Establishment and Characterization of a Skeletal Muscle-Derived Myogenic Cell Line from Black Sea Bream (Acanthopagrus schlegelii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Primary Explant Culture and Subculture
2.3. Analysis of the Growth Characteristics of Cell Lines
2.4. Induction of Differentiation
2.5. Measurement of Fusion Index
2.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
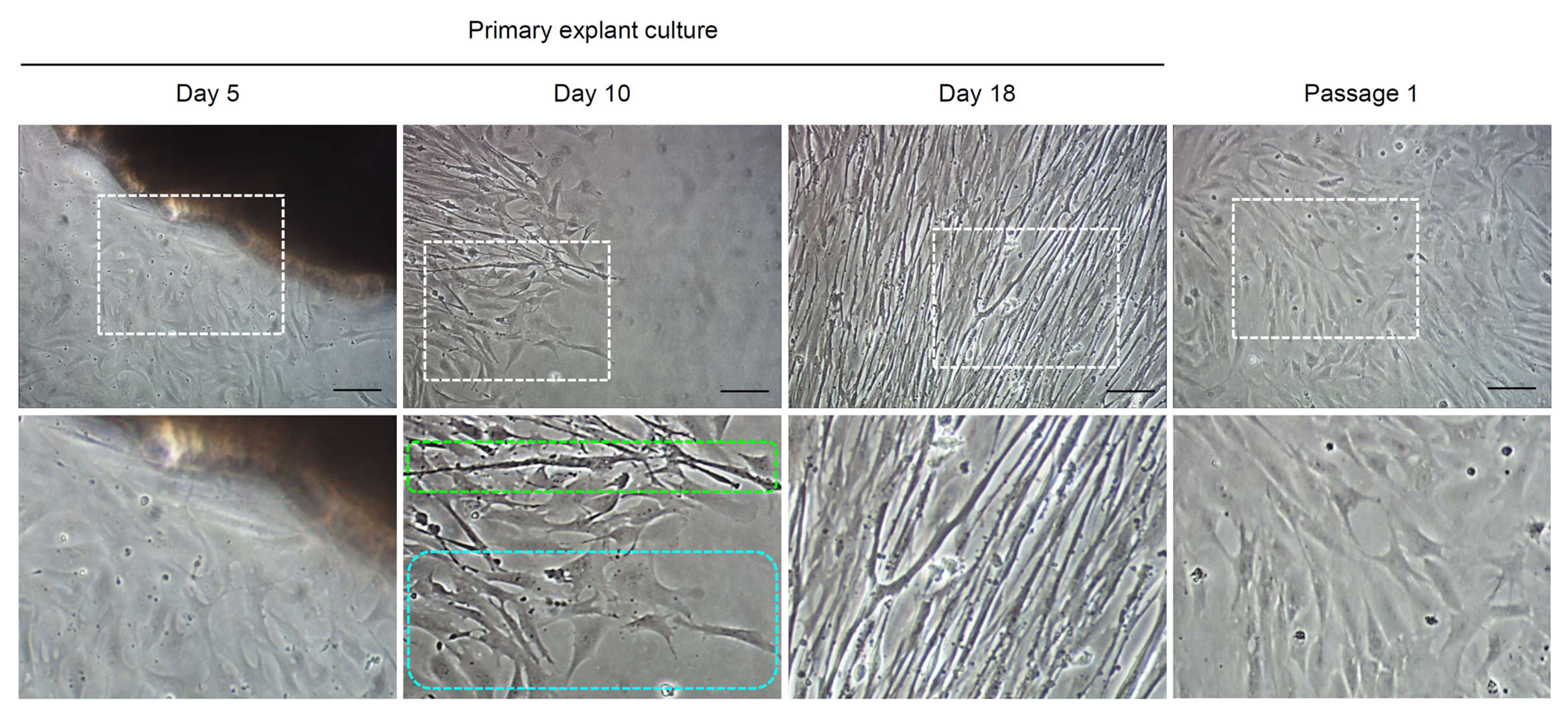
3.1. Outcomes of Primary Explant Culture of A. schlegelii Muscle Tissues
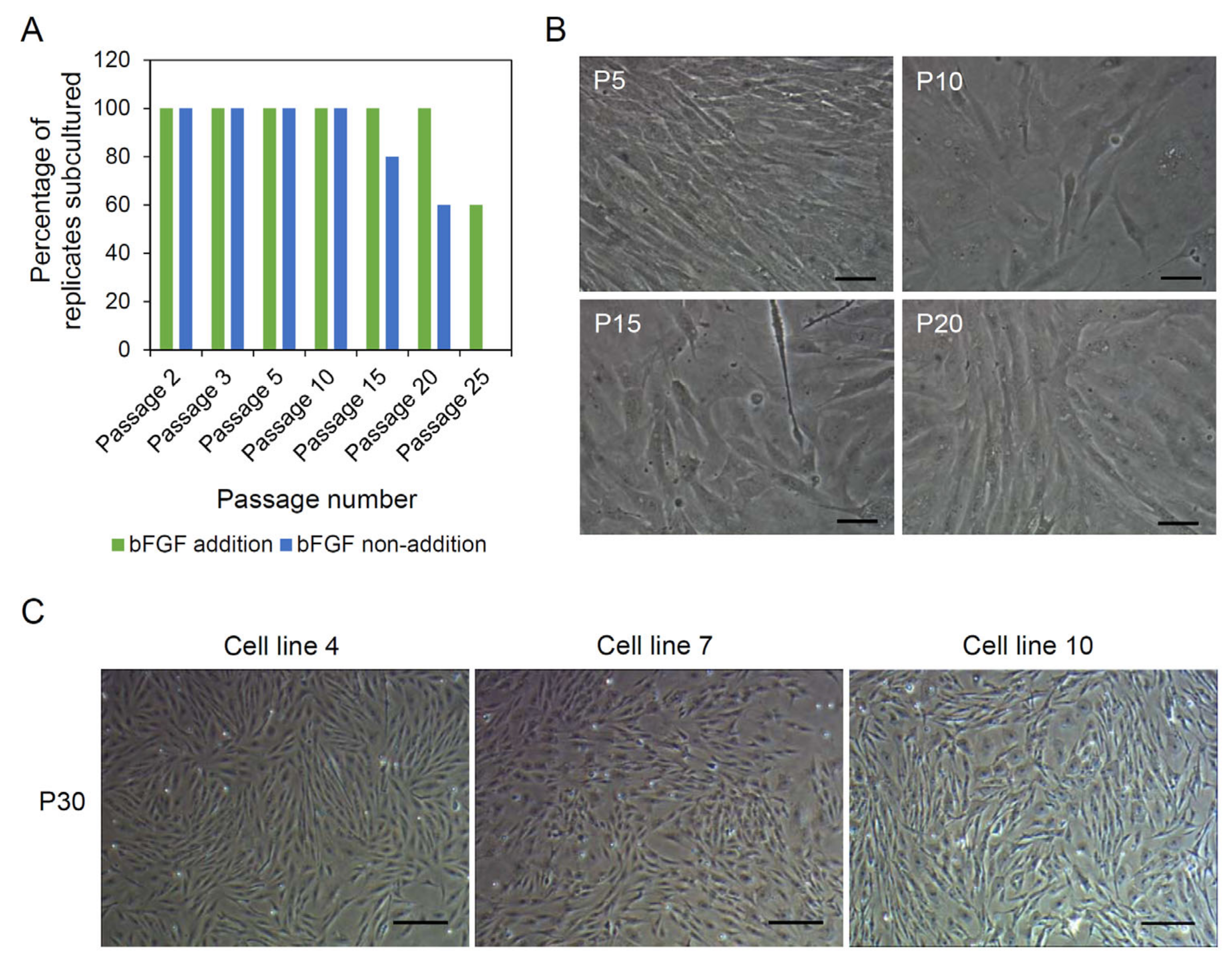
3.2. Derivation of Cell Lines from A. schlegelii Muscle Tissues
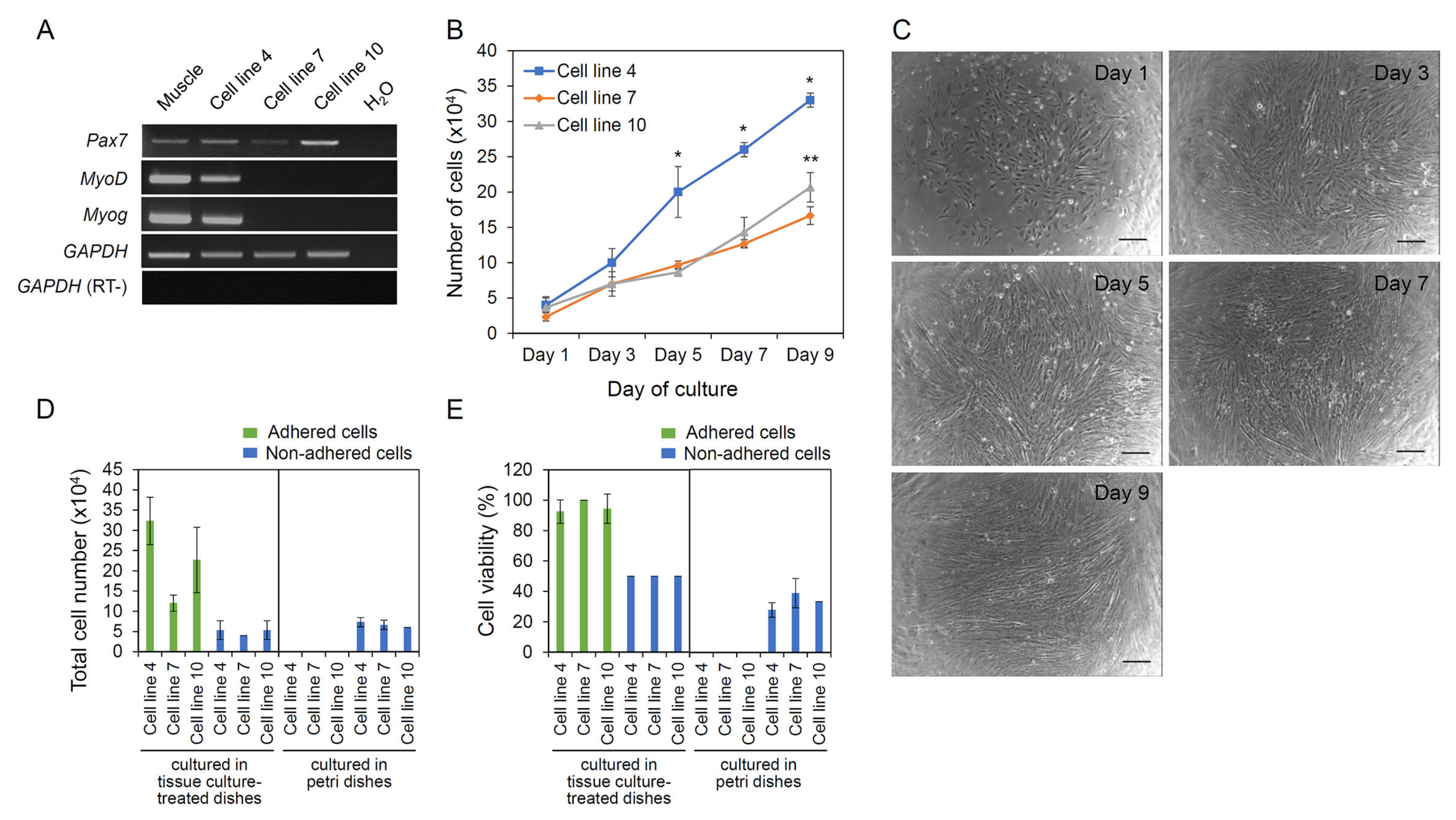
3.3. Characterization of A. schlegelii Muscle-Derived Cell Lines
3.4. Evaluation of Differentiation Capacity of A. schlegelii Muscle-Derived Cell Lines
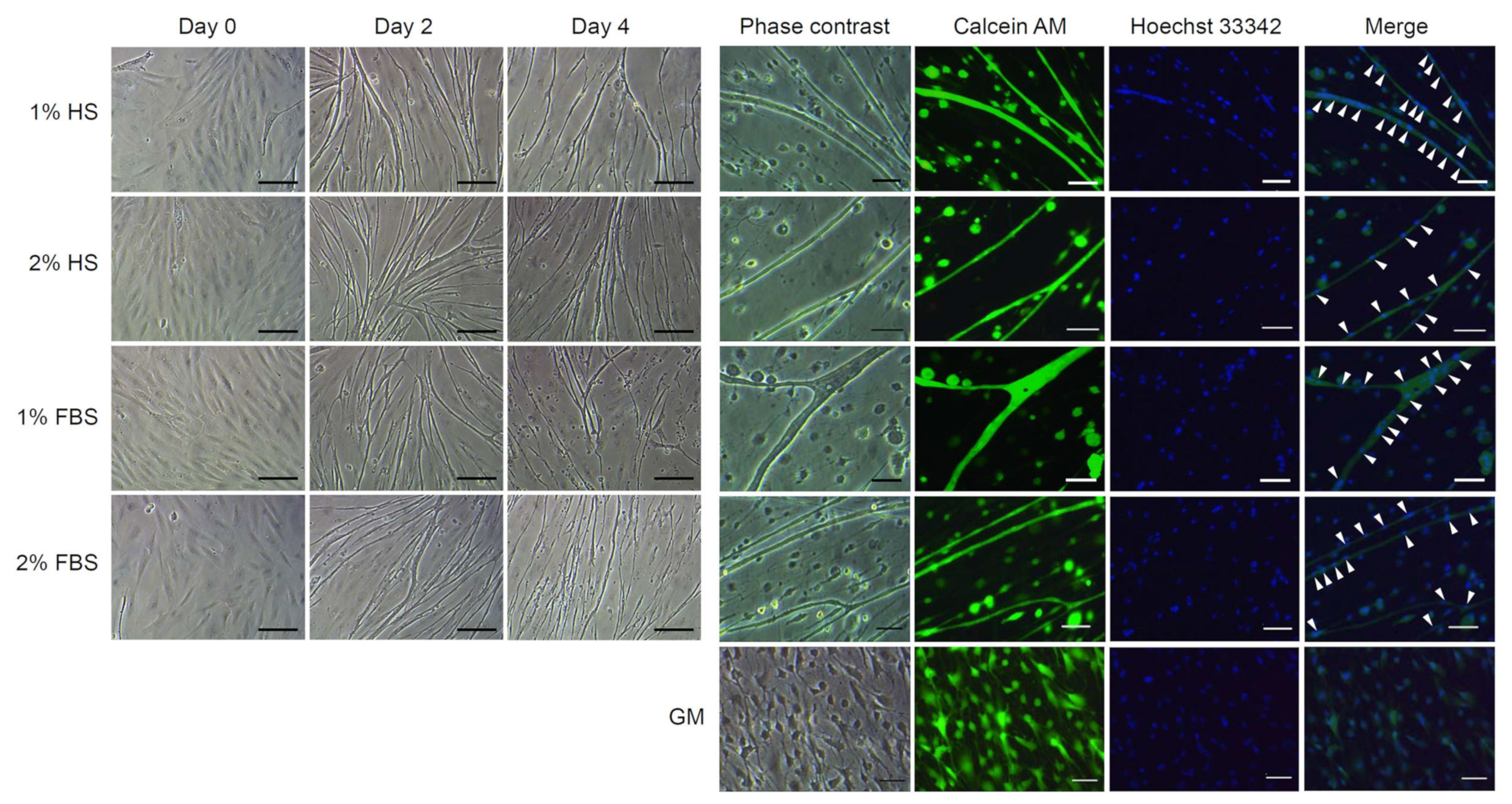
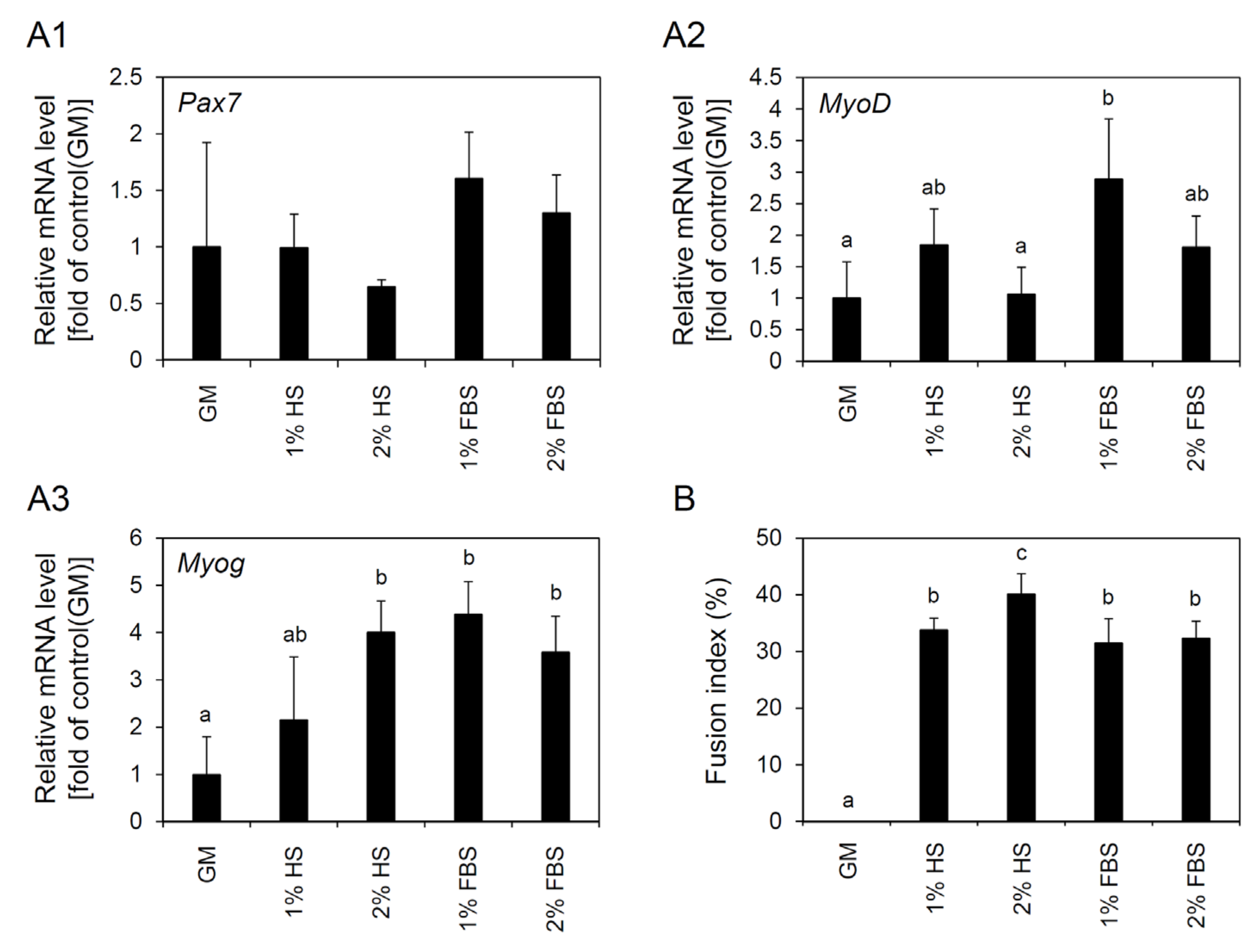
3.5. Effects of Serum Type and Concentration on Differentiation
3.6. Differentiation of a Muscle Satellite Cell Line after Long-Term Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koganti, P.; Yao, J.; Cleveland, B.M. Molecular mechanisms regulating muscle plasticity in fish. Animals 2020, 11, 61. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Choi, K.H.; Yoon, J.W.; Kim, M.; Lee, H.J.; Jeong, J.; Ryu, M.; Lee, C.K. Muscle stem cell isolation and in vitro culture for meat production: A methodological review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 429–457. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.Y.; Jung, J.W.; Kim, J.H.; Oh, D.H.; Kim, H.W.; Hur, S.J. Review of technology and materials for the development of cultured meat. Crit. Rev. Food Sci. Nutr. 2023, 63, 8591–8615. [Google Scholar] [CrossRef]
- Potter, G.; Smith, A.S.; Vo, N.T.; Muster, J.; Weston, W.; Bertero, A.; Rostain, A. A more open approach is needed to develop cell-based fish technology: It starts with zebrafish. One Earth 2020, 3, 54–64. [Google Scholar] [CrossRef]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. USA 1968, 61, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Saxel, O.R.A. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and plant-derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Zschüntzsch, J.; Meyer, S.; Shahriyari, M.; Kummer, K.; Schmidt, M.; Kummer, S.; Tiburcy, M. The evolution of complex muscle cell In vitro models to study Pathomechanisms and drug development of neuromuscular disease. Cells 2022, 11, 1233. [Google Scholar] [CrossRef] [PubMed]
- Mau, M.; Oksbjerg, N.; Rehfeldt, C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017, 3, 17003. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Post, M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018, 8, 10808. [Google Scholar] [CrossRef] [PubMed]
- Will, K.; Schering, L.; Albrecht, E.; Kalbe, C.; Maak, S. Differentiation of bovine satellite cell-derived myoblasts under different culture conditions. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, N.; Gabillard, J.C.; Capilla, E.; Navarro, M.I.; Gutiérrez, J. Role of insulin, insulin-like growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2007, 150, 462–472. [Google Scholar] [CrossRef]
- Bower, N.I.; Johnston, I.A. Paralogs of Atlantic salmon myoblast determination factor genes are distinctly regulated in proliferating and differentiating myogenic cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1615–1626. [Google Scholar] [CrossRef]
- Seiliez, I.; Sabin, N.; Gabillard, J.C. Myostatin inhibits proliferation but not differentiation of trout myoblasts. Mol. Cell. Endocrinol. 2012, 351, 220–226. [Google Scholar] [CrossRef]
- Codina, M.; Capilla, E.; Jiménez-Amilburu, V.; Navarro, I.; Du, S.J.; Johnston, I.A.; Gutiérrez, J. Characterisation and expression of myogenesis regulatory factors during in vitro myoblast development and in vivo fasting in the gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 2014, 167, 90–99. [Google Scholar]
- Gabillard, J.C.; Sabin, N.; Paboeuf, G. In vitro characterization of proliferation and differentiation of trout satellite cells. Cell Tissue Res. 2010, 342, 471–477. [Google Scholar] [CrossRef]
- Peng, L.M.; Zheng, Y.; You, F.; Wu, Z.H.; Tan, X.; Jiao, S.; Zhang, P.J. Comparison of growth characteristics between skeletal muscle satellite cell lines from diploid and triploid olive flounder Paralichthys olivaceus. PeerJ 2016, 4, e1519. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, X.; Li, M.; Song, W.; Huang, K.; Zhang, F.; Zhang, Q.; Qi, J.; He, Y. Establishment of myoblast cell line and identification of key genes regulating myoblast differentiation in a marine teleost, Sebastes schlegelii. Gene 2021, 802, 145869. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.L.; Böhmert, B.; Lee, L.E.; Bols, N.C.; Dowd, G.C. A continuous myofibroblast precursor cell line from the tail muscle of Australasian snapper (Chrysophrys auratus) that responds to transforming growth factor beta and fibroblast growth factor. In Vitro Cell. Dev. Biol. Anim. 2022, 58, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.K.; Yuen, J.S., Jr.; Joyce, C.M.; Li, X.; Lim, T.; Wolfson, T.L.; Kaplan, D.L. Continuous fish muscle cell line with capacity for myogenic and adipogenic-like phenotypes. Sci. Rep. 2023, 13, 5098. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Niu, R.; Lao, J.; Zhang, S.; Li, J.; Zhu, Y.; Liu, D. Tissue-like cultured fish fillets through a synthetic food pipeline. NPJ Sci. Food 2023, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sagada, G.; Xu, B.; Chao, W.; Zou, F.; Ng, W.K.; Sun, Y.; Wang, L.; Zhong, Z.; Shao, Q. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2019, 51, 1000–1011. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Gladstone, S.; Ng, W.K.; Zhang, J.; Shao, Q. Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 2019, 513, 734397. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Song, K.D.; Kim, S.J.; Kim, D.C.; Lee, S.C.; Shim, K. Growth factors improve the proliferation of Jeju black pig muscle cells by regulating myogenic differentiation 1 and growth-related genes. Anim. Biosci. 2021, 34, 1392. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 107–114. [Google Scholar]
- Lakra, W.S.; Swaminathan, T.R.; Joy, K.P. Development, characterization, conservation and storage of fish cell lines: A review. Fish. Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef]
- Feige, P.; Rudnicki, M.A. Isolation of satellite cells and transplantation into mice for lineage tracing in muscle. Nat. Protoc. 2022, 15, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.R.; Passey, S.L.; Player, D.J.; Khodabukus, A.; Ferguson, R.A.; Sharples, A.P.; Lewis, M.P. Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials 2013, 34, 5759–5765. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.W.; Yoon, J.W.; Kim, M.S.; Jeong, J.S.; Ryu, M.K.; Park, S.K.; Jo, C.R.; Lee, C.K. Optimization of culture conditions for maintaining pig muscle Stem cells In vitro. Food Sci. Anim. Resour. 2020, 40, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, F.; Wu, P.; Li, T.; He, M.; Yin, X.; Dai, G. MicroRNA-7 targets the KLF4 gene to regulate the proliferation and differentiation of chicken primary myoblasts. Front. Genet. 2020, 11, 842. [Google Scholar] [CrossRef]
- Van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.; Fu, J.; Gstraunthaler, G. Fetal bovine serum (FBS): Past–present–future. Altex 2018, 35, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, F.; Ye, X.; Wang, L.; Yang, J.; Zhang, J.; Liu, L. KSR-based medium improves the generation of high-quality mouse iPS cells. PLoS ONE 2014, 9, e105309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Zhang, X.; Ren, L.; Shi, W.; Tian, Y.; Zhang, T. The use of KnockOut serum replacement (KSR) in three dimensional rat testicular cells co-culture model: An improved male reproductive toxicity testing system. Food Chem. Toxicol. 2017, 106, 487–495. [Google Scholar] [CrossRef]
- Zammit, P.; Beauchamp, J. The skeletal muscle satellite cell: Stem cell or son of stem cell? Differentiation 2001, 68, 193–204. [Google Scholar] [CrossRef]
- Montarras, D.; L’honore, A.; Buckingham, M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013, 280, 4036–4050. [Google Scholar] [CrossRef]
- Bischoff, R.; Heintz, C. Enhancement of skeletal muscle regeneration. Dev. Dyn. 1994, 201, 41–54. [Google Scholar] [CrossRef]
- Shi, X.; Garry, D.J. Muscle stem cells in development, regeneration, and disease. Genes. Dev. 2006, 20, 1692–1708. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, S.H.; Lim, J.A.; Froehner, S.C.; Adams, M.E.; Kim, H.S. α-Syntrophin modulates myogenin expression in differentiating myoblasts. PLoS ONE 2010, 5, e15355. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef]
- Berberoglu, M.A.; Gallagher, T.L.; Morrow, Z.T.; Talbot, J.C.; Hromowyk, K.J.; Tenente, I.M.; Amacher, S.L. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Dev. Biol. 2017, 424, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Ziman, M. Pax genes during neural development and their potential role in neuroregeneration. Prog. Neurobiol. 2011, 95, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; D’Addabbo, P.; Fornetti, E.; Belli, R.; Fuoco, C.; Bernardini, S.; Gargioli, C. Myoblast myogenic differentiation but not fusion process is inhibited via MyoD tetraplex interaction. Oxid. Med. Cell. Longev. 2018, 2018, 7640272. [Google Scholar] [CrossRef]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef]
- Zhang, P.; Wong, C.; Liu, D.; Finegold, M.; Harper, J.W.; Elledge, S.J. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes. Dev. 1999, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer Sequences (5′ → 3′) | Product Size (bp) | Usage | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Pax7 | GGTTCCTTCAGGTGAGGCTT | GGATGCCGACTTTACCCACA | 475 | RT-PCR |
| MyoD | GCAAGAGGAAGACCACCAAC | TTTAACGGACCGAGCTGTCA | 313 | |
| Myog | GGTTACCATGGACCGTCGAA | TCCTGAGTGCTACCGACTCT | 376 | |
| GAPDH | GCATCTTGCACGACTAACTGC | TCCTCCGACTTCATCGGTGA | 419 | |
| Pax7 | GCAACTCCAGACGTGGAGAA | GGCTTCATCTGTGAGCTCCA | 148 | qRT-PCR |
| MyoD | CAGGACGACGGCTACTACC | GGACTTTAACGGACCGAGCT | 109 | |
| Myog | CCTGAGTGCTACCGACTCTC | GAGCTGTGGCCTTTCCAATG | 100 | |
| GAPDH | ACAGTCCATGCATACACAGC | CAAGGCAGTCGGCAAAGTTA | 133 | |
| Substrates | Media | Serum | bFGF Addition | No. of Replicates | No. (%) a of Replicates That Showed Primary Cell Adherence | No. (%) a of Replicates That Accomplished First Subculture |
|---|---|---|---|---|---|---|
| Gelatin | L15 | FBS | Yes | 5 | 4 (80) | 4 (80) |
| No | 3 | 1 (33) | 1 (33) | |||
| KSR | Yes | 3 | 2 (67) | 0 (0) | ||
| No | 3 | 1 (33) | 0 (0) | |||
| DMEM | FBS | Yes | 4 | 4 (100) | 4 (100) | |
| No | 3 | 3 (100) | 3 (100) | |||
| KSR | Yes | 3 | 1 (33) | 1(33) | ||
| No | 5 | 4 (80) | 1 (20) | |||
| α-MEM | FBS | Yes | 3 | 2 (67) | 1 (33) | |
| No | 4 | 4 (100) | 2 (50) | |||
| KSR | Yes | 5 | 5 (100) | 1 (20) | ||
| No | 5 | 5 (100) | 0 (0) | |||
| Matrigel | L15 | FBS | Yes | 3 | 2 (67) | 1 (33) |
| No | 3 | 3 (100) | 1 (33) | |||
| KSR | Yes | 3 | 0 (0) | 0 (0) | ||
| No | 3 | 2 (67) | 0 (0) | |||
| DMEM | FBS | Yes | 3 | 3 (100) | 3 (100) | |
| No | 4 | 4 (100) | 4 (100) | |||
| KSR | Yes | 3 | 1 (33) | 0 (0) | ||
| No | 3 | 1 (33) | 0 (0) | |||
| α-MEM | FBS | Yes | 5 | 4 (80) | 2 (40) | |
| No | 3 | 2 (67) | 2 (67) | |||
| KSR | Yes | 3 | 2 (67) | 2 (67) | ||
| No | 3 | 2 (67) | 2 (67) | |||
| Total | 85 | 62 (73) | 35 (41) | |||
| Factors | Groups | No. of Replicates | No. (%) a of Replicates That Showed Primary Cell Adherence | No. (%) a of Replicates That Accomplished First Subculture |
|---|---|---|---|---|
| Whole replicates | Merged | 85 | 62 (73) | 35 (41) |
| Substrates | Gelatin | 46 | 36 (78) | 18 (39) |
| Matrigel | 39 | 26 (67) | 17 (44) | |
| p-value | 0.2355 | 0.6816 | ||
| Media | L15 | 26 | 15 (58) | 7 (27) |
| DMEM | 28 | 21 (75) | 16 (57) | |
| α-MEM | 31 | 26 (84) | 12 (39) | |
| p-value | 0.0833 | 0.0749 | ||
| Serum | FBS | 43 | 36 (84) | 28 (65) |
| KSR | 42 | 26 (62) | 7 (17) | |
| p-value | 0.0235 | <0.0001 | ||
| bFGF | Addition | 43 | 30 (70) | 19 (44) |
| No addition | 42 | 32 (76) | 16 (38) | |
| p-value | 0.5109 | 0.5737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.H.; Gong, S.P. Establishment and Characterization of a Skeletal Muscle-Derived Myogenic Cell Line from Black Sea Bream (Acanthopagrus schlegelii). J. Mar. Sci. Eng. 2024, 12, 249. https://doi.org/10.3390/jmse12020249
Han DH, Gong SP. Establishment and Characterization of a Skeletal Muscle-Derived Myogenic Cell Line from Black Sea Bream (Acanthopagrus schlegelii). Journal of Marine Science and Engineering. 2024; 12(2):249. https://doi.org/10.3390/jmse12020249
Chicago/Turabian StyleHan, Dan Hee, and Seung Pyo Gong. 2024. "Establishment and Characterization of a Skeletal Muscle-Derived Myogenic Cell Line from Black Sea Bream (Acanthopagrus schlegelii)" Journal of Marine Science and Engineering 12, no. 2: 249. https://doi.org/10.3390/jmse12020249
APA StyleHan, D. H., & Gong, S. P. (2024). Establishment and Characterization of a Skeletal Muscle-Derived Myogenic Cell Line from Black Sea Bream (Acanthopagrus schlegelii). Journal of Marine Science and Engineering, 12(2), 249. https://doi.org/10.3390/jmse12020249






