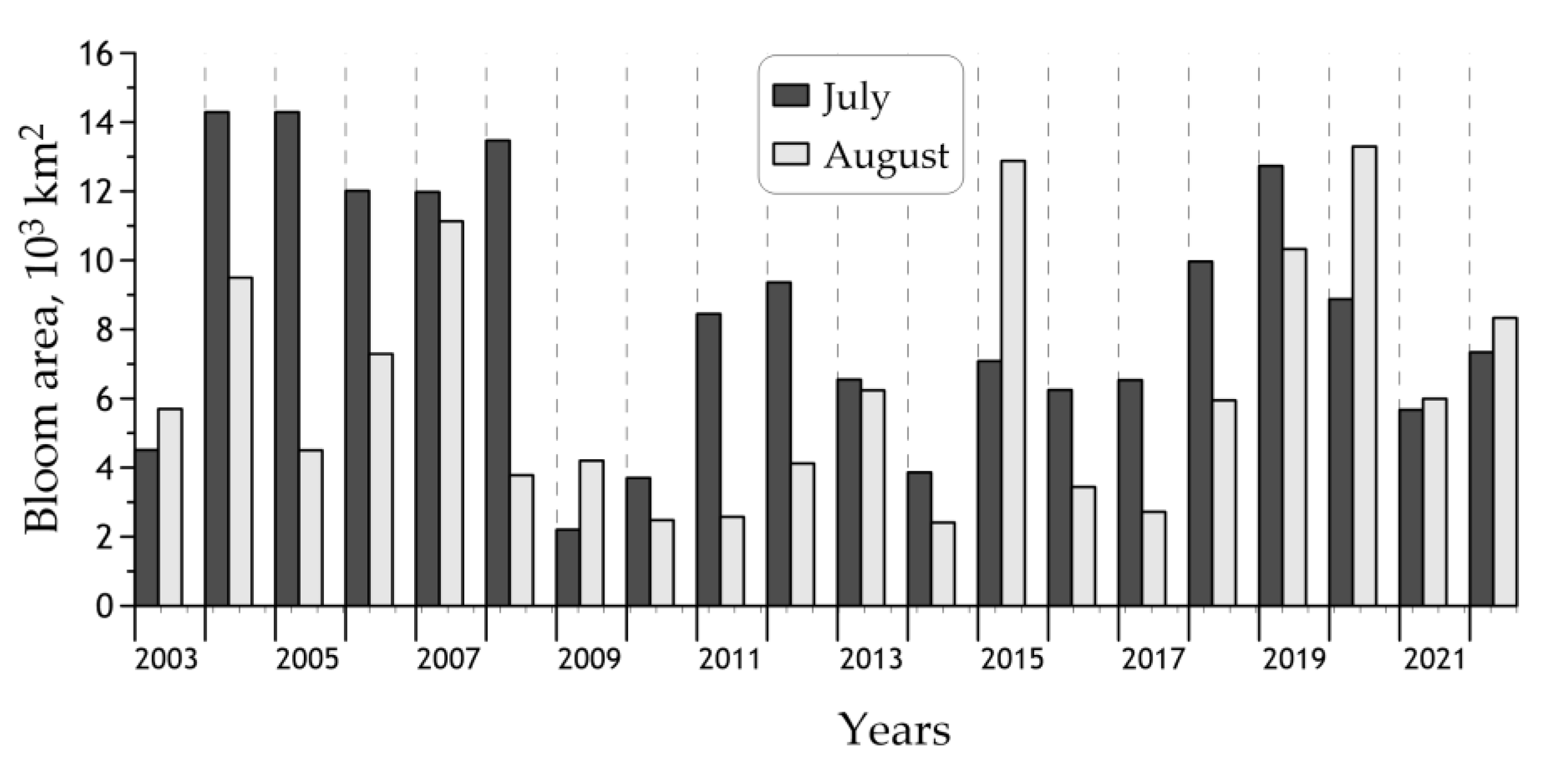
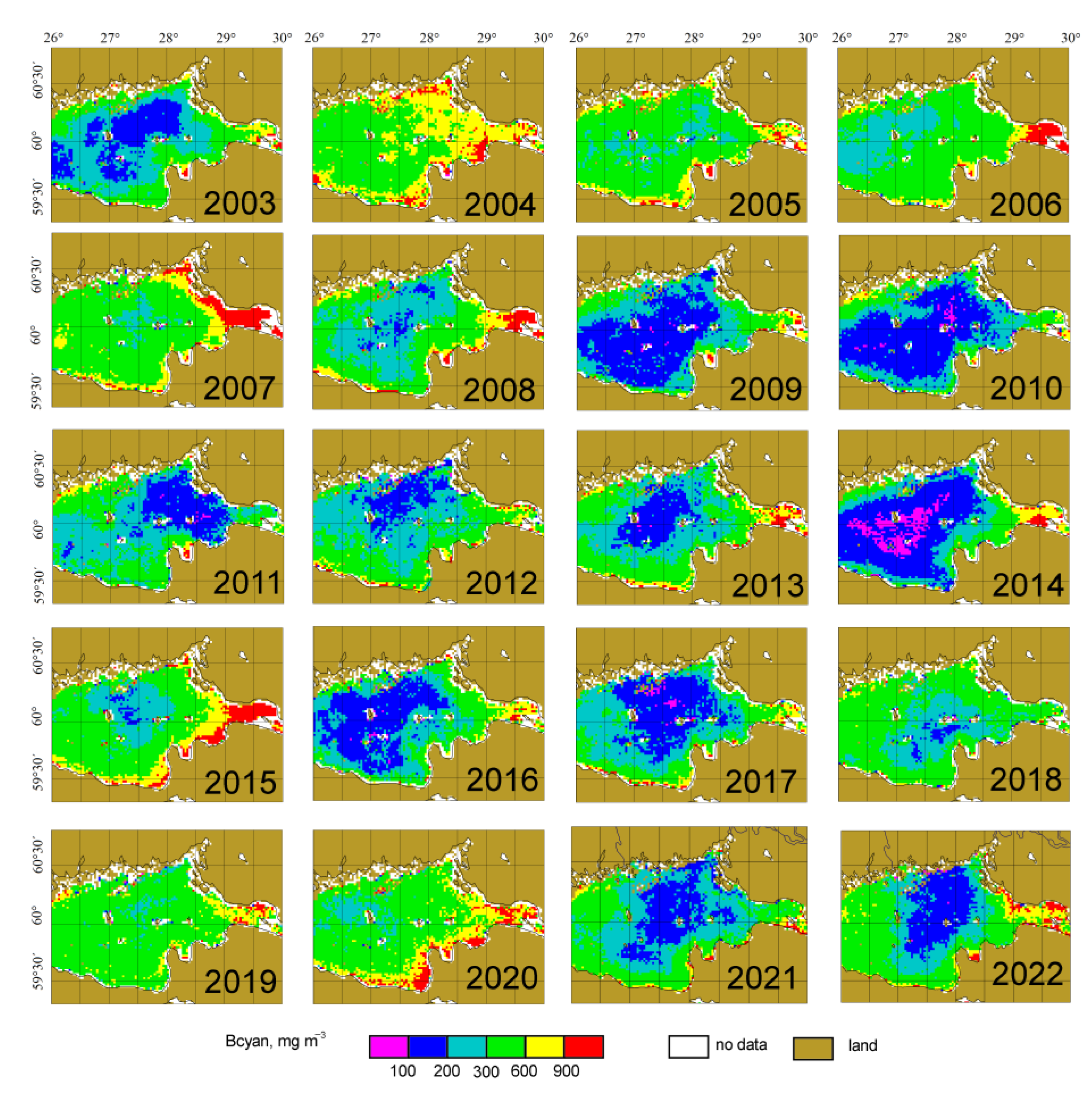
3.3. Interannual Changes of the Cyanobacteria Blooms’ Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Gower, J.; King, S. New results from a global survey using MERIS MCI. In Proceedings of the 2nd MERIS/(A)ATSR User Workshop, Frascati, Italy, 22–26 September 2008. [Google Scholar]
- Gower, J.; King, S.; Goncalves, P. Global monitoring of plankton blooms using MERIS MCI. Int. J. Remote Sens. 2008, 29, 6209–6216. [Google Scholar] [CrossRef]
- Hansson, M.; Hakansson, B. The Baltic Algae Watch System—A remote sensing application for monitoring cyanobacterial blooms in the Baltic Sea. J. Appl. Remote Sens. 2007, 1, 011507. [Google Scholar] [CrossRef]
- Hansson, M.; Pemberton, P.; Håkansson, B.; Reinart, A.; Alikas, K. Operational Nowcasting of Algal Blooms in the Baltic Sea Using MERIS and MODIS. In Proceedings of the ESA Living Planet Symposium, Bergen, Norway, 28 June–2 July 2010. Special Publication SP-686. [Google Scholar]
- Kahru, M.; Savchuk, O.P.; Elmgren, R. Satellite measurements of cyanobacterial bloom frequency in the Baltic Sea: Interannual and spatial variability. Mar. Ecol. Prog. Ser. 2007, 343, 15–23. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R. Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences 2014, 11, 3619–3633. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R.; Kaiser, J.; Wasmund, N.; Savchuk, O. Cyanobacterial blooms in the Baltic Sea: Correlations with environmental factors. Harmful Algae 2020, 92, 101739. [Google Scholar] [CrossRef]
- Kutser, T.; Metsamaa, L.; Strömbeck, N.; Vahtmäe, E. Monitoring cyanobacterial blooms by satellite remote sensing. Estuar. Coast. Shelf Sci. 2006, 67, 303–312. [Google Scholar] [CrossRef]
- Reinart, A.; Kutser, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ. 2006, 102, 74–85. [Google Scholar] [CrossRef]
- Riha, S.; Krawczyk, H. Development of a remote sensing algorithm for cyanobacterial phycocyanin pigment in the Baltic Sea using neural network approach. In Proceedings of the Remote Sensing of the Ocean, Sea Ice, Coastal Waters, and Large Water Regions, Prague, Czech Republic, 7 October 2011. [Google Scholar] [CrossRef]
- Woźniak, M.; Bradtke, K.M.; Darecki, M.; Krężel, A. Empirical Model for Phycocyanin Concentration Estimation as an Indicator of Cyanobacterial Bloom in the Optically Complex Coastal Waters of the Baltic Sea. Remote Sens. 2016, 8, 212. [Google Scholar] [CrossRef]
- HELCOM Thematic Assessment of Eutrophication 2011–2016. Baltic Sea Environment Proceedings No. 156. Available online: http://www.helcom.fi/baltic-sea-trends/holistic-assessments/state-of-the-baltic-sea-2018/reports-and-materials (accessed on 27 July 2022).
- Kownacka, J.; Busch, S.; Göbel, J.; Gromisz, S.; Hällfors, H.; Höglander, H.; Huseby, S.; Jaanus, A.; Jakobsen, H.H.; Johansen, M.; et al. Cyanobacteria biomass, 1990–2019. HELCOM Baltic Sea Environment Fact Sheets 2020. Available online: https://helcom.fi/wp-content/uploads/2020/09/BSEFS-Cyanobacteria-biomass-1990-2019-1.pdf (accessed on 27 July 2022).
- Öberg, J. Cyanobacteria Blooms in the Baltic Sea. HELCOM Baltic Sea Environment Fact Sheets 2017. Available online: https://helcom.fi/wp-content/uploads/2020/06/BSEFS-Cyanobacteria-blooms-in-the-Baltic-Sea.pdf (accessed on 27 July 2022).
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Soja-Woźniak, M.; Craig, S.E.; Kratzer, S.; Wojtasiewicz, B.; Darecki, M.; Jones, C.T. A Novel Statistical Approach for Ocean Colour Estimation of Inherent Optical Properties and Cyanobacteria Abundance in Optically Complex Waters. Remote Sens. 2017, 9, 343. [Google Scholar] [CrossRef]
- Gower, J.; King, S.; Borstad, G.; Brown, L. Detection of intense plankton blooms using the 709 nm band of the MERIS imaging spectrometer. Int. J. Remote Sens. 2005, 26, 2005–2012. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Warner, R.A.; Tester, P.A.; Dyble, J.; Fahnenstiel, G.L. Relating spectral shape to cyanobacterial blooms in the Laurentian Great Lakes. Int. J. Remote Sens. 2008, 29, 3665–3672. [Google Scholar] [CrossRef]
- Gower, J.F.R.; Doerffer, R.; Borstad, G.A. Interpretation of the 685 nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens. 1999, 20, 1771–1786. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Dyble, J. Characterizing a cyanobacterial bloom in Western Lake Erie using satellite imagery and meteorological data. Limnol. Oceanogr. 2010, 55, 2025–2036. [Google Scholar] [CrossRef]
- Wynne, T.; Stumpf, R.; Briggs, T. Comparing MODIS and MERIS spectral shapes for cyanobacterial bloom detection. Int. J. Remote Sens. 2013, 34, 6668–6678. [Google Scholar] [CrossRef]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Report of SCOR-UNESCO Working Group 17 on Determination of Photosynthetic Pigments. In Determination of Photosynthetic Pigments in Sea-Water; UNESCO: Paris, France, 1966; pp. 9–16.
- Artemiev, V.A.; Burenkov, V.I.; Vortman, M.I.; Grigoriev, A.V.; Kopelevich, O.V.; Khrapko, A.N. Sea-truth measurements of ocean color: A new floating spectroradiometer and its metrology. Oceanology 2000, 40, 139–145. [Google Scholar]
- Vazyulya, S.; Khrapko, A.; Kopelevich, O.; Burenkov, V.; Eremina, T.; Isaev, A. Regional algorithms for the estimation of chlorophyll and suspended matter concentration in the Gulf of Finland from MODIS-Aqua satellite data. Oceanologia 2014, 56, 737–756. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters I A semianalytical model. Appl. Opt. 1998, 37, 6329–6338. [Google Scholar] [CrossRef]
- Seppälä, J.; Ylöstalo, P.; Kaitala, S.; Hällfors, S.; Raateoja, M.; Maunula, P. Ship-of-opportunity based phycocyanin fluorescence monitoring of the filamentous cyanobacteria bloom dynamics in the Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 73, 489–500. [Google Scholar] [CrossRef]
- Maximov, A.A.; Eremina, T.R.; Lange, E.K.; Litvinchuk, L.F.; Maximova, O.B. Regime shift in the ecosystem of the eastern Gulf of Finland caused by the invasion of the polychaete Marenzelleria arctia. Oceanology 2014, 54, 46–53. [Google Scholar] [CrossRef]
- Burenkov, V.I.; Ershova, S.V.; Kopelevich, O.V.; Sheberstov, S.V.; Shevchenko, V.P. An estimate of the distribution of sus-pended matter in the Barents Sea waters on the basis of the SeaWiFS satellite ocean color scanner. Oceanology 2001, 41, 622–628. [Google Scholar]
- Wasmund, N. Occurrence of cyanobacterial blooms in the baltic sea in relation to environmental conditions. Int. Rev. Hydrobiol. 1997, 82, 169–184. [Google Scholar] [CrossRef]
- Tiede, J.; Jordan, C.; Moghimi, A.; Schlurmann, T. Long-term shoreline changes at large spatial scales at the Baltic Sea: Remote-sensing based assessment and potential drivers. Front. Mar. Sci. 2023, 10, 1207524. [Google Scholar] [CrossRef]
- Cazzaniga, I.; Zibordi, G.; Mélin, F. Spectral features of ocean colour radiometric products in the presence of cyanobacteria blooms in the Baltic Sea. Remote Sens. Environ. 2023, 287, 113464. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Pardo, S.; Simis, S.G.H.; Qin, P.; Selmes, N.; Dessailly, D.; Kwiatkowska, E. Consistency between Satellite Ocean Colour Products under High Coloured Dissolved Organic Matter Absorption in the Baltic Sea. Remote Sens. 2021, 14, 89. [Google Scholar] [CrossRef]
- Qin, P.; Simis, S.G.; Tilstone, G.H. Radiometric validation of atmospheric correction for MERIS in the Baltic Sea based on continuous observations from ships and AERONET-OC. Remote Sens. Environ. 2017, 200, 263–280. [Google Scholar] [CrossRef]
- Chen, C.; Liang, J.; Yang, G.; Sun, W. Spatio-temporal distribution of harmful algal blooms and their correlations with marine hydrological elements in offshore areas, China. Ocean Coast. Manag. 2023, 238, 106554. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Sahling, I.V.; Vazyulya, S.V.; Glukhovets, D.I.; Sheberstov, S.V.; Burenkov, V.I.; Karalli, P.G.; Yushmanova, A.V. Bio-Optical Characteristics of the Seas, Surrounding the Western Part of Russia, from Data of the Satellite Ocean Color Scanners of 1998–2017; VASh FORMAT, OOO: Moscow, Russia, 2018. [Google Scholar]
| Algorithm | R2 | RMSE, mg m−3 | CV, % | Ratio | MPD, % |
|---|---|---|---|---|---|
| bbp based: Bcyan = 64.6 bbp 103 − 263 | 0.55 | 282 | 75 | 1.5 | 60 |
| Chl based: Bcyan = 92 Chl + 6 | 0.50 | 304 | 81 | 1.1 | 52 |
| Multi-regression: Bcyan = 45 bbp 103 + 38.5 Chl − 227 | 0.61 | 272 | 72 | 1.4 | 54 |
| Input Dataset | <model> *, mg m−3 | <model>/ <measured> | R2 | RMSE, mg m−3 | Ratio | Range of ‘mod/meas’ |
|---|---|---|---|---|---|---|
| #1 in situ | 522 | 0.83 | 0.59 | 428 | 1.31 | 0.34–3.61 |
| #2 MODIS L2, 1 px | 527 | 0.84 | 0.66 | 393 | 1.53 | 0.53–3.41 |
| #3 MODIS L2, 9 px | 564 | 0.90 | 0.75 | 332 | 1.45 | 0.58–4.18 |
| #4 MODIS L3 | 506 | 0.81 | 0.56 | 443 | 1.22 | 0.42–7.40 |
| Year | <Bcyan>, mg m−3 | Bloom Area, 103 km2 | |
|---|---|---|---|
| >300 mg m−3 | >600 mg m−3 | ||
| 2003 | 297 ± 148 | 5.1 (35%) | 0.7 (5%) |
| 2004 | 598 ± 205 | 14.5 (100%) | 6.2 (43%) |
| 2005 | 434 ± 182 | 12.6 (87%) | 2.1 (15%) |
| 2006 | 422 ± 219 | 11.2 (77%) | 1.6 (11%) |
| 2007 | 543 ± 308 | 13.6 (94%) | 3.3 (22%) |
| 2008 | 401 ± 265 | 8.2 (56%) | 1.9 (13%) |
| 2009 | 247 ± 150 | 3.0 (21%) | 0.5 (3%) |
| 2010 | 231 ± 135 | 2.7 (19%) | 0.4 (3%) |
| 2011 | 289 ± 150 | 5.2 (36%) | 0.4 (3%) |
| 2012 | 325 ± 181 | 6.2 (43%) | 0.8 (5%) |
| 2013 | 347 ± 209 | 7.1 (49%) | 1.4 (9%) |
| 2014 | 240 ± 205 | 3.0 (20%) | 0.9 (6%) |
| 2015 | 498 ± 313 | 11.3 (78%) | 3.3 (23%) |
| 2016 | 283 ± 173 | 4.4 (30%) | 0.8 (6%) |
| 2017 | 289 ± 204 | 4.3 (30%) | 0.9 (6%) |
| 2018 | 360 ± 121 | 9.9 (68%) | 0.4 (3%) |
| 2019 | 451 ± 163 | 13.6 (94%) | 1.5 (10%) |
| 2020 | 518 ± 256 | 13.1 (91%) | 3.7 (26%) |
| 2021 | 302 ± 142 | 6.1 (42%) | 0.6 (4%) |
| 2022 | 365 ± 217 | 8.4 (58%) | 1.6 (11%) |
| 2003–2022 | 372 ± 107 | 8.2 (56%) | 1.6 (11%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazyulya, S.; Kopelevich, O.; Sahling, I.; Kochetkova, E.; Lange, E.; Khrapko, A.; Eremina, T.; Glukhovets, D. Regional Algorithm of Quantitative Assessment of Cyanobacteria Blooms in the Eastern Part of the Gulf of Finland Using Satellite Ocean Color Data. J. Mar. Sci. Eng. 2023, 11, 1746. https://doi.org/10.3390/jmse11091746
Vazyulya S, Kopelevich O, Sahling I, Kochetkova E, Lange E, Khrapko A, Eremina T, Glukhovets D. Regional Algorithm of Quantitative Assessment of Cyanobacteria Blooms in the Eastern Part of the Gulf of Finland Using Satellite Ocean Color Data. Journal of Marine Science and Engineering. 2023; 11(9):1746. https://doi.org/10.3390/jmse11091746
Chicago/Turabian StyleVazyulya, Svetlana, Oleg Kopelevich, Inna Sahling, Ekaterina Kochetkova, Evgenia Lange, Alexander Khrapko, Tatyana Eremina, and Dmitry Glukhovets. 2023. "Regional Algorithm of Quantitative Assessment of Cyanobacteria Blooms in the Eastern Part of the Gulf of Finland Using Satellite Ocean Color Data" Journal of Marine Science and Engineering 11, no. 9: 1746. https://doi.org/10.3390/jmse11091746








