Population Genetic Structure of Sargassum horneri, the Dominant Species of Golden Tide in the Yellow Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Satellite Remote Sensing
2.3. Molecular Identification of Sargassum Samples
2.4. Haplotype Statistics and Analysis of cob-cox2 and cox3 Partial Sequences
3. Results
3.1. Blooming Process of Drifting Sargassum Species in 2021
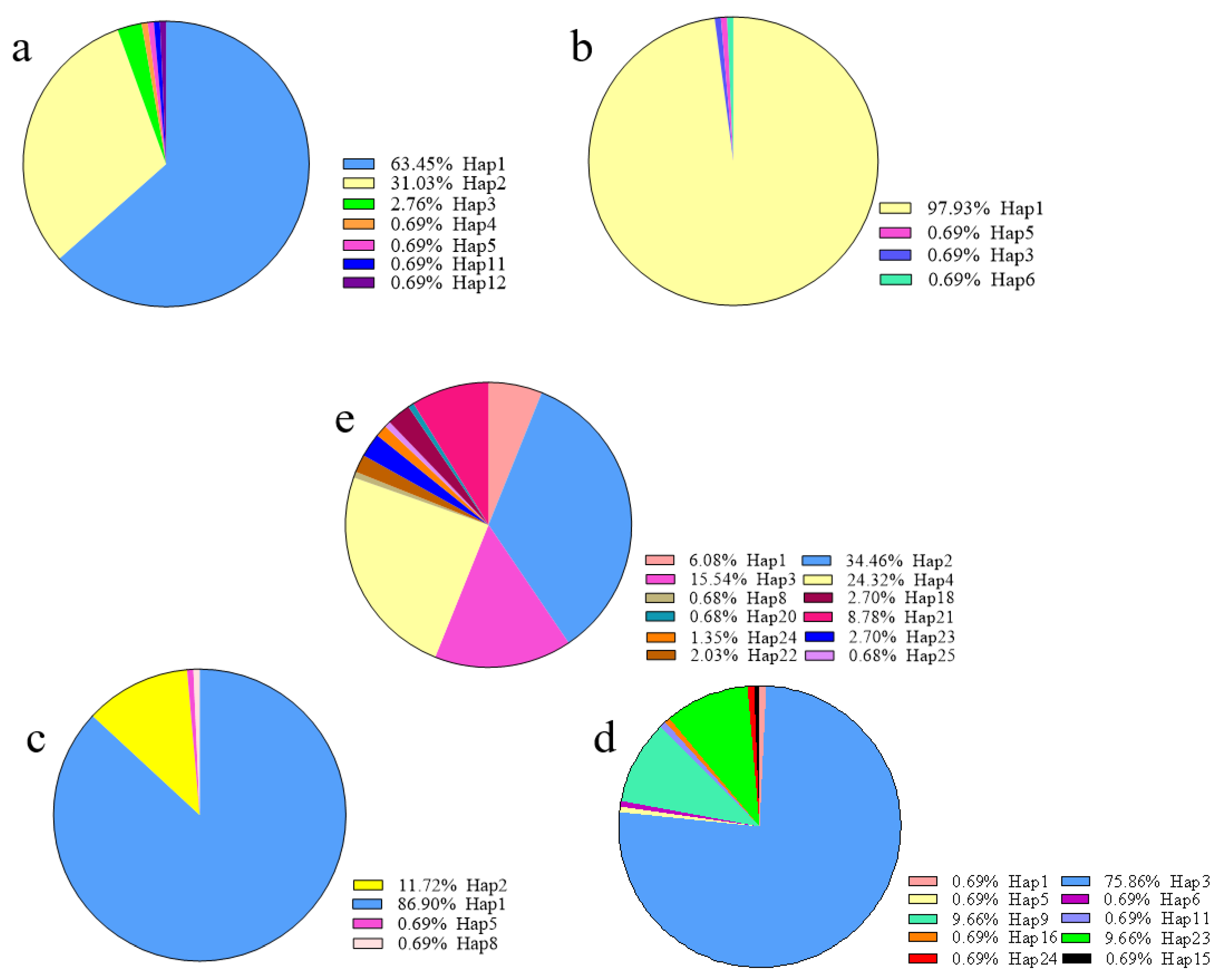
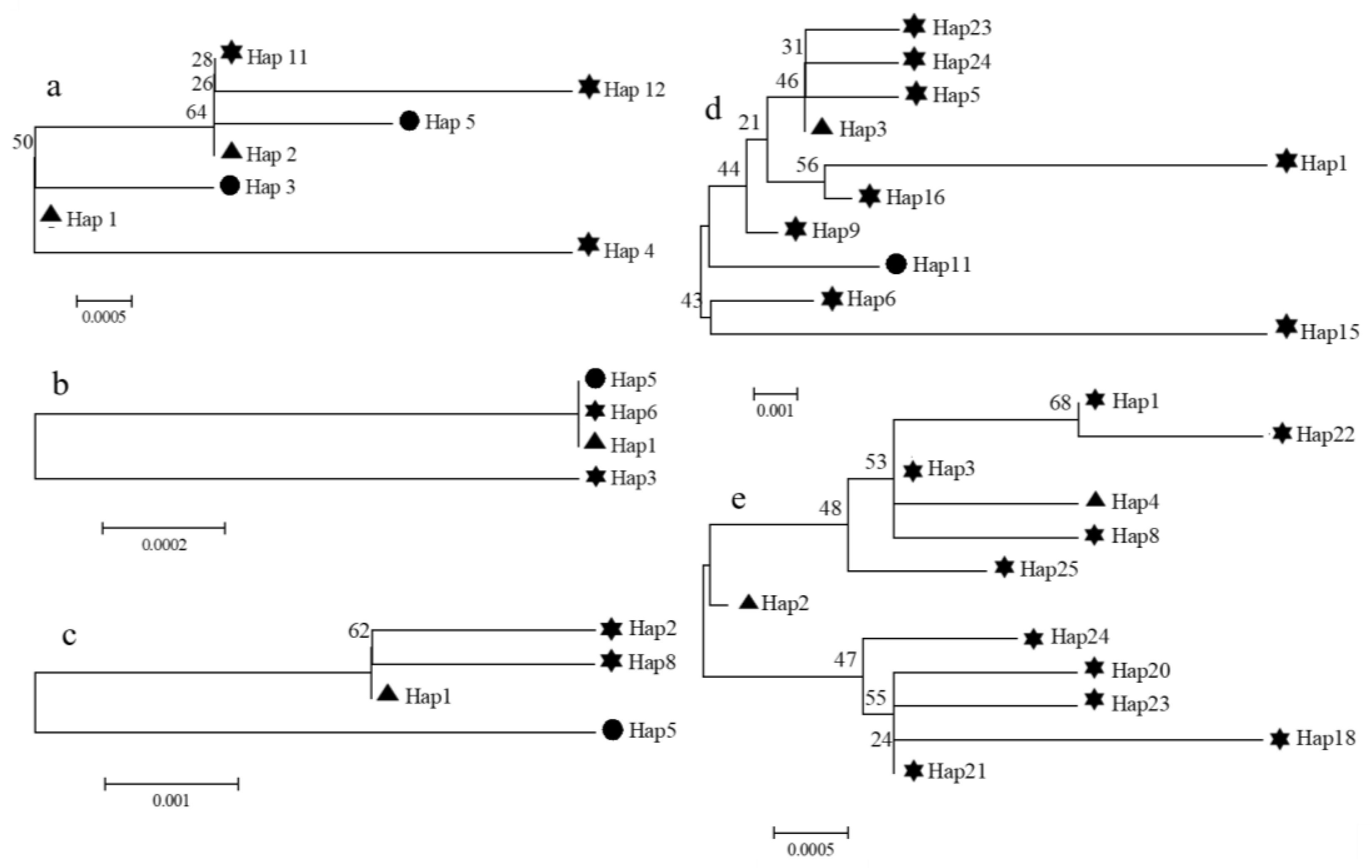
3.2. Molecular Analysis Based on Marker Genes in Ribosomes, Mitochondria, and Chloroplasts
 ), and all were at the position where the evolutionary tree branching began. In addition, many of the haplotypes that accounted for a relatively small proportion of the total were derived from the major haplotypes, and there was a relatively close genetic evolution relationship with the major haplotypes.
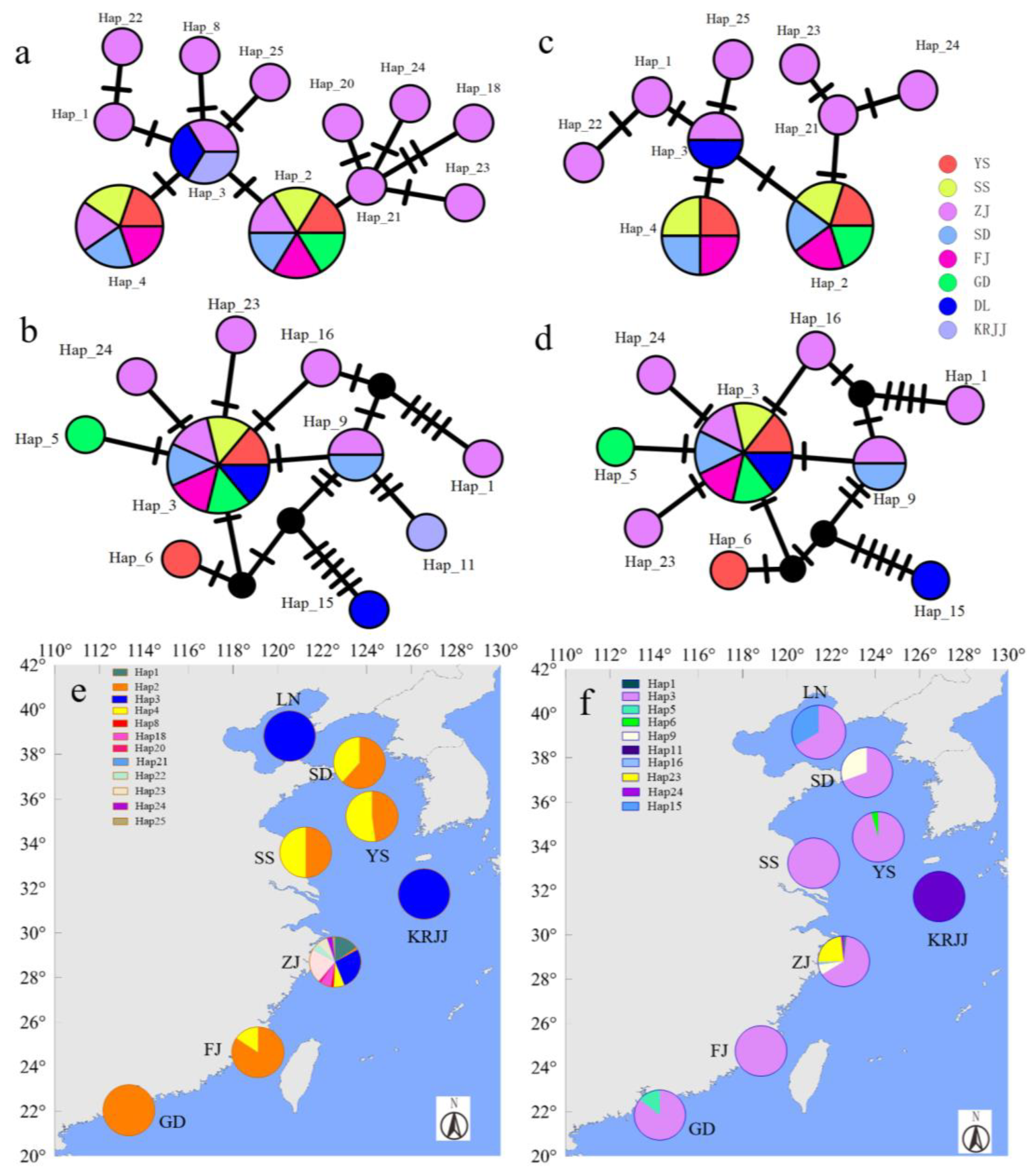
), and all were at the position where the evolutionary tree branching began. In addition, many of the haplotypes that accounted for a relatively small proportion of the total were derived from the major haplotypes, and there was a relatively close genetic evolution relationship with the major haplotypes.3.3. Haplotype Network Analysis Based on the Partial cob-cox2 and cox3 Sequences
3.4. Analysis of Different Haplotype Occupancy in Different Geographic Regions Based on the Partial cob-cox2 and cox3 Sequences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.L.; Wan, J.H.; Zhang, J.; Ma, Y.J.; Wang, L.; Zhao, J.H.; Wang, Z.Z. Spatial-temporal distribution of golden tide based on high-resolution satellite remote sensing in the South Yellow Sea. J. Coast. Res. 2019, 90, 221–227. [Google Scholar] [CrossRef]
- Mattio, L.; Payri, C.E. 190 Years of Sargassum taxonomy, facing the advent of DNA phylogenies. Bot Rev. 2011, 77, 31–70. [Google Scholar] [CrossRef]
- Liu, F.; Pan, J.; Zhang, Z.; Moejes, W.F. Organelle genomes of Sargassum confusum (Fucales, Phaeophyceae): mtDNA vs cpDNA. J. Appl. Phycol. 2018, 30, 2715–2722. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Republic of Ireland, 2014. [Google Scholar]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, J.; Zhuang, M.; Zhang, J.; Sun, Y.; Tong, Y.; Zhao, S.; He, P. Golden seaweed tides accumulated in Pyropia aquaculture areas are becoming a normal phenomenon in the Yellow Sea of China. Sci. Total Environ. 2021, 774, 145726. [Google Scholar] [CrossRef]
- Yan, F.; Li, L.; Yu, D.; Cui, C.; Zang, S.; Xu, Z.; Wu, H. Physiological responses of Sargassum muticum, a potential golden tide species, to different levels of light and nitrogen. Front. Ecol. Evol. 2021, 9, 759732. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef]
- Haro, S.; Bermejo, R.; Wilkes, R.; Bull, L.; Morrison, L. Monitoring intertidal golden tides dominated by Ectocarpus siliculosus using Sentinel-2 imagery. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103451. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, H.; Ma, Y.; Cui, C.; Qin, H.; Liu, L.; Zang, S.; Xing, H.; Xu, Z.; Wu, H. Combined Influences of Light and Nitrogen Enrichment on the Physiological Performance of a Golden Tide Alga (Sargassum horneri). J. Mar. Sci. Eng. 2022, 10, 1195. [Google Scholar] [CrossRef]
- Baker, P.; Minzlaff, U.; Schoenle, A.; Schwabe, E.; Hohlfeld, M.; Jeuck, A.; Brenke, N.; Prausse, D.; Rothenbeck, M.; Brix, S.; et al. Potential contribution of surface-dwelling Sargassum algae to deep-sea ecosystems in the southern North Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 21–34. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Liang, Z. A preliminary study of the Enteromorpha prolifera drift gathering causing the green tide phenomenon. J. Ocean Univ. China 2008, 38, 601–604. [Google Scholar] [CrossRef]
- Niu, J.F. Observation of Enteromorpha prolifera floating in Qingdaosea area by microscope. Mar. Sci. 2008, 32, 30–33. [Google Scholar]
- Yi, J.T.; Huang, J.T.; Song, J.L. Initial understand of Enteromorpha prolifera Occurred in Yancheng Coastal Waters in 2008. Mar. Environ. Res. 2009, 28, 57–58. [Google Scholar]
- Zhang, H. Analysis of pigments in floating and fixed Sargassum horneri by high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry. J. Nucl. Agric. Sci. 2019, 33, 1173–1180. [Google Scholar] [CrossRef]
- Zhuang, M.; Liu, J.; Ding, X.; He, J.; Zhao, S. Sargassum blooms in the East China Sea and Yellow Sea: Formation and management. MPB 2021, 162, 111845. [Google Scholar] [CrossRef]
- Huang, B.X.; Ding, L.P.; Qin, S. The taxonomical status and biogeographical distribution of sargassum horneri with the origin analysis of its drifting population in the end of 2016 at the western yellow sea. Oceanol. Et Limnol. Sin. 2018, 49, 214–223. [Google Scholar] [CrossRef]
- Wang, T. Macroalgae in Dongtou City, Zhejiang Province; China Ocean Press: Beijing, China, 2012. [Google Scholar]
- Zhang, P.; Cai, Y.F.; Wang, T.G.; Zhong, C.H. AFLP analysis of different geographic populations of Sargassum horneri along the coast of Zhejiang Province. Acta Agric. Zhejiangensis 2015, 27, 1586–1592. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, X.; Zhuang, M.; Wang, S. An increase in new Sargassum (Phaeophyceae) blooms along the coast of the East China Sea and Yellow Sea. Phycologia 2019, 58, 374–381. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Chen, Y.; Zhao, X.; Zhou, X.; Chen, Y. Life history and morphology of Sargassum horneri from the Sargassum seaweed bed of Gouqi Island. J. Fish. China 2015, 39, 1218–1229. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.R.; Wang, K.; Zhang, S.Y. Morphological characteristics of vesicle of Sargassum horneri andits relationship to environmental factors in Gouqi Island. J. Fish. China 2020, 44, 793–804. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, X.; Tang, H. Research on genecology of benthic macroalgae. Fish. Inf. Strategy 2018, 33, 36–44. [Google Scholar] [CrossRef]
- Sun, J.; Chen, D.; Zhuang, D.; Zheng, H.; Feng, S. In situ ecological studies of the subtidal brown alga Sargasssum horneri at Nanji Island of China. Fish Sci. 2008, 4, 58–63. [Google Scholar]
- Liu, Y.J.; Xiao, J.; Fan, S.L.; Miao, X.X.; Yuan, C.; Zang, Y.; Wang, Z.L.; Zhang, B.T.; Ma, X.J.; Zhang, X.L. Distribution and diversity of the sympatric macroalgae of the pelagic Sargassum horneri in the Yellow and East China seas. Aquat. Bot. 2023, 188, 103683. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Yu, Q.; Bi, Y.; He, P. Molecular phylogenetic analysis of floating Sargassum horneri associated with green tides in coastal area of Qingdao. J. Biol. 2016, 33, 39–42. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Wang, Y.; Jin, Z.; Moejes, F.W.; Sun, S. Insights on the Sargassum horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol Oceanogr. 2018, 63, 1762–1773. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S. Complete mitochondrial genome of the brown alga Sargassum horneri (Sargassaceae, Phaeophyceae): Genome organization and phylogenetic analyses. J. Appl. Phycol. 2016, 27, 469–478. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S. Chloroplast genome of Sargassum horneri (Sargassaceae, Phaeophyceae): Comparative chloroplast genomics of brown algae. J. Appl. Phycol. 2016, 28, 1419–1426. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cheon, K.S.; Kim, S.; Yun, S.H.; Oh, H.J.; Park, S.R.; Kim, T.H.; Kim, J.K.; Lee, H.J. Comparative analysis of sequence polymorphism in complete organelle genomes of the ‘Golden Tide’ Seaweed Sargassum horneri between Korean and Chinese forms. Sustainability 2020, 12, 7280. [Google Scholar] [CrossRef]
- Rothman, M.D.; Mattio, L.; Anderson, R.J.; Bolton, J.J. A phylogeographic investigation of the kelp genus Laminaria (Laminariales, Phaeophyceae), with emphasis on the South Atlantic Ocean. J. Phycol. 2017, 53, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Okuda, S.; Kasahara, M.; Takahashi, F.; Yoshikawa, S.; Uwai, S. Phenological shifts and genetic differentiation between sympatric populations of Sargassum horneri (Fucales, Phaeophyceae) in Japan. Mar. Ecol. Prog. Ser. 2020, 642, 103–116. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Stiger, V.; Horiguchi, T. Sargassum boreale sp. nov. (Fucales, Phaeophyceae) from Hokkaido, Japan. Phycological Res. 2000, 48, 125–131. [Google Scholar] [CrossRef]
- Baylis, A. Advances in precision farming technologies for crop protection. Outlooks Pest Manag. 2017, 28, 158–161. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, J.; Zhuang, M.; Kang, X.; Zhao, X. Growth of Sargassum horneri distribution properties of golden tides in the Yangtze Estuary and adjacent waters. Mar. Fish. 2019, 41, 188–196. [Google Scholar] [CrossRef]
- Xing, Q.; Guo, R.; Wu, L.; An, D.; Cong, M.; Qin, S.; Li, X. High-resolution satellite observations of a new hazard of golden tides caused by floating Sargassum in winter in the Yellow Sea. IEEE Geosci. Remote Sens. Lett. 2017, 14, 1815–1819. [Google Scholar] [CrossRef]
- Xiao, J.; Fan, S.; Wang, Z.; Fu, M.; Song, H.; Wang, X.; Yuan, C.; Pang, M.; Miao, X.; Zhang, X. Decadal characteristics of the floating Ulva and Sargassum in the Subei Shoal, Yellow Sea. Acta Oceanol. Sin. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Mizuno, S.; Ajisaka, T.; Lahbib, S.; Kokkubu, Y.; Alabsi, M.N.; Komatsu, T. Spatial distributions of floating seaweeds in the East China Sea from late winter to early spring. J. Appl. Phycol. 2014, 26, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Zhang, S.; Feng, M. Distribution and flora of seaweed beds in the coastal waters of China. Sustainability 2021, 13, 3009. [Google Scholar] [CrossRef]


 ), attached samples only (
), attached samples only ( ), and floating samples only (
), and floating samples only ( ).
).
 ), attached samples only (
), attached samples only ( ), and floating samples only (
), and floating samples only ( ).
).
 : floating samples found during reinvestigation;
: floating samples found during reinvestigation;  : attached samples found during reinvestigation) (a) and an overview of culture area and total production of mussels and S. japonica along the coast of Zhejiang (b) and Shandong Provinces (c) over successive years.
: attached samples found during reinvestigation) (a) and an overview of culture area and total production of mussels and S. japonica along the coast of Zhejiang (b) and Shandong Provinces (c) over successive years.
 : floating samples found during reinvestigation;
: floating samples found during reinvestigation;  : attached samples found during reinvestigation) (a) and an overview of culture area and total production of mussels and S. japonica along the coast of Zhejiang (b) and Shandong Provinces (c) over successive years.
: attached samples found during reinvestigation) (a) and an overview of culture area and total production of mussels and S. japonica along the coast of Zhejiang (b) and Shandong Provinces (c) over successive years.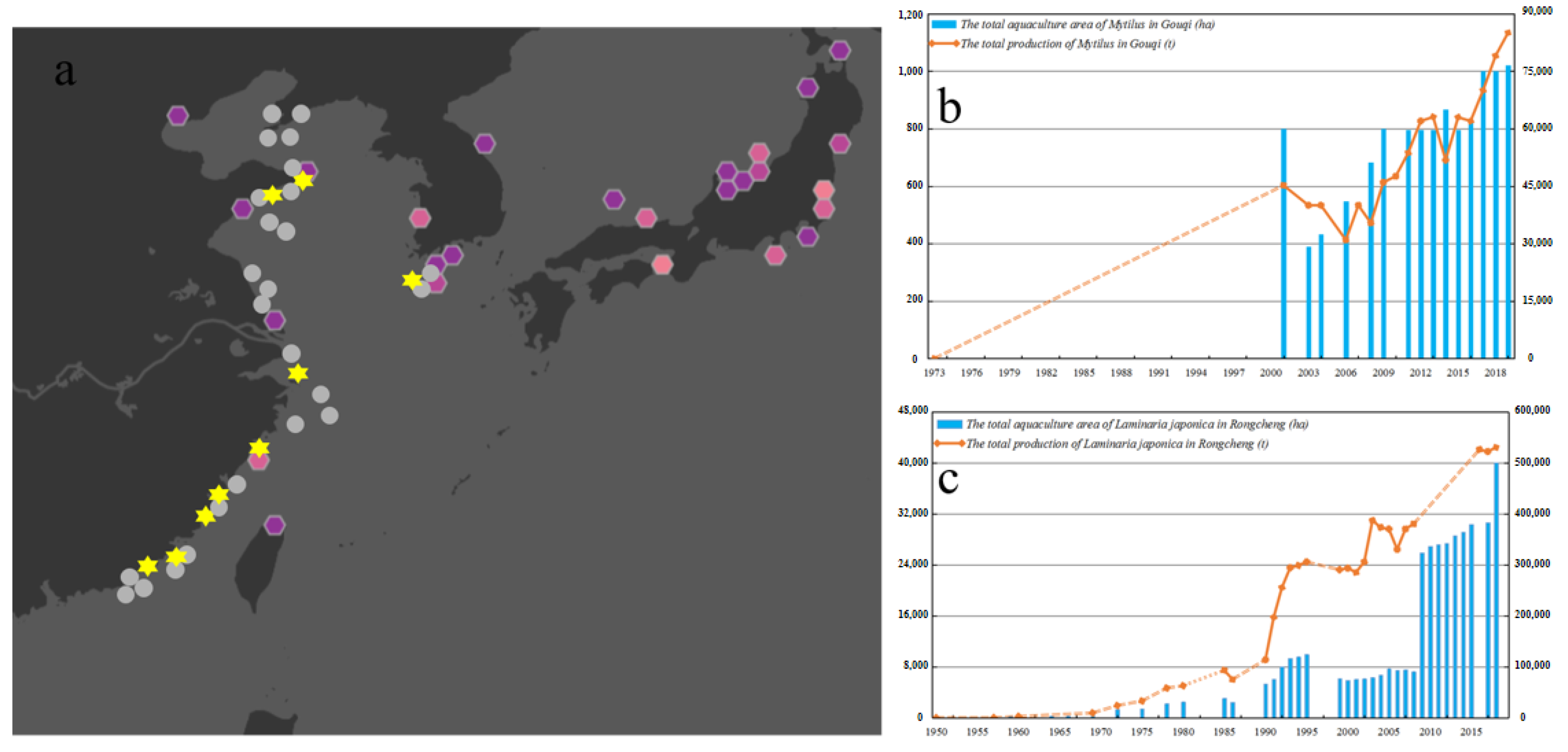
| Source ID | Sample Number | Dates | Collection Sites | Geographical Coordinate | State |
|---|---|---|---|---|---|
| 2017-A-ZJZS | 2 | 26 November 2017 | ZJ | 122°45′ E 30°43′ N | Attached |
| 2018-A-ZJNJ | 5 | 23 December 2018 | ZJ | 120°83′ E 27°32′ N | Attached |
| 2018-A-ZJZS | 9 | 11 November 2017–24 May 2018 | ZJ | 122°45′ E 30°43′ N | Attached |
| 2019-A-ZJZS | 3 | 22 January–18 October 2019 | ZJ | 122°43′ E 30°43′ N | Attached |
| 2019-A-SDWH | 5 | 10 January–31 October 2019 | SD | 122°34′ E 37°17′8″–37°17′11″ N | Attached |
| 2019-A-KRJJ | 1 | 30 April 2019 | KR | 126°46′ E 33°51′ N | Attached |
| 2019-F-YS | 3 | 3–4 June 2019 | YS | 121°08′–121°20′ E 30°43′–33°04′ N | Floating |
| 2019-F-JSNT | 1 | 4 June 2019 | SS | 120°58′ E 32°36′ N | Floating |
| 2020-A-ZJZS | 3 | 27 July–17 November 2020 | ZJ | 122°43′ E 30°43′ N | Attached |
| 2020-A-SDQD | 5 | 1 May 2020 | SD | 120°21′ E 36°02′ E | Attached |
| 2020-F-YS | 11 | 1 May–7 June 2020 | YS | 121°43′–120°56′ E 35°13′–35°57′ N | Floating |
| 2020-F-JSYC | 5 | 12 May 2020 | SS | 33°53′ N 120°52′ E | Floating |
| 2020-F-JSNT | 11 | 17 January–13 May 2020 | SS | 120°59′ E 33°12′ N | Floating |
| 2021-A-SDWH | 7 | 15 January–29 April 2021 | SD | 122°34′ E 37°17′8″–37°17′11″ N | Attached |
| 2021-A-FJND | 6 | 10–12 April 2021 | FJ | 119°44′–119°46′ E 26°36′–26°35′ N | Attached |
| 2021-A-FJPT | 5 | 13 April 2021 | FJ | 25°15′ N 119°28′ E | Attached |
| 2021-A-FJFZ | 5 | 11 April 2021 | FJ | 26°21′ N 119°42′ E | Attached |
| 2021-A-FJZZ | 5 | 14–15 April 2021 | FJ | 23°34′ N 117°22′ E | Attached |
| 2021-A-GDCZ | 5 | 14 May 2021 | GD | 117°03′ E 23°33′ N | Attached |
| 2021-A-GDST | 2 | 15 May 2021 | GD | 116°56′ E 23°26′ N | Attached |
| 2021-A-ZJZS | 41 | 24 June–25 October 2021 | ZJ | 122°45′ E 53°43′ N | Attached |
| 2021-F-YS | 9 | 6–9 May 2021 | YS | 119°45′–122°58′ E 33°58′–35°14′ N | Floating |
| 2021-F-JSNT | 13 | 25 March–30 April 2021 | SS | 120°59′–121°39′ E 32°05′–32°36′ N | Floating |
| 2021-F-LNDL | 3 | 7 July 2021 | LN | 121°34′ E 38°52′ N | Attached |
| Primers | Sequences (5′-3′) | Size of Products (bp) | References [28,29,30,34,35,36,37] |
|---|---|---|---|
| rbcL-F | 5’-TTAGGGTTATTTGTAAATGGATGCG-3′ | 700 | This study |
| rbcL-R | 5′-ACATCCTTGTGTAAGTCTCATTACT-3′ | ||
| ITS2-F | 5′-CGATGAAGAACGCAGCGAAATGCGAT-3′ | 582 | Yoshida et al., 2000 [36] |
| ITS2-R | 5′-TCCTCCGCTTAGTATATGCTTAA-3′ | ||
| cox1-F | 5′-CAGCGATGTCTGTTCTTATAAGG-3′ | 1027 | This study |
| cox1-R | 5′-TAATAAGTATCGTGAAGAGCAATATCTACAC-3′ | ||
| cob-cox2-F | 5′-GTTACCTTTTTTAATTACGGGTAT-3′ | 890 | Liu et al., 2018 [29] |
| cob-cox2-R | 5ʹ-AAGAAATAATACCTTCCATAATCGG-3′ | ||
| cox3-F | 5′-GCGGTTCAACAAGGTTTGC-3′ | 469 | This study |
| cox3-R | 5′-GACGACTAAAATGATCTAGATATAACCGAAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, W.; Zhuang, M.; Wu, T.; Zhao, C.; Dai, W.; Zhang, J. Population Genetic Structure of Sargassum horneri, the Dominant Species of Golden Tide in the Yellow Sea. J. Mar. Sci. Eng. 2024, 12, 900. https://doi.org/10.3390/jmse12060900
Wang X, Zhao W, Zhuang M, Wu T, Zhao C, Dai W, Zhang J. Population Genetic Structure of Sargassum horneri, the Dominant Species of Golden Tide in the Yellow Sea. Journal of Marine Science and Engineering. 2024; 12(6):900. https://doi.org/10.3390/jmse12060900
Chicago/Turabian StyleWang, Xiaoran, Weiqian Zhao, Minmin Zhuang, Tingjian Wu, Chunyan Zhao, Wei Dai, and Jianheng Zhang. 2024. "Population Genetic Structure of Sargassum horneri, the Dominant Species of Golden Tide in the Yellow Sea" Journal of Marine Science and Engineering 12, no. 6: 900. https://doi.org/10.3390/jmse12060900





