Physiological and Biochemical Responses of the Green Tide-Forming Algae, Ulva Species, under Different Nutrient Conditions on Jeju Island, Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Environmental Parameters
2.3. Sample Preparations and Nitrate Uptake Efficiency
2.4. Nitrate Reductase Activity
2.5. Elemental Composition and Isotope Analysis
2.6. Statistical Analyses
3. Results
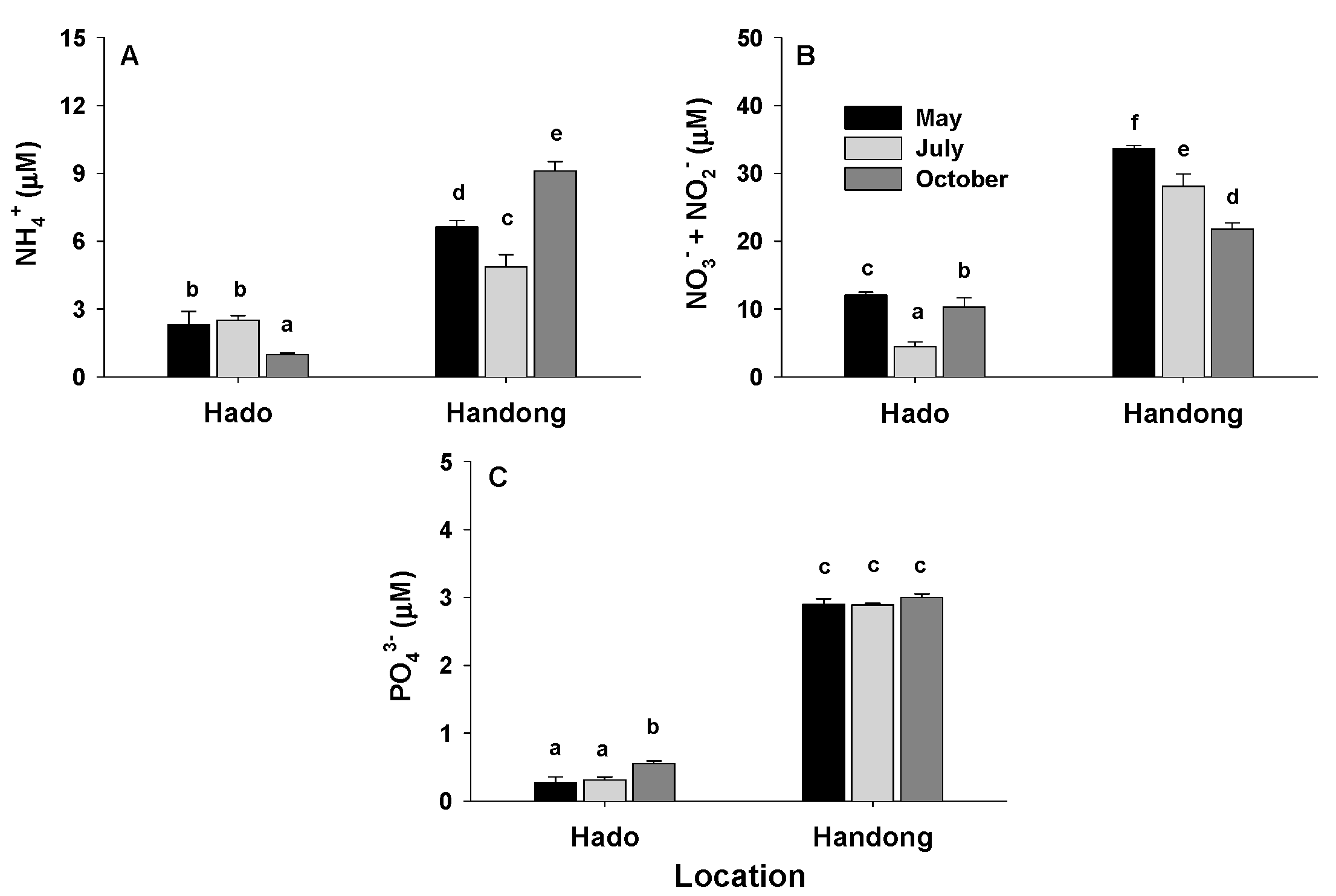
3.1. Environmental Parameters
3.2. Nitrate Reductase Activity and Nitrate Uptake Efficiency
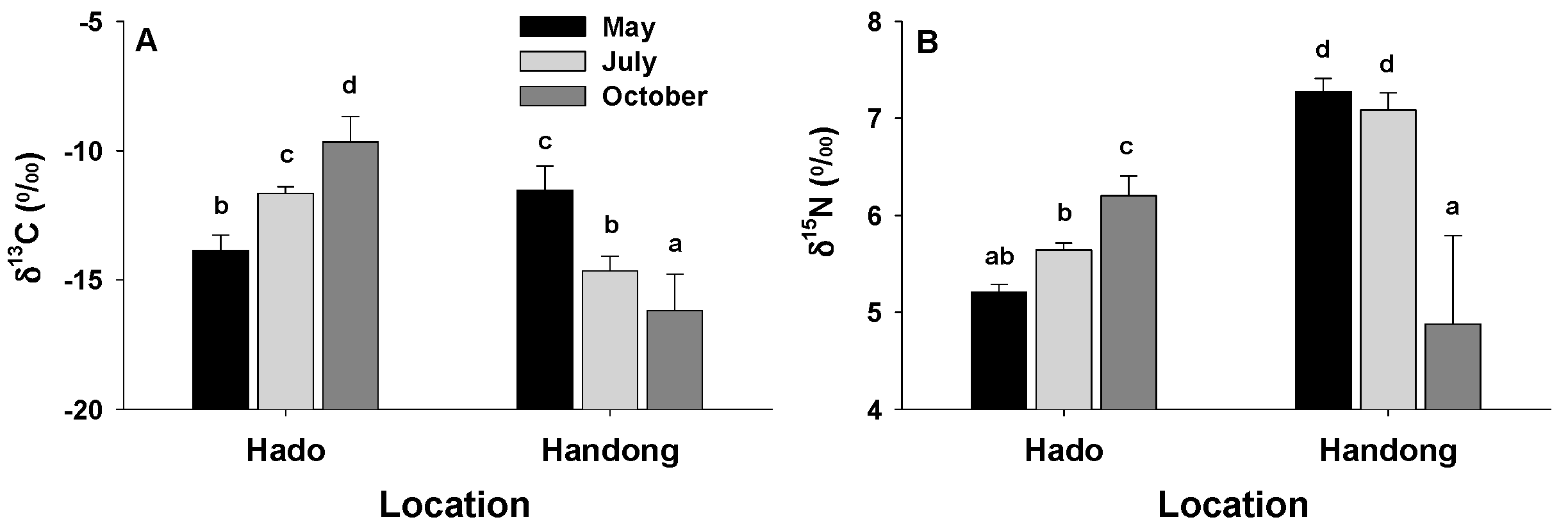
3.3. Elemental and Isotope Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nixon, S.W.; Pilson, M.E. Nitrogen in estuarine and coastal marine ecosystems. Nitrogen Mar. Environ. 1983, 565, 648. [Google Scholar]
- Howarth, R.W. Nutrient limitation of net primary production in marine ecosystems. Annu. Rev. Ecol. Syst. 1988, 19, 89–110. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Clark, M.W. Nutrient inputs from the watershed and coastal eutrophication in the Florida Keys. Estuaries 1992, 15, 465–476. [Google Scholar] [CrossRef]
- Smith, S.V. Phosphorus versus nitrogen limitation in the marine environment. Limnol. Oceanogr. 1984, 29, 1149–1160. [Google Scholar] [CrossRef]
- Valiela, I.; Collins, G.; Kremer, J.; Lajtha, K.; Geist, M.; Seely, B.; Brawley, J.; Sham, C.H. Nitrogen loading from coastal watersheds to receiving estuaries: New method and application. Ecol. Appl. 1998, 7, 358–380. [Google Scholar] [CrossRef]
- Howarth, R.W.; Sharpley, A.; Walker, D. Sources of nutrient pollution to coastal waters in the United States: Implications for achieving coastal water quality goals. Estuaries 2002, 25, 656–676. [Google Scholar] [CrossRef]
- Cho, H.-M.; Kim, G.; Shin, K.-H. Tracing nitrogen sources fueling coastal green tides off a volcanic island using radon and nitrogen isotopic tracers. Sci. Total Environ. 2019, 665, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, T.H.; Kang, Y.H.; Kim, S.; Kim, T.-H.; Kim, J.K.; Lee, T.; Son, Y.B.; Lee, H.J.; Park, S.R. Changes in the Dynamics and Nutrient Budget of a Macroalgal Community Exposed to Land-Based Fish Farm Discharge Off Jeju Island, Korea. Sustainability 2021, 13, 11793. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justić, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Azanza, R.V.; Benico, G.A. Toxic Alexandrium blooms in fish farming sites in Bolinao, Pangasinan. J. Environ. Sci. Manag. 2013, 44–49. [Google Scholar]
- Troell, M.; Rönnbäck, P.; Halling, C.; Kautsky, N.; Buschmann, A. Ecological engineering in aquaculture: Use of seaweeds for removing nutrients from intensive mariculture. In Proceedings of the Sixteenth International Seaweed Symposium, Cebu City, Philippines, 12–18 April 1998. [Google Scholar]
- Read, P.; Fernandes, T. Management of environmental impacts of marine aquaculture in Europe. Aquaculture 2003, 226, 139–163. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Neori, A.; Chopin, T.; Buschmann, A.; Kautsky, N.; Yarish, C. Integrated mariculture: Asking the right questions. Aquaculture 2003, 226, 69–90. [Google Scholar] [CrossRef]
- Neori, A.; Krom, M.D.; van Rijn, J. Biogeochemical processes in intensive zero-effluent marine fish culture with recirculating aerobic and anaerobic biofilters. J. Exp. Mar. Biol. Ecol. 2007, 349, 235–247. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. 2022. Available online: https://reliefweb.int/report/world/state-world-fisheries-and-aquaculture-2022 (accessed on 16 October 2023).
- Korea, S. Korean Statistical Information System (KOSIS). Available online: http://kostat.go.kr (accessed on 16 October 2023).
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Kim, J.; Song, B.-C.; Lee, M.-Y.; Kim, T.-H. Monthly variation in flux of inorganic nutrients from submarine groundwater discharge in a volcanic island: Significant nitrogen contamination in groundwater. Front. Mar. Sci. 2022, 9, 835207. [Google Scholar] [CrossRef]
- Oh, Y.H.; Kim, Y.; Park, S.R.; Lee, T.; Son, Y.B.; Park, S.-E.; Lee, W.C.; Im, D.-H.; Kim, T.-H. Spatiotemporal change in coastal waters caused by land-based fish farm wastewater-borne nutrients: Results from Jeju Island, Korea. Mar. Pollut. Bull. 2021, 170, 112632. [Google Scholar] [CrossRef] [PubMed]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.A.V.; Corbisier, T.N.; Soto-jiménez, M.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Chang. Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Berges, J.A.; Harrison, P.J. Nitrate reductase activity quantitatively predicts the rate of nitrate incorporation under steady state light limitation: A revised assay and characterization of the enzyme in three species of marine phytoplankton. Limnol. Oceanogr. 1995, 40, 82–93. [Google Scholar] [CrossRef]
- Chow, F. Applied Photosynthesis New Progress; InTechOpen: London, UK, 2012; pp. 105–120. [Google Scholar]
- Teichberg, M.; Heffner, L.; Fox, S.; Valiela, I. Nitrate reductase and glutamine synthetase activity, internal N pools, and growth of Ulva lactuca: Responses to long and short-term N supply. Mar. Biol. 2007, 151, 1249–1259. [Google Scholar] [CrossRef]
- Gordillo, F.J. Environment and algal nutrition. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 67–86. [Google Scholar]
- Amato, D.W.; Bishop, J.M.; Glenn, C.R.; Dulai, H.; Smith, C.M. Impact of submarine groundwater discharge on marine water quality and reef biota of Maui. PLoS ONE 2016, 11, e0165825. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.R.; Kang, Y.H.; Kim, G.-Y.; Lee, K.-S.; Lee, H.J.; Won, N.-I.; Kil, H.-J. Usefulness of tissue nitrogen content and macroalgal community structure as indicators of water eutrophication. J. Appl. Phycol. 2014, 26, 1149–1158. [Google Scholar] [CrossRef]
- Samanta, P.; Shin, S.; Jang, S.; Song, Y.-C.; Oh, S.; Kim, J.K. Stable carbon and nitrogen isotopic characterization and tracing nutrient sources of Ulva blooms around Jeju coastal areas. Environ. Pollut. 2019, 254, 113033. [Google Scholar] [CrossRef]
- Orlandi, L.; Bentivoglio, F.; Carlino, P.; Calizza, E.; Rossi, D.; Costantini, M.L.; Rossi, L. δ15N variation in Ulva lactuca as a proxy for anthropogenic nitrogen inputs in coastal areas of Gulf of Gaeta (Mediterranean Sea). Mar. Pollut. Bull. 2014, 84, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lemesle, S.; Erraud, A.; Mussio, I.; Rusig, A.-M.; Claquin, P. Dynamics of δ15N isotopic signatures of different intertidal macroalgal species: Assessment of bioindicators of N sources in coastal areas. Mar. Pollut. Bull. 2016, 110, 470–483. [Google Scholar] [CrossRef]
- Parsons, T.R. A Manual of Chemical & Biological Methods for Seawater Analysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, S.; Choi, S.K.; Lee, H.J.; Chung, I.K.; Park, S.R. A comparison of the bioremediation potential of five seaweed species in an integrated fish-seaweed aquaculture system: Implication for a multi-species seaweed culture. Rev. Aquac. 2021, 13, 353–364. [Google Scholar] [CrossRef]
- Corzo, A.; Niell, F. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J. Exp. Mar. Biol. Ecol. 1991, 146, 181–191. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Young, E.B.; Dring, M.J.; Savidge, G.; Birkett, D.A.; Berges, J.A. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant Cell Environ. 2007, 30, 764–774. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Krause-Jensen, D. Monitoring nutrient release from fish farms with macroalgal and phytoplankton bioassays. Aquaculture 2006, 256, 302–310. [Google Scholar] [CrossRef]
- Tsagkamilis, P.; Danielidis, D.; Dring, M.J.; Katsaros, C. Removal of phosphate by the green seaweed Ulva lactuca in a small-scale sewage treatment plant (Ios Island, Aegean Sea, Greece). J. Appl. Phycol. 2010, 22, 331–339. [Google Scholar] [CrossRef]
- Cohen, R.A.; Fong, P. Physiological responses of a bloom-forming green macroalga to short-term change in salinity, nutrients, and light help explain its ecological success. Estuaries 2004, 27, 209–216. [Google Scholar] [CrossRef]
- Cabello-Pasini, A.; Macías-Carranza, V.; Abdala, R.; Korbee, N.; Figueroa, F.L. Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). J. Appl. Phycol. 2011, 23, 363–369. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Z.; Shao, H.; Jin, Y. Effects of nitrogen and phosphate enrichment on the activity of nitrate reductase of Ulva prolifera in coastal zone. Acta Physiol. Plant. 2016, 38, 169. [Google Scholar] [CrossRef]
- Lartigue, J.; Sherman, T.D. Response of Enteromorpha sp.(Chlorophyceae) to a nitrate pulse: Nitrate uptake, inorganic nitrogen storage and nitrate reductase activity. Mar. Ecol. Prog. Ser. 2005, 292, 147–157. [Google Scholar] [CrossRef]
- Sanford, L.P.; Crawford, S.M. Mass transfer versus kinetic control of uptake across solid-water boundaries. Limnol. Oceanogr. 2000, 45, 1180–1186. [Google Scholar] [CrossRef]
- Zollmann, M.; Liberzon, A.; Golberg, A. Modeling of growth of the macroalga Ulva sp. in a controlled photobioreactor based on nitrogen accumulation dynamics. Appl. Phycol. 2023, 4, 121–140. [Google Scholar] [CrossRef]
- Alsufyani, T.; Weiss, A.; Wichard, T. Time course exo-metabolomic profiling in the green marine macroalga Ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar. Drugs. 2017, 15, 14. [Google Scholar] [CrossRef]
- Naldi, M.; Viaroli, P. Nitrae uptake and storage in the seaweed Ulva rigida C. Agardh in relation to nitrate availability and thallus nitrate content in a eutrophic coastal lagoon (Sacca di Goro, Po River Delta, Italy). J. Exp. Mar. Bio. Ecol. 2002, 269, 65–83. [Google Scholar] [CrossRef]
- Streicher, M.D.; Reiss, H.; Reiss, K. Impact of aquaculture and agriculture nutrient sources on macroalgae in a bioassay study. Mar. Pollut. Bull. 2021, 173, 113025. [Google Scholar] [CrossRef] [PubMed]
- Hanisak, M.D. The use of Gracilaria tikvahiae (Gracilariales, Rhodophyta) as a model system to understand the nitrogen nutrition of cultured seaweeds. Hydrobiologia 1990, 204, 79–87. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Björnsäter, B.R. Seasonal fluctuations in tissue nitrogen, phosphorus, and N:P for five macroalgal species common to the pacific northwest coast. J. Phycol. 1992, 28, 1–6. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, X.; Jiang, Y.; Luo, P.; Hu, C. Bioremediation and fodder potentials of two Sargassum spp. in coastal waters of Shenzhen, South China. Mar. Pollut. Bull. 2014, 85, 797–802. [Google Scholar] [CrossRef]
- Atkinson, M.; Smith, S. C: N: P ratios of benthic marine plants. Limnol. Oceanogr. 1983, 28, 568–574. [Google Scholar] [CrossRef]
- Björnsäter, B.R.; Wheeler, P.A. Effect of nitrogen and phosphorus supply on growth and tissue composition of Ulva fenestrata and Enteromorpha intestinalis (ulvales, chlorophyta). J. Phycol. 1990, 26, 603–611. [Google Scholar] [CrossRef]
- Lin, D.T.; Fong, P. Macroalgal bioindicators (growth, tissue N, δ15N) detect nutrient enrichment from shrimp farm effluent entering Opunohu Bay, Moorea, French Polynesia. Mar. Pollut. Bull. 2008, 56, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Albertin, A.R.; Sickman, J.O.; Pinowska, A.; Stevenson, R.J. Identification of nitrogen sources and transformations within karst springs using isotope tracers of nitrogen. Biogeochemistry 2012, 108, 219–232. [Google Scholar] [CrossRef]
- Viana, I.G.; Bode, A. Variability in δ15N of intertidal brown algae along a salinity gradient: Differential impact of nitrogen sources. Sci. Total Environ. 2015, 512, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L. Seasonal changes in nutrient limitation and nitrate sources in the green macroalga Ulva lactuca at sites with and without green tides in a northeastern Pacific embayment. Mar. Pollut. Bull. 2016, 103, 186–194. [Google Scholar] [CrossRef]
- Ghaderiardakni, F.; Califiano, G.; Mohr, J.F.; Abreu, M.H.; Coates, J.C.; Wichard, T. Analysis of algal growth-and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp. Aquaculture 2019, 11, 375–391. [Google Scholar] [CrossRef]




| Parameter | Time | Site | |
|---|---|---|---|
| Hado | Handong | ||
| Water temperature (°C) | May | 18.5 | 17.0 |
| July | 25.9 | 20.4 | |
| October | 18.6 | 17.9 | |
| Salinity (PSU) | May | 34.5 | 33.4 |
| July | 33.1 | 33.2 | |
| October | 31.2 | 29.8 | |
| pH | May | 8.28 | 7.82 |
| July | 8.62 | 7.88 | |
| October | 8.35 | 7.86 | |
| Parameter | Source | df | MS | F-Ratio | p-Value |
|---|---|---|---|---|---|
| NH4+ | Site | 1 | 131.794 | 815.524 | <0.001 |
| Time | 2 | 3.445 | 21.319 | <0.001 | |
| Site × Time | 2 | 15.615 | 96.624 | <0.001 | |
| NO3− + NO2− | Site | 1 | 2142.882 | 1807.797 | <0.001 |
| Time | 2 | 119.197 | 100.558 | <0.001 | |
| Site × Time | 2 | 84.155 | 70.996 | <0.001 | |
| PO43− | Site | 1 | 39.066 | 5162.910 | <0.001 |
| Time | 2 | 0.091 | 11.980 | <0.001 | |
| Site × Time | 2 | 0.019 | 2.493 | <0.001 | |
| Nitrate reductase | Site | 1 | 96.520 | 87.212 | <0.001 |
| activity | Time | 2 | 18.983 | 17.153 | 0.002 |
| Site × Time | 2 | 12.151 | 10.979 | <0.001 | |
| Nitrate uptake efficiency | Site | 1 | 6.534 | 715.159 | <0.001 |
| (May) | Time | 2 | 0.013 | 1.450 | 0.257 |
| Site × Time | 2 | 0.004 | 0.455 | 0.641 | |
| Nitrate uptake efficiency | Site | 1 | 2.574 | 278.060 | <0.001 |
| (July) | Time | 2 | 0.685 | 74.002 | <0.001 |
| Site × Time | 2 | 0.074 | 8.041 | 0.003 | |
| Tissue C content | Site | 1 | 47.704 | 94.620 | <0.001 |
| Time | 2 | 21.345 | 42.336 | <0.001 | |
| Site × Time | 2 | 44.071 | 87.414 | <0.001 | |
| Tissue N content | Site | 1 | 9.441 | 542.336 | <0.001 |
| Time | 2 | 0.188 | 10.771 | <0.001 | |
| Site × Time | 2 | 0.347 | 19.963 | <0.001 | |
| Tissue P content | Site | 1 | 0.090 | 488.876 | <0.001 |
| Time | 2 | 0.002 | 9.433 | <0.001 | |
| Site × Time | 2 | 0.0002 | 0.833 | <0.001 | |
| C:N ratio | Site | 1 | 162.691 | 727.260 | <0.001 |
| Time | 2 | 4.737 | 21.177 | <0.001 | |
| Site × Time | 2 | 17.231 | 77.024 | <0.001 | |
| C:P ratio | Site | 1 | 1,746,261.600 | 1062.402 | <0.001 |
| Time | 2 | 40,411.851 | 24.586 | <0.001 | |
| Site × Time | 2 | 57,225.171 | 34.815 | <0.001 | |
| N:P ratio | Site | 1 | 3248.704 | 210.584 | <0.001 |
| Time | 2 | 173.035 | 11.216 | 0.001 | |
| Site × Time | 2 | 97.810 | 6.340 | 0.008 | |
| δ13C | Site | 1 | 31.061 | 44.155 | <0.001 |
| Time | 2 | 0.443 | 0.630 | 0.544 | |
| Site × Time | 2 | 42.409 | 60.288 | <0.001 | |
| δ15N | Site | 1 | 2.891 | 26.950 | <0.001 |
| Time | 2 | 1.267 | 11.816 | 0.001 | |
| Site × Time | 2 | 5.907 | 55.072 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.; Choi, S.K.; Ham, S.B.; Son, Y.B.; Kang, Y.H.; Park, S.R. Physiological and Biochemical Responses of the Green Tide-Forming Algae, Ulva Species, under Different Nutrient Conditions on Jeju Island, Korea. J. Mar. Sci. Eng. 2024, 12, 959. https://doi.org/10.3390/jmse12060959
Moon K, Choi SK, Ham SB, Son YB, Kang YH, Park SR. Physiological and Biochemical Responses of the Green Tide-Forming Algae, Ulva Species, under Different Nutrient Conditions on Jeju Island, Korea. Journal of Marine Science and Engineering. 2024; 12(6):959. https://doi.org/10.3390/jmse12060959
Chicago/Turabian StyleMoon, Kyeonglim, Sun Kyeong Choi, Seong Bin Ham, Young Baek Son, Yun Hee Kang, and Sang Rul Park. 2024. "Physiological and Biochemical Responses of the Green Tide-Forming Algae, Ulva Species, under Different Nutrient Conditions on Jeju Island, Korea" Journal of Marine Science and Engineering 12, no. 6: 959. https://doi.org/10.3390/jmse12060959
APA StyleMoon, K., Choi, S. K., Ham, S. B., Son, Y. B., Kang, Y. H., & Park, S. R. (2024). Physiological and Biochemical Responses of the Green Tide-Forming Algae, Ulva Species, under Different Nutrient Conditions on Jeju Island, Korea. Journal of Marine Science and Engineering, 12(6), 959. https://doi.org/10.3390/jmse12060959






