Investigation on the Influence of Different Coating Surfaces on the Adhesive Force of Hydrate Particles
Abstract
:1. Introduction
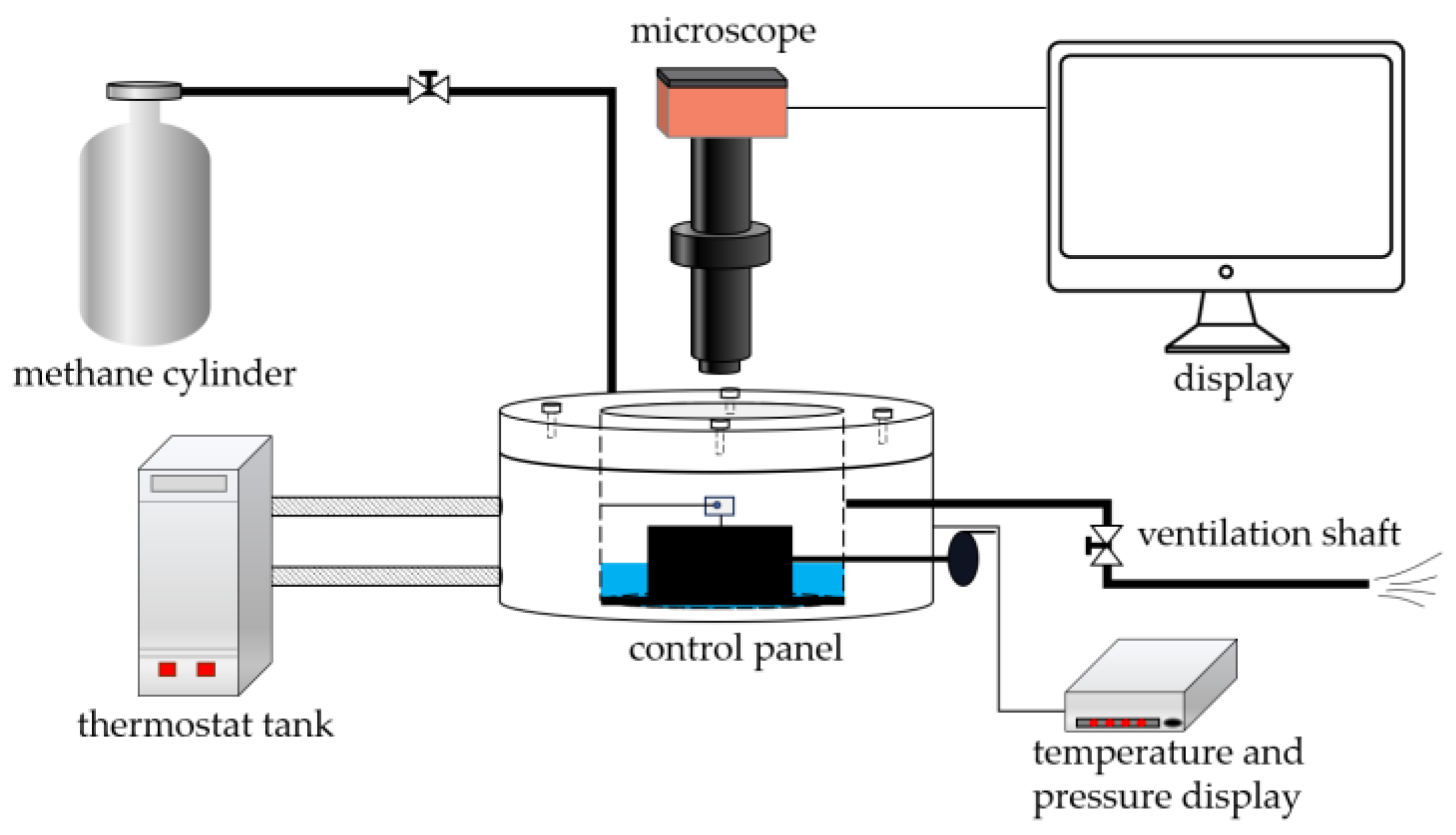
2. Experimental Setup and Methods
2.1. Experimental Materials and Instruments
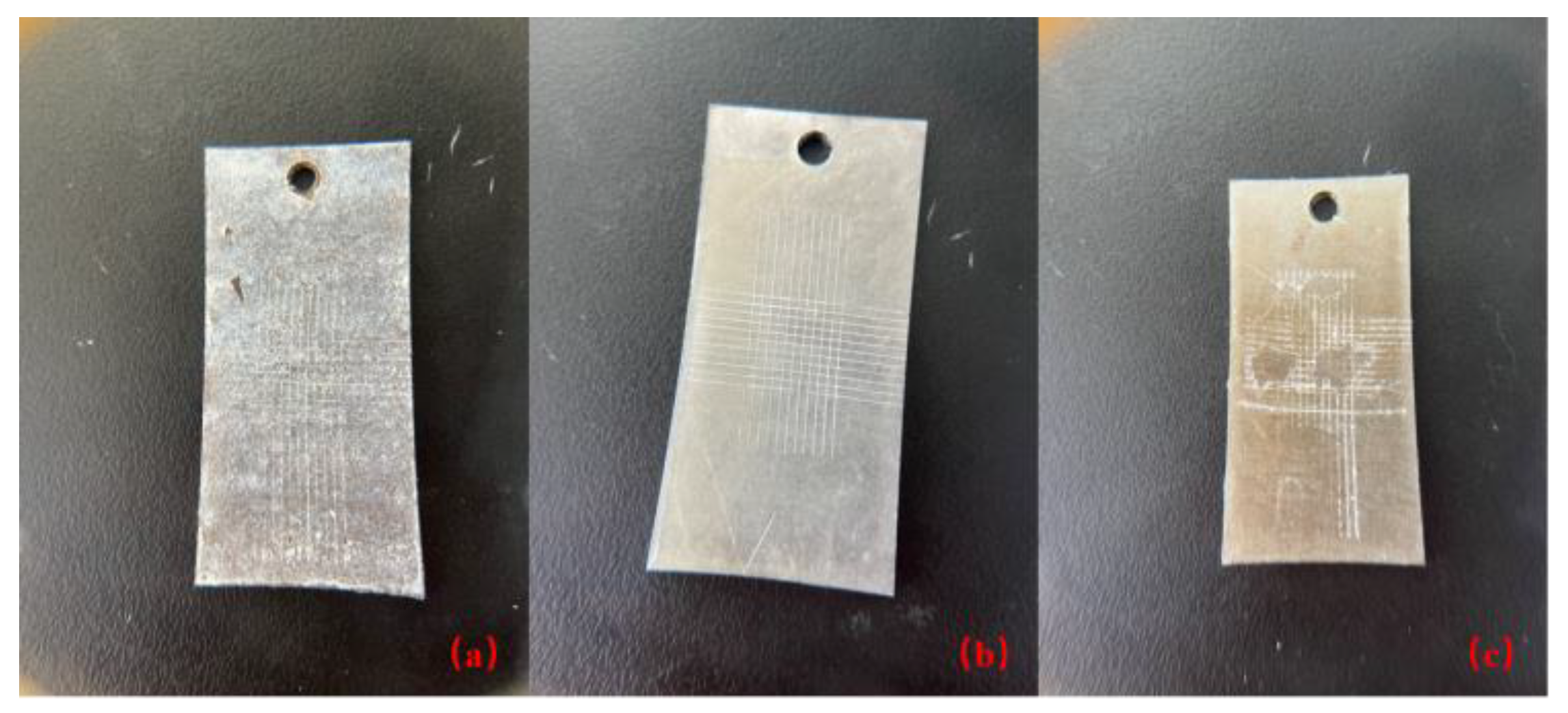
2.2. Preparation and Testing of Coatings
- (1)
- Good corrosion resistance.
- (2)
- Strong pressure resistance.
- (3)
- Easy to paint.
- (4)
- Chemically stable.
- (5)
- Strong adhesion and bending resistance.
- (6)
- Abrasion resistance.
- (7)
- Heat resistance.
- (8)
- Smooth coating surface.
- (1)
- Weigh the coating materials and add them to a beaker, incorporating leveling agents, dispersants, and defoaming agents. Stir thoroughly until all components are uniformly mixed, filter, and let the coating mature before use.
- (2)
- Mechanically clean the coating substrate by sanding, then remove any particles and rust resulting from the sanding process. Control the surface roughness of the substrate within a certain range; a certain degree of roughness is retained to aid in the adhesion of the coating to the substrate surface.
- (3)
- Wash with deionized water and dry in a high-temperature oven.
- (4)
- Apply the coating to the substrate uniformly in three separate spray applications.
- (5)
- After spraying, dry the coating in a high-temperature oven for 2 h.
2.3. Coating Adhesion Test
- (1)
- Sample Preparation: The substrate with the coating to be tested must be dry, cured, and under appropriate conditions for testing. Typically, the coating should be fully dried to ensure the accuracy of the test results.
- (2)
- Scribing: Use a crosshatch cutter (Figure 3) or other appropriate tool to scribe a series of perpendicular lines onto the surface of the coating, creating a lattice or grid pattern. The spacing between lines is usually 1 to 2 mm in each direction.
- (3)
- Cleaning: Ensure that the substrate is thoroughly cleaned before and after scribing to remove any impurities that may affect the test outcome.
- (4)
- Applying Tape: Apply a piece of transparent adhesive tape over the scribed grid, making sure the tape makes tight contact with the coating surface and press down gently to ensure adhesion.
- (5)
- Tape Removal: Quickly and evenly remove the adhesive tape. This will cause parts of the coating to be detached along with the tape.
- (6)
- Evaluation: Examine the area from which the tape was removed to check the condition of the peeled coating. The quality of the coating adhesion is assessed by visual inspection or with a microscope, depending on the extent of the detachment and the condition of the flaked-off coating.
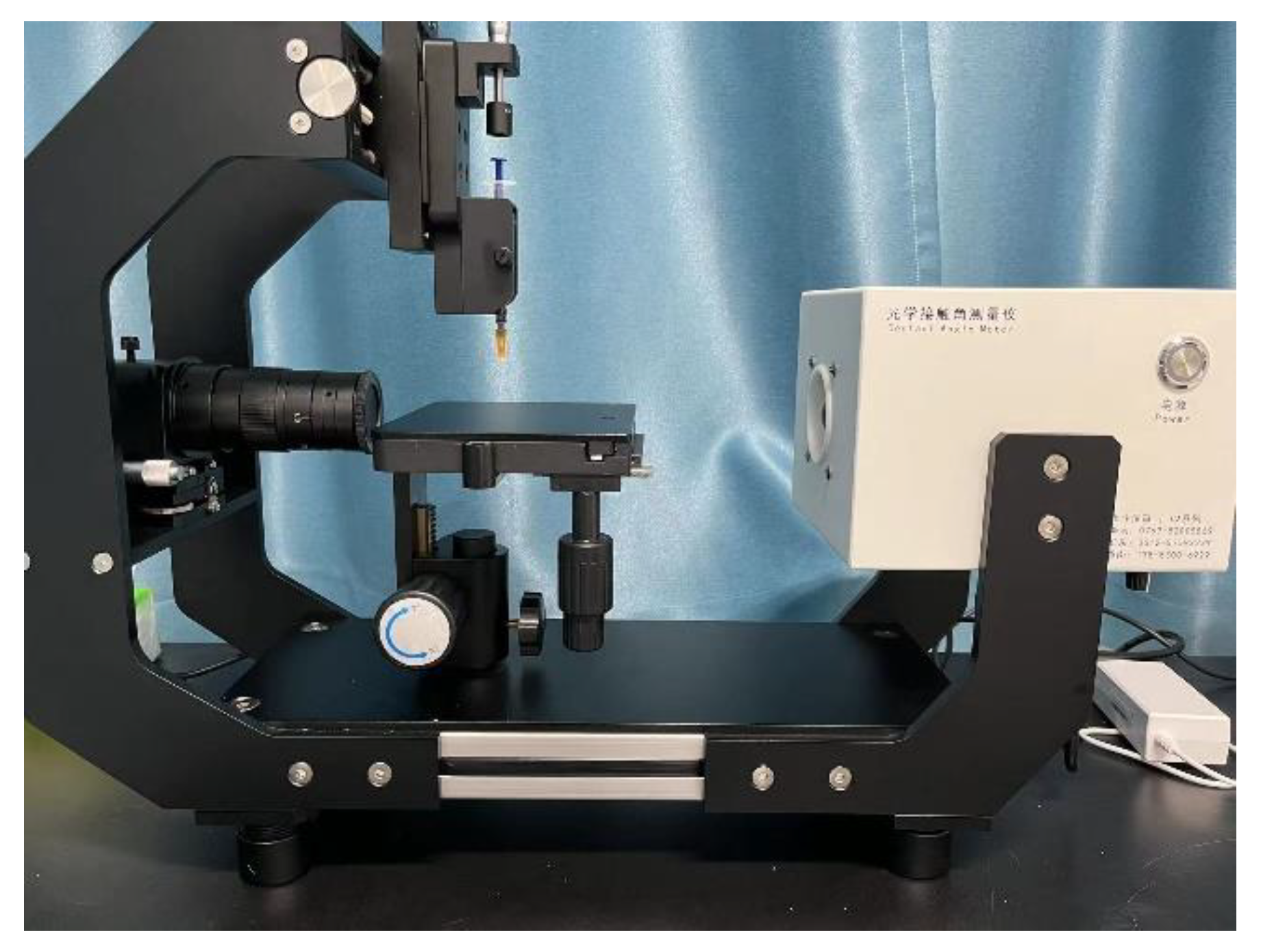
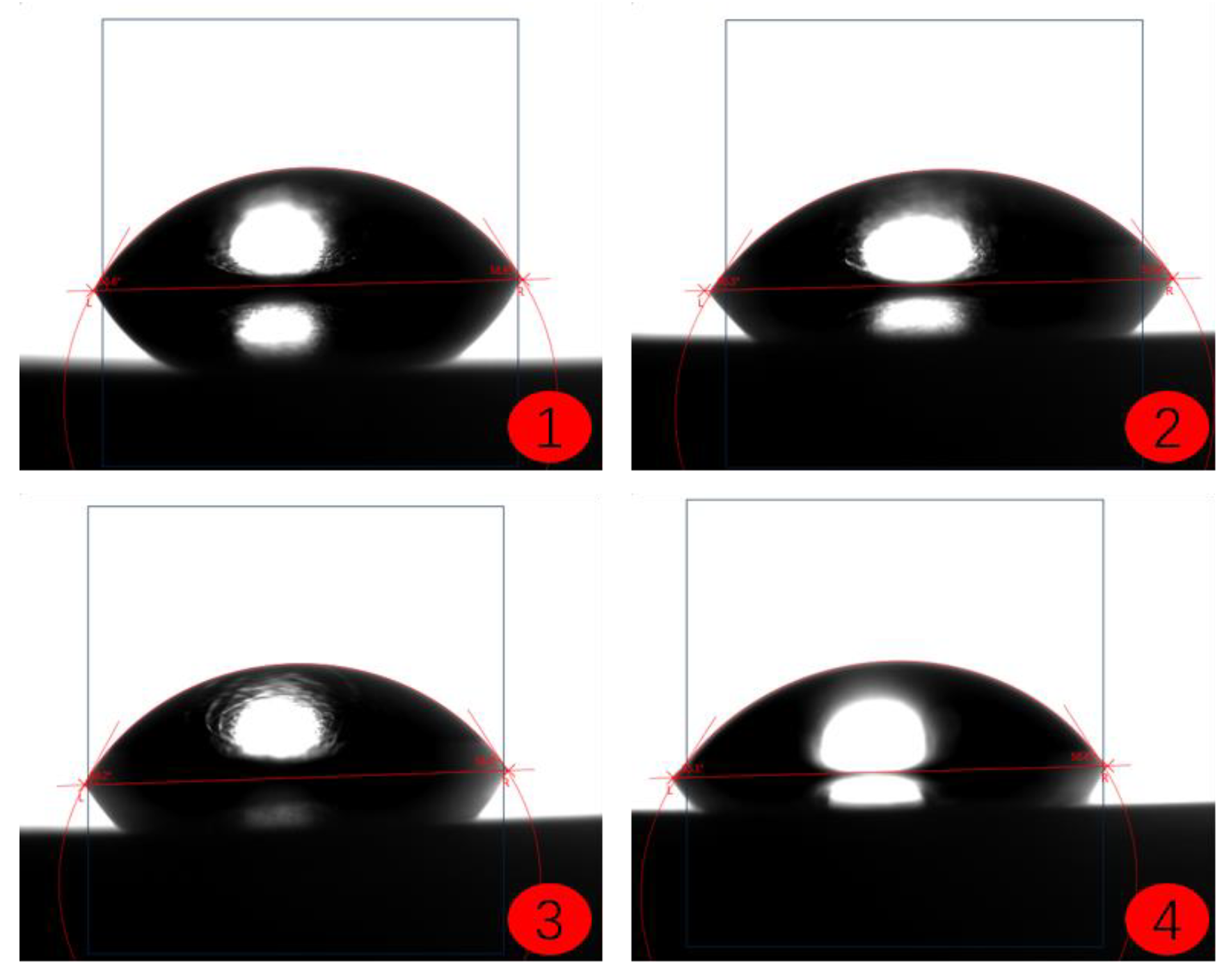
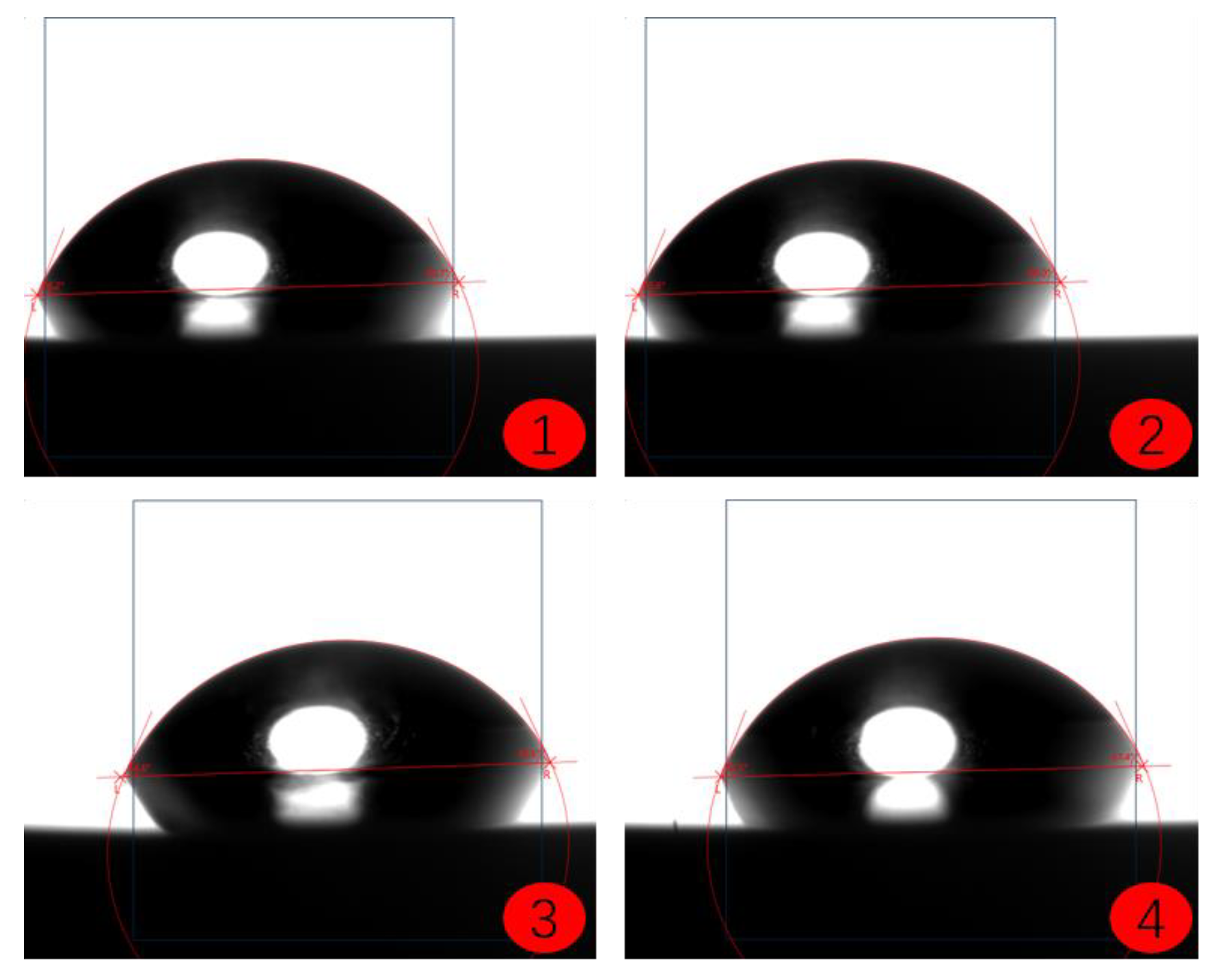
2.4. Coating Contact Angle Test
- (1)
- Sample Preparation: Prepare the substrate coated with the coating to be tested, ensuring that the surface is clean, dry, and free from impurities such as dust or dirt.
- (2)
- Droplet Deposition: Deposit a liquid droplet onto the surface of the coating, making sure that the size and shape of the droplet are uniform and stable. A single droplet is commonly used for testing to ensure consistency.
- (3)
- Image Acquisition: Use an optical contact angle meter (Figure 4) to capture the image of the droplet on the coating surface. Ensure the image quality is high enough for accurate contact angle measurement.
- (4)
- Contact Angle Measurement: Measure the contact angle on the captured image using image analysis software. The contact angle is the angle at which the liquid droplet edge meets the coating surface and is typically expressed in degrees (°).
- (5)
- Repeat Tests: To obtain more reliable results, perform multiple tests on the same coating sample and calculate the average contact angle.
- (1)
- Contact angles less than 90 degrees are typically indicative of hydrophilic surfaces due to the liquid’s ability to spread across the surface.
- (2)
- Contact angles greater than 90 degrees are usually indicative of hydrophobic surfaces, as the liquid does not spread across the surface, but instead forms spherical beads.
2.5. Testing of the Adhesion of Coatings to Hydrate Particles
- (1)
- Start the thermostatic water bath to cool the solution to the specified temperature and maintain a constant temperature.
- (2)
- Fix the substrate coating (5 mm× 5 mm× 0.3 mm) on the fixed operating arm, ensuring that the coating attachment surface is perpendicular to the overhead microscope.
- (3)
- The experiment utilizes ice particles to induce the formation of hydrate particles. Firstly, place a spherical droplet on the tip of the cantilever glass fiber, freeze it in liquid nitrogen, expose it to air for 2 s, freeze it in liquid nitrogen for 2 s, and repeat this process several times until the surface of the ice particle becomes smooth.
- (4)
- Rapidly place the ice particle into the reaction vessel and tightly seal the lid.
- (5)
- Open the inlet valve and slowly increase the pressure to 0.5 MPa.
- (6)
- Open the outlet valve and slowly release the methane gas.
- (7)
- Close the outlet valve, open the inlet valve, and gradually increase the pressure to the experimental pressure during the gas introduction process, maintaining the temperature of the reaction vessel below the freezing point of the reagent.
- (8)
- After the gas introduction is completed, start heating, and gradually raise the temperature of the sealed chamber to the experimental temperature.
- (9)
- During the heating process, a thin layer of hydrate forms gradually on the surface of the ice particle. The ice particle gradually transforms into hydrate from the outside to the inside, and the hydrate layer thickens while the particle transparency decreases.
- (10)
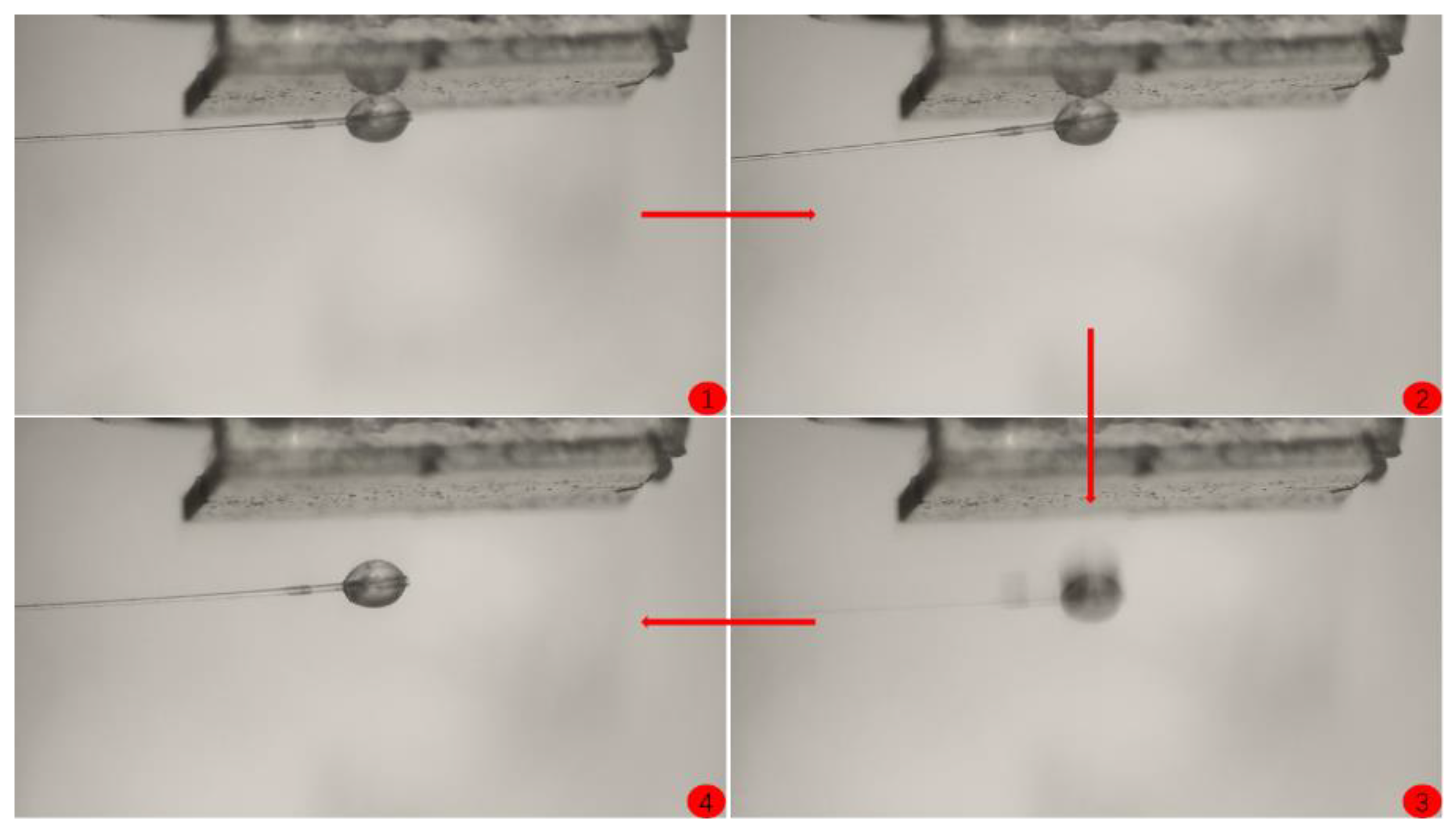
- Move the hydrate particle at the end of the glass fiber within the line of sight and ensure that the glass fiber is parallel to the substrate coating.
- (11)
- Keep the position of the substrate fixed, move the manual operating arm to make the hydrate particle contact the substrate, and apply a certain pressure for 5 s. Slowly pull the manual cantilever, causing the glass fiber at the end of the operating arm to bend, resulting in the separation of the hydrate particle from the substrate.
3. Results and Discussion
3.1. Coating Adhesion Test Results
3.2. Coating Contact Angle Test Results
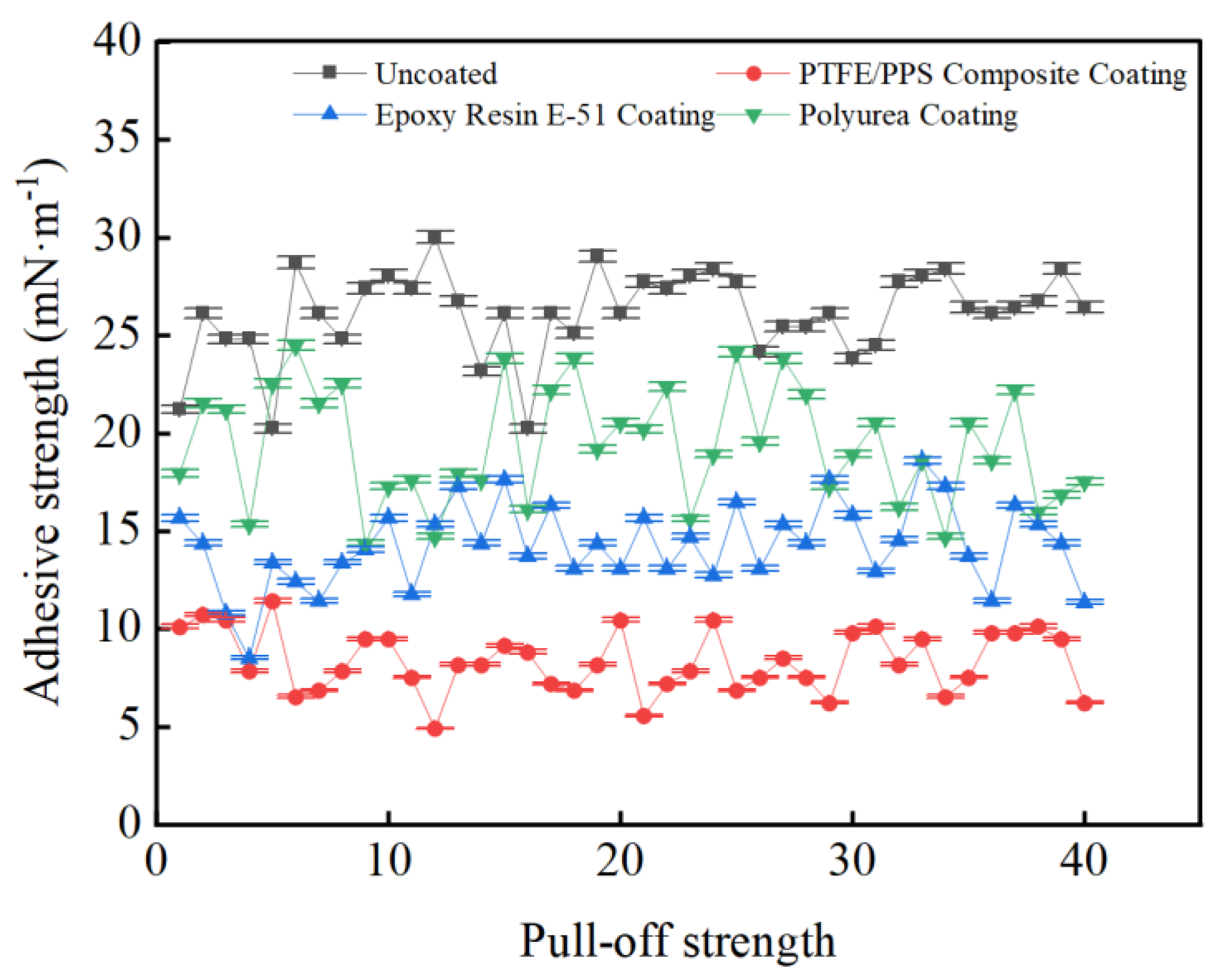
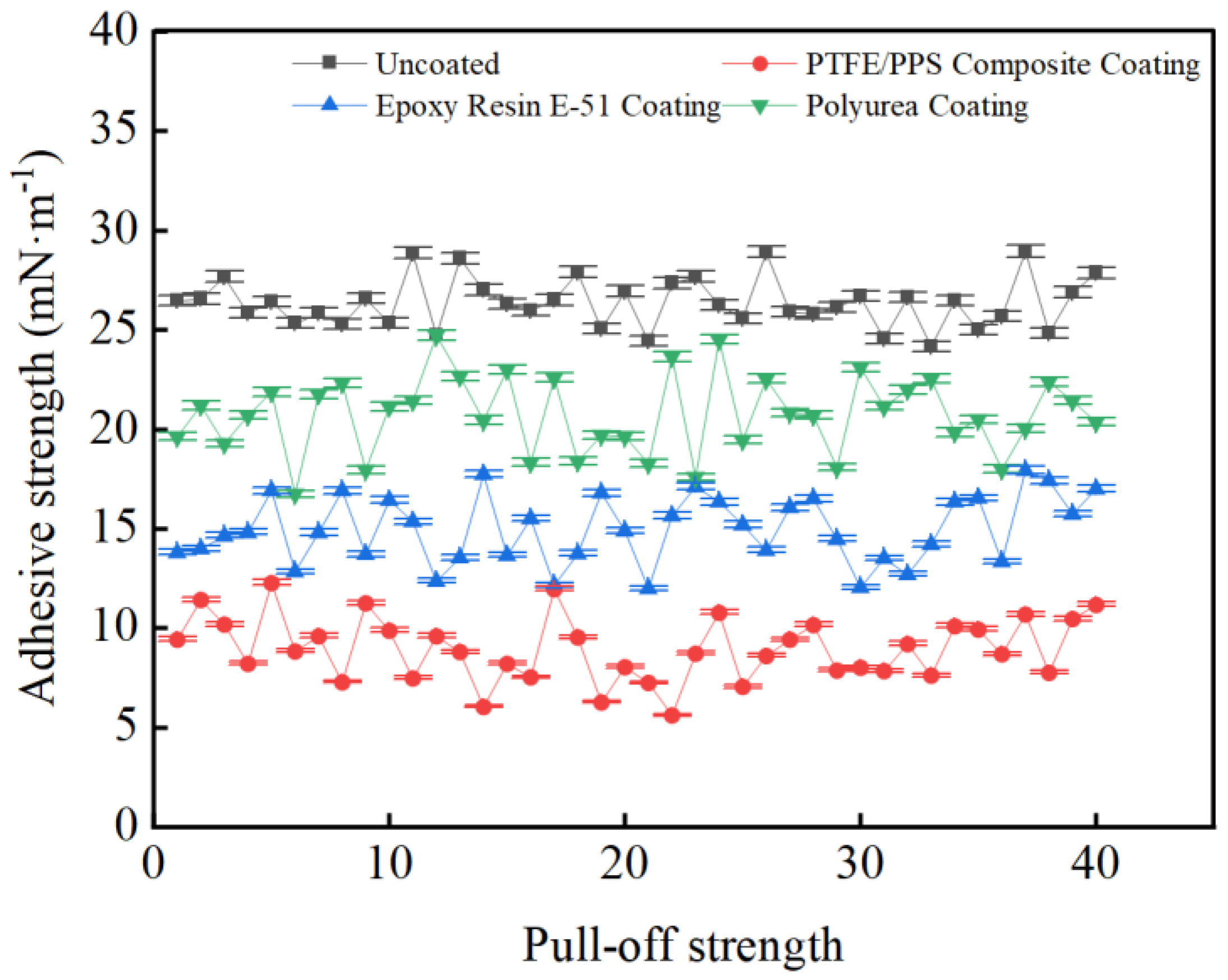
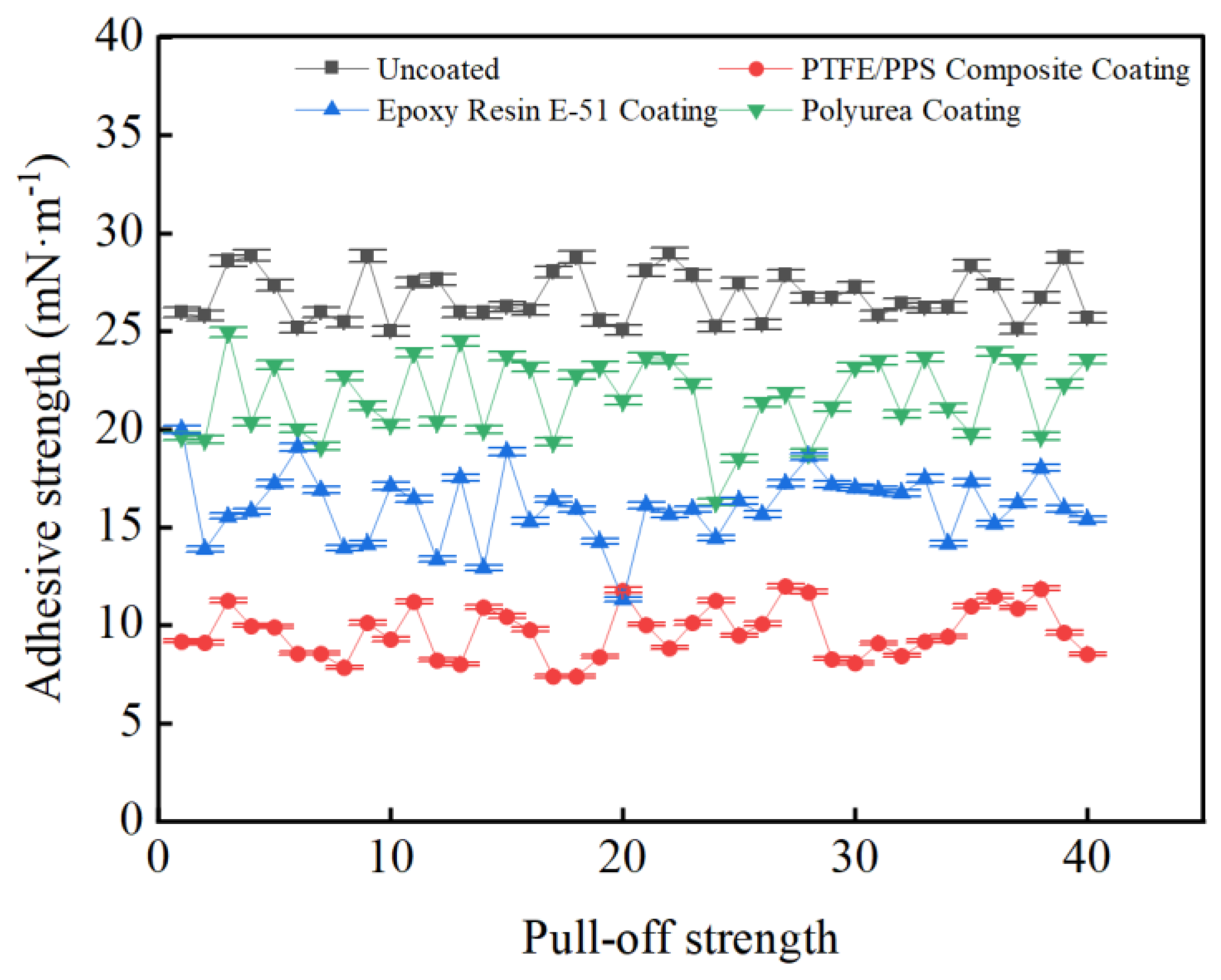
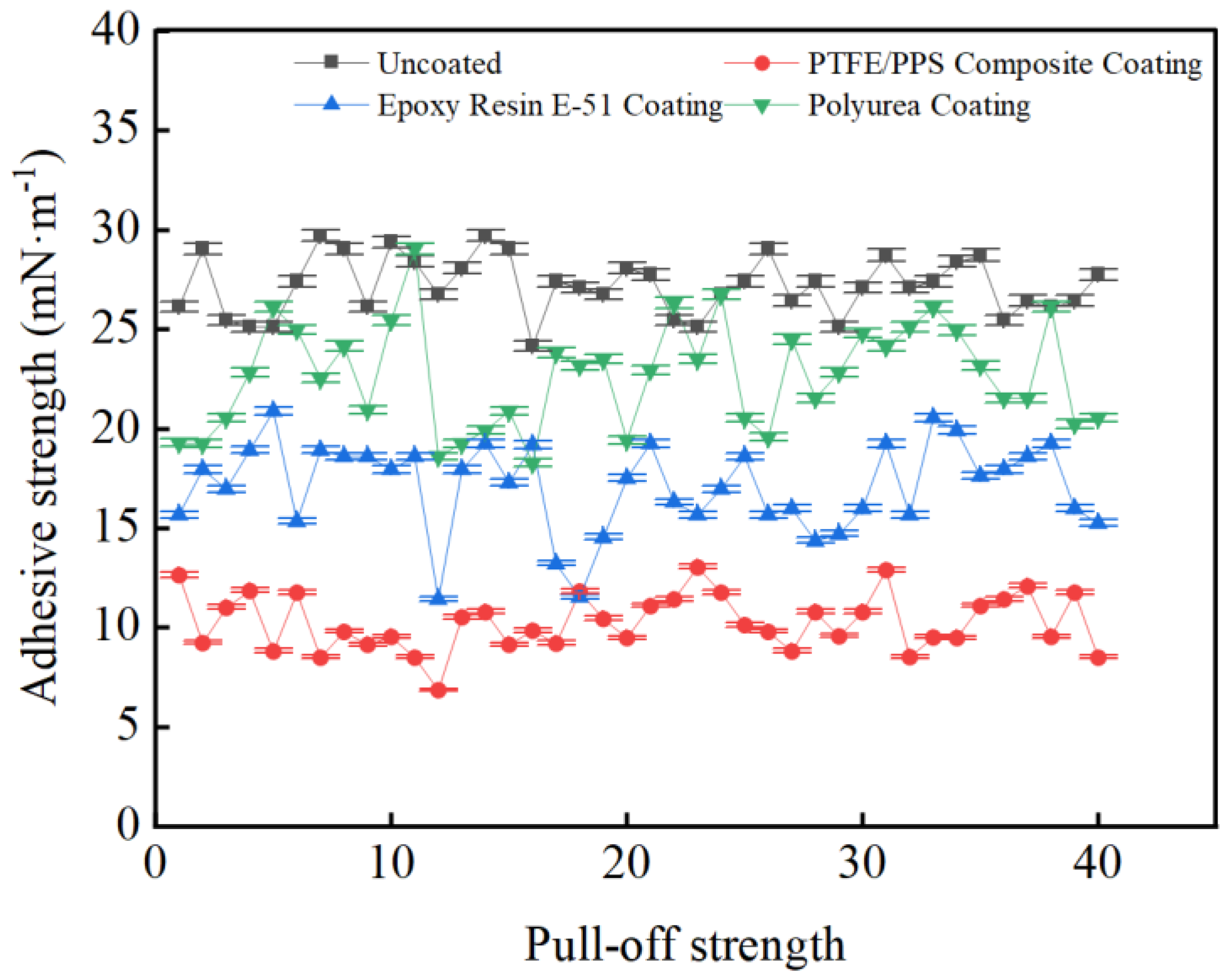
3.3. Coating Adhesion to Hydrate Particles Test Results
4. Conclusions
- (1)
- The adhesion test results indicate that among the three selected coating materials, the PTFE/PPS composite coating has an adhesion grade of 1, while the epoxy resin E-51 coating has an adhesion grade of 0, demonstrating good adhesion performance and meeting the adhesion requirements of practical working conditions. In comparison, the polyurea coating has an adhesion grade of 3, indicating poor adhesion and a significant peeling phenomenon, thus not meeting the requirements for coating usage.
- (2)
- The contact angle test results of the coatings show that among the three selected coating materials, the PTFE/PPS composite coating has an average contact angle of 105.63°, exhibiting the best hydrophobicity. The epoxy resin E-51 coating has an average contact angle of 67.75°, and the polyurea coating has an average contact angle of 66.04°, indicating some hydrophobicity compared to the uncoated substrate. In comparison, the PTFE/PPS composite coating is more suitable for the coating method to prevent hydrate formation in gas pipelines.
- (3)
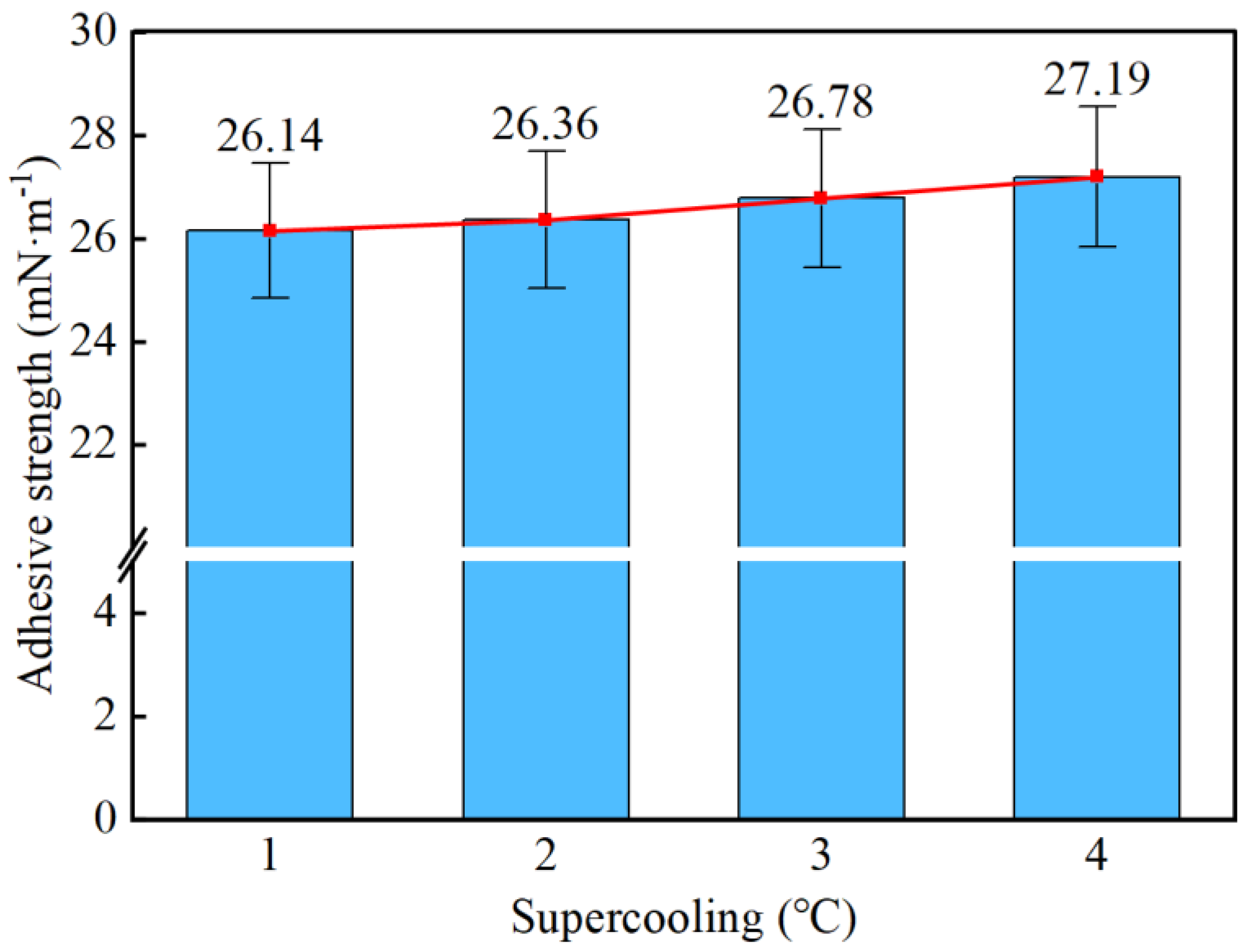
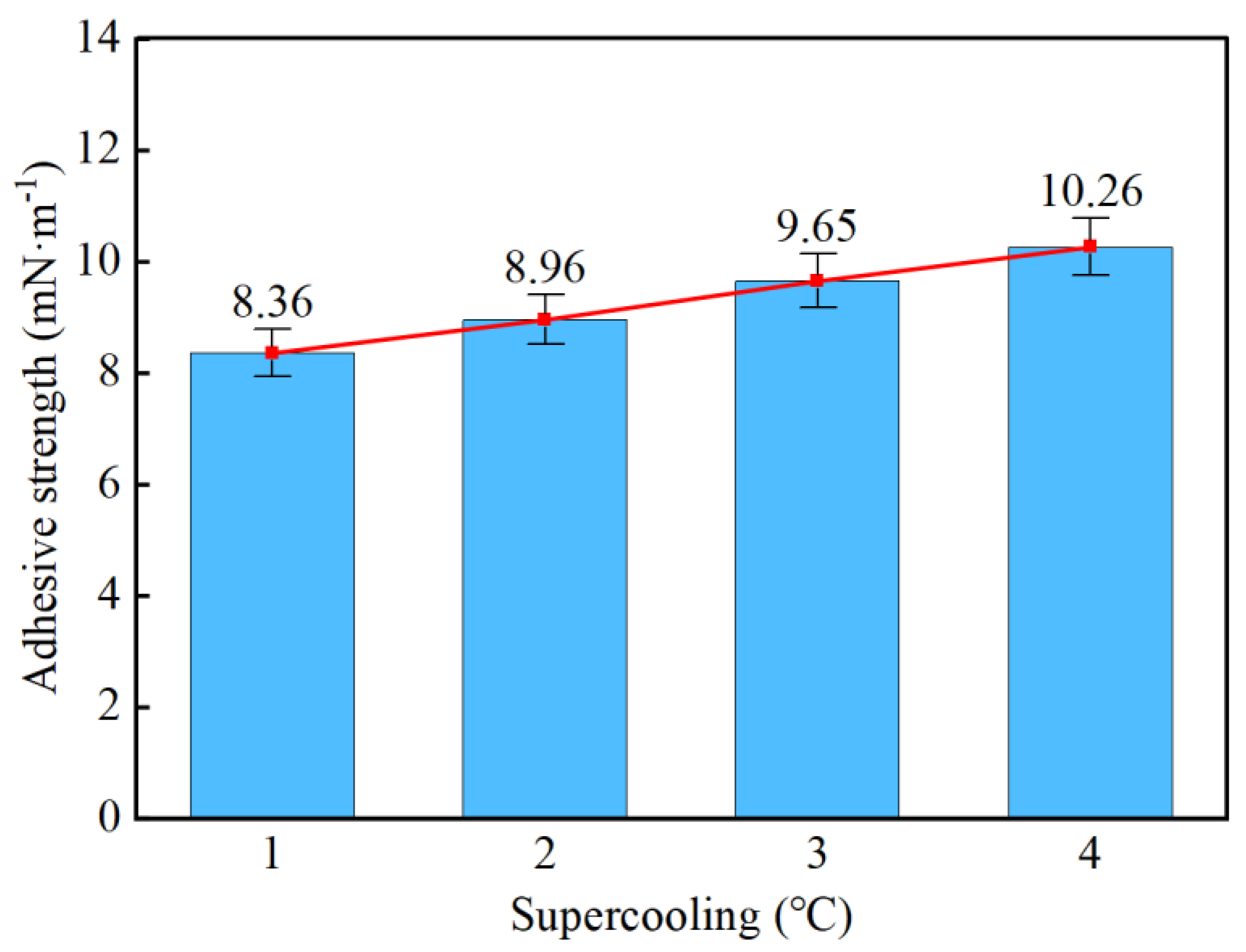
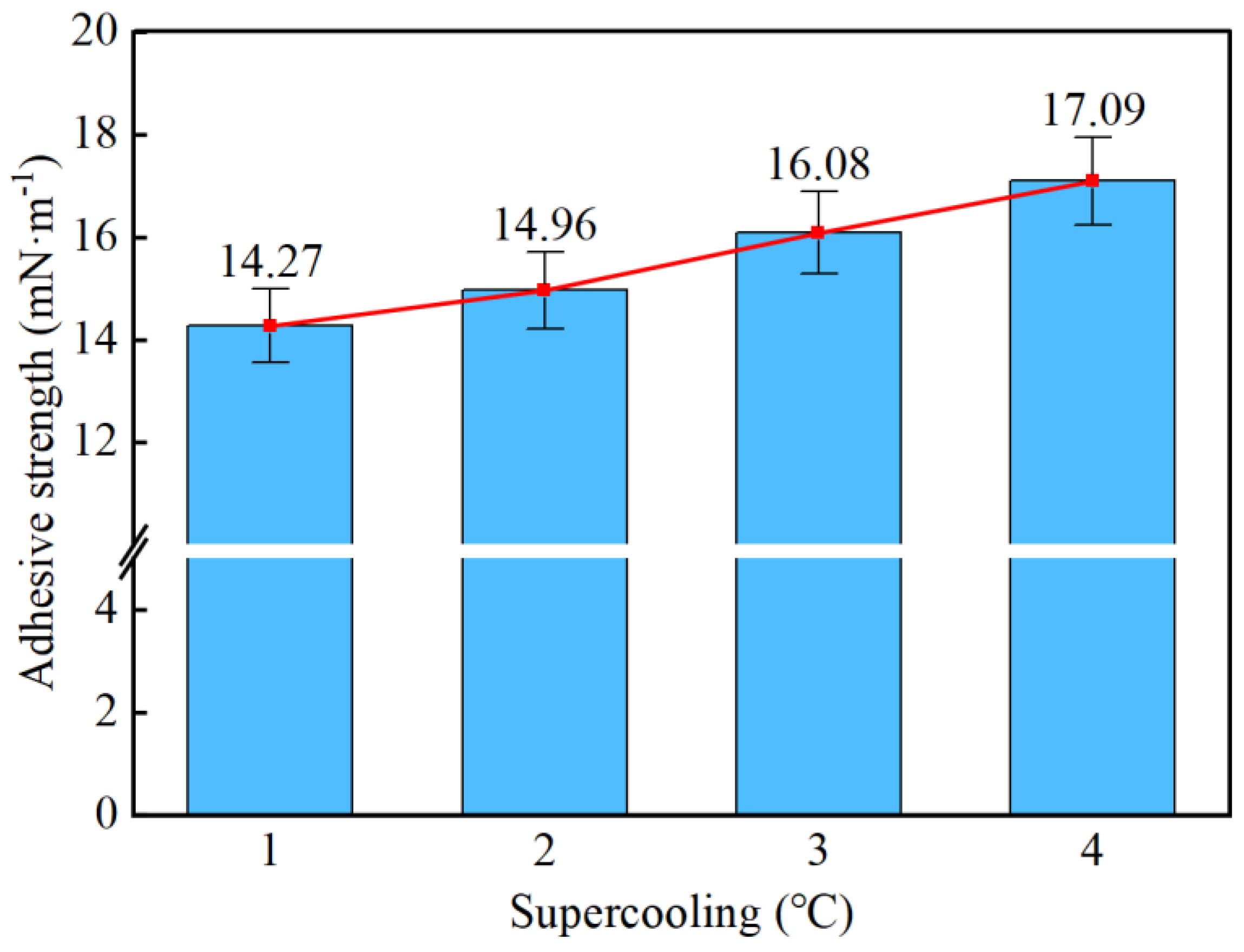
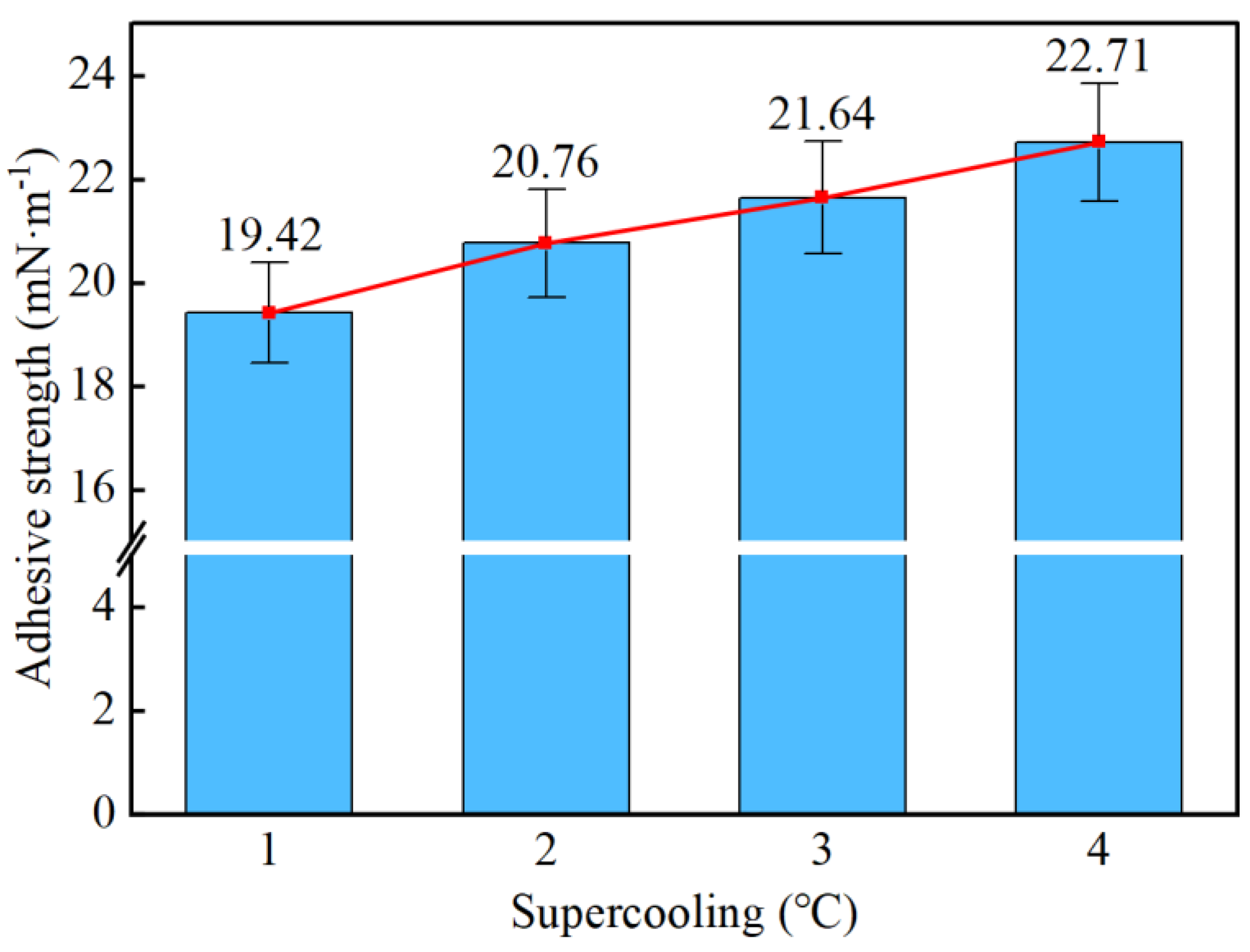
- Taking a subcooling temperature of 1 °C as an example, the average adhesion force between hydrate particles and the uncoated conventional substrate is 26.14 mN·m−1. The average adhesion force between hydrate particles and the PTFE/PPS composite-coated substrate is 8.36 mN·m−1, between hydrate particles and the epoxy resin E-51-coated substrate is 14.27 mN·m−1, and between hydrate particles and the polyurea-coated substrate is 19.42 mN·m−1. This indicates that the PTFE/PPS composite coating has an advantage in reducing the adhesion force of hydrate particles under low temperatures and different subcooling conditions. Choosing the PTFE/PPS composite coating as the wall coating is a preferable option for preventing hydrate accumulation. Additionally, the epoxy resin E-51 coating and polyurea coating also demonstrate certain anti-adhesion performance characteristics, but their performance is slightly inferior compared to the PTFE/PPS composite coating.
- (4)
- The epoxy resin E-51 coating and polyurea coating also exhibit some resistance to hydrate adhesion, especially under lower subcooling conditions. Although their performance is relatively inferior to the PTFE/PPS composite coating, they still have the ability to reduce the adhesion force of hydrate particles in certain specific application scenarios.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sojoudi, H.; Walsh, M.R.; Gleason, K.K.; McKinley, G.H. Designing Durable Vapor-Deposited Surfaces for Reduced Hydrate Adhesion. Adv. Mater. Interfaces 2015, 2, 1500003. [Google Scholar] [CrossRef]
- Smith, J.D.; Meuler, A.J.; Bralower, H.L.; Venkatesan, R.; Subramanian, S.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Hydrate-phobic surfaces: Fundamental studies in clathrate hydrate adhesion reduction. Phys. Chem. Chem. Phys. 2012, 14, 6013–6020. [Google Scholar] [CrossRef]
- Sojoudi, H.; Walsh, M.R.; Gleason, K.K.; McKinley, G.H. Investigation into the Formation and Adhesion of Cyclopentane Hdyrates on Mechanically Robust Vapor-Deposited Polymeric Coatings. Langmuir 2015, 31, 6186–6196. [Google Scholar] [CrossRef]
- Aspenes, G.; Dieker, L.E.; Aman, Z.M.; Høiland, S.; Sum, A.K.; Koh, C.A.; Sloan, E.D. Adhesion force between cyclopentane hydrates and solid surface materials. J. Colloid Interface Sci. 2009, 343, 529–536. [Google Scholar] [CrossRef]
- Aman, Z.M.; Leith, W.J.; Grasso, G.A.; Sloan, E.D.; Sum, A.K.; Koh, C.A. Adhesion force between cyclopentane hydrate and mineral surfaces. Langmuir 2013, 29, 15551–15557. [Google Scholar] [CrossRef]
- Aman, Z.M.; Sloan, E.D.; Sum, A.K.; Koh, C.A. Adhesion force interactions between cyclopentane hydrate and physically and chemically modified surfaces. Phys. Chem. Chem. Phys. 2014, 16, 25121–25128. [Google Scholar] [CrossRef]
- Das, A.; Farnham, T.A.; Bengaluru Subramanyam, S.; Varanasi, K.K. Designing Ultra-Low Hydrate Adhesion Surfaces by Interfacial Spreading of Water-Immiscible Barrier Films. ACS Appl. Mater. Interfaces 2017, 9, 21496–21502. [Google Scholar] [CrossRef]
- Sojoudi, H.; Arabnejad, H.; Raiyan, A.; Shirazi, S.A.; McKinley, G.H.; Gleason, K.K. Scalable and durable polymeric icephobic and hydrate-phobic coatings. Soft Matter 2018, 14, 3443–3454. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a Surface-Active Coating for Promoted Gas Hydrate Formation. Chem. Ing. Tech. 2019, 91, 85–91. [Google Scholar] [CrossRef]
- Wang, S.; Fan, S.; Song, Y.; Lang, X.; Wang, Y.; Li, G.; Chen, J. The effect of NaCl concentration and corrosion on cohesion/adhesion forces of structure-II hydrate particles. Sci. Sin. Phys. Mech. Astron. 2018, 49, 034608. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Pei, H.; Liu, W.; Guo, W.; Fang, M.; Liu, N.; Mo, Z. Polyphenylene sulfide hydrophobic composite coating with high stability, corrosion resistance and antifouling performance. Surf. Interfaces 2021, 27, 101577. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Tian, J.; Yan, C.; Li, M. Fundamental investigation of the adhesion strength between cyclopentane hydrate deposition and solid surface materials. Chem. Eng. Sci. 2020, 217, 115524. [Google Scholar]
- Zhao, J.; Liu, Y.; Yang, L.; Zhang, L.; Song, Y. Organics-coated nanoclays further promote hydrate formation kinetics. J. Phys. Chem. Lett. 2021, 12, 3464–3467. [Google Scholar] [CrossRef]
- Ma, R.; Wang, F.; Chang, Y.; Xiao, S.; English, N.J.; He, J.; Zhang, Z. Unraveling adhesion strength between gas hydrate and solid surfaces. Langmuir 2021, 37, 13873–13881. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.I.; Qian, T.; Ahmed, W.; Zhan, X.; Chen, F.; Cheng, D.; Zhang, Q. Durable Hydrate-phobic Coating with In Situ Self-Replenishing Hydrocarbon Barrier Films for Low Clathrate Hydrate Adhesion. Langmuir 2022, 38, 11621–11630. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between water wettability and ice adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D. Hydrate-Phobic Surfaces. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2011. [Google Scholar]
- Ning, L. Application research on inner coating of natural gas pipeline. Coal & Chemicals, 2021. Available online: http://www.mtyhg.com.cn/EN/10.19286/j.cnki.cci.2021.03.043 (accessed on 1 November 2023).
- Asante, B. Justification for Internal Coating of Natural Gas Pipelines. ASME 1995, 211, 1111–1130. [Google Scholar]
- Raymond, H. Crowe: Internal Coating of Gas Pipelines. OilGas J. 1959, 4, 233–251. [Google Scholar]
- Conradi, M.; Kocijan, A.; Zorko, M.; Jerman, I. Effect of silica/PVC composite coatings on steel-substrate corrosion protection. Prog. Org. Coat. 2012, 75, 392–397. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; Zhou, Q. Preparation of nanocomposite inner coating for natural gas pipeline. Ferroelectrics 2021, 581, 287–301. [Google Scholar] [CrossRef]
- Xiao, W.; Shen, Z. Research progress of diamond-like carbon films for biomedical use. Int. J. Biomed. Eng. 2008, 31, 123–125. [Google Scholar]
- Al-Hadhrami, L.M.; Maslehuddin, M.; Ali, M.R. Chemical resistance and mechanical properties of glass fiber–reinforced plastic pipes for oil, gas, and power-plant applications. J. Compos. Constr. 2016, 20, 04015031. [Google Scholar] [CrossRef]
- Yang, F.; Feng, L.; Li, G.; Luo, H.; Zhai, Z. Performance experiment on preparation of composite SiO2/EP wear-resistant coating through electrostatic spraying. Oil Gas Storage Transp. 2016, 1, 63–67. [Google Scholar]
- Lin, Z.; Qin, Y.; Huang, X. Research and Application of Flow Coat for Natural Gas Pipeline. Corros. Prot. -Nanchang 2003, 24, 206–209. [Google Scholar]
- ISO 2409:2007; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2007.
- Dong, S.; Firoozabadi, A. Hydrate anti-agglomeration and synergy effect in normal octane at varying water cuts and salt concentrations. J. Chem. Thermodyn. 2018, 117, 214–222. [Google Scholar] [CrossRef]




| Name | Model Number |
|---|---|
| Microscopic force test system | 4700 m |
| Magnetic stirrer | LC-DMS-PRONI |
| High-temperature drying oven | XMTA-600 |
| Electronic multifunction oven | K-98-II |
| Electronic balances | YP 1002 |
| Constant temperature water bath box | TMS8035-R30 |
| Cross-cut tester | QFH-A |
| Optical contact angle measuring instrument | CA-Series |
| Microscopic force test system | 4700 m |
| Coating Type | Adhesion Level |
|---|---|
| PTFE/PPS composite coating | 1 |
| Epoxy resin E-51 coating | 0 |
| Polyurea coating | 3 |
| Coating Type | Group 1 | Group 2 | Group 3 | Group 4 | Average Contact Angle (°) |
|---|---|---|---|---|---|
| Uncoated | 58.73 | 55.66 | 59.04 | 56.82 | 57.56 |
| PTFE/PPS | 103.97 | 106.88 | 102.95 | 108.74 | 105.63 |
| Epoxy resin E-51 | 69.40 | 67.33 | 67.59 | 66.68 | 67.75 |
| Polyurea | 65.84 | 65.37 | 65.50 | 67.43 | 66.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Gao, Y.; Yin, F.; Li, S. Investigation on the Influence of Different Coating Surfaces on the Adhesive Force of Hydrate Particles. J. Mar. Sci. Eng. 2024, 12, 232. https://doi.org/10.3390/jmse12020232
Su X, Gao Y, Yin F, Li S. Investigation on the Influence of Different Coating Surfaces on the Adhesive Force of Hydrate Particles. Journal of Marine Science and Engineering. 2024; 12(2):232. https://doi.org/10.3390/jmse12020232
Chicago/Turabian StyleSu, Xinyao, Yonghai Gao, Faling Yin, and Shaoqiang Li. 2024. "Investigation on the Influence of Different Coating Surfaces on the Adhesive Force of Hydrate Particles" Journal of Marine Science and Engineering 12, no. 2: 232. https://doi.org/10.3390/jmse12020232






