Study on the Characteristics and Mechanism of Inorganic Nitrogen Nutrients Preferential Assimilation by Phaeocystis globosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Culture
2.2. Experimental Setup
2.3. Sample Collection
2.4. Sample Analysis
2.5. Data Processing
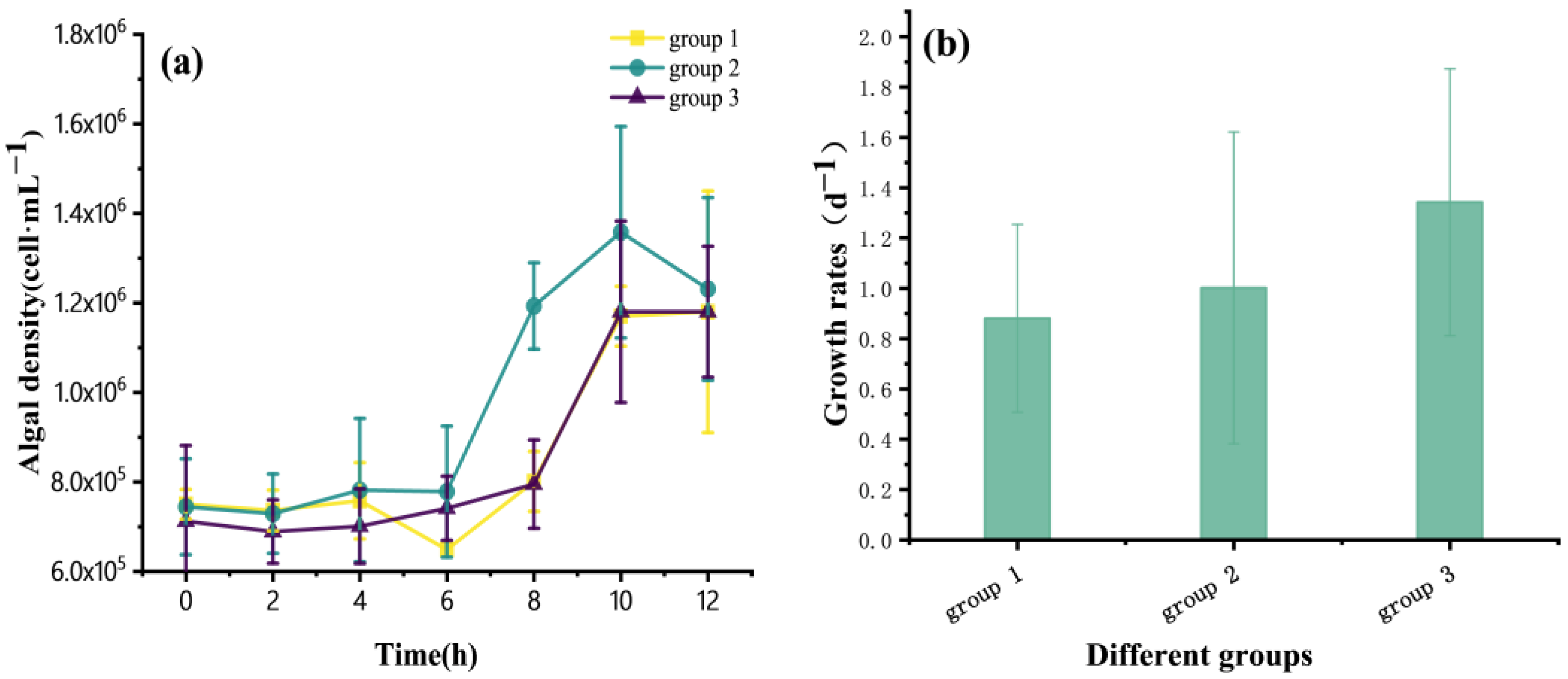
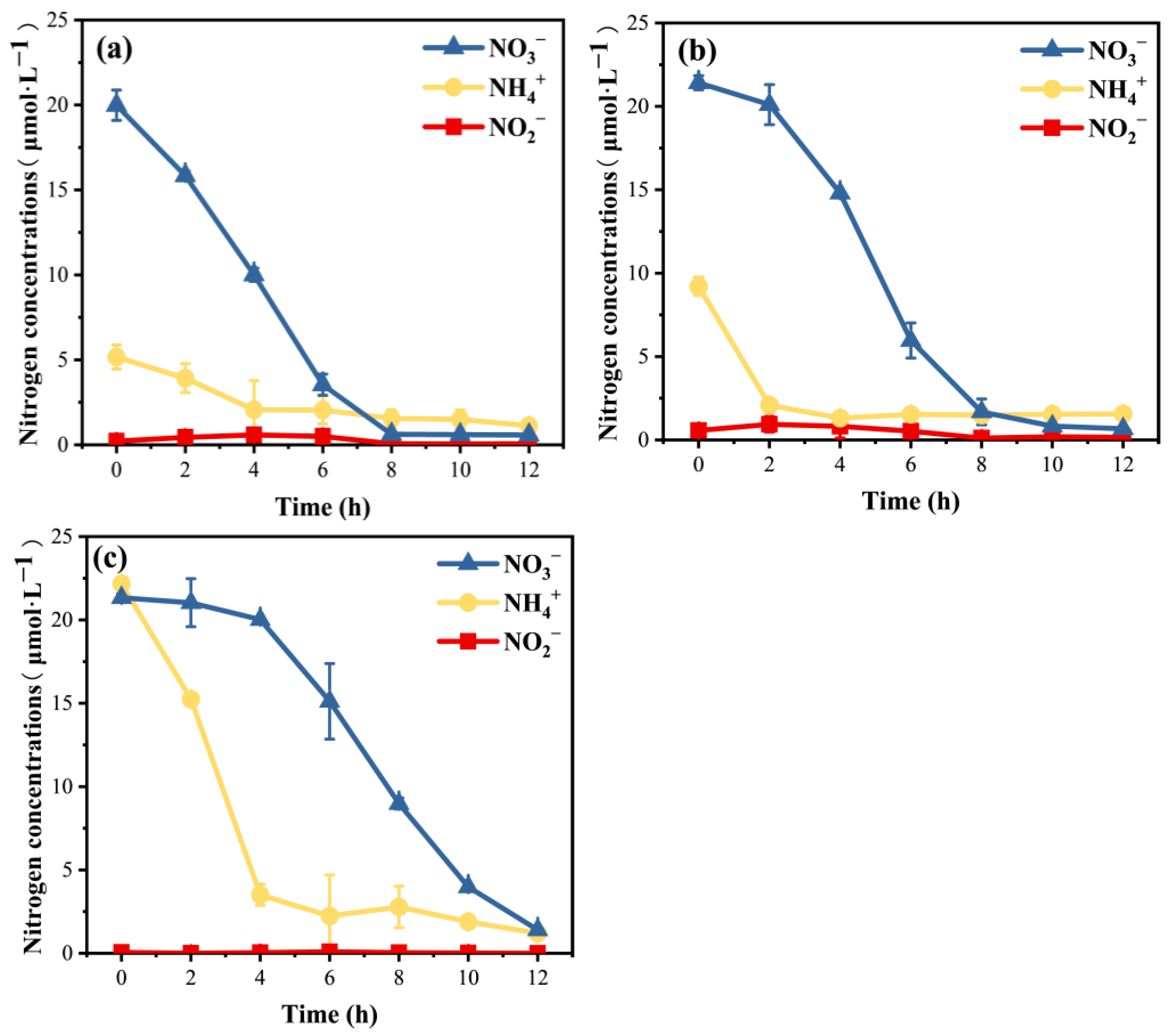
3. Results
3.1. Growth of P. globosa
3.2. Variations in Inorganic N Nutrient Concentrations
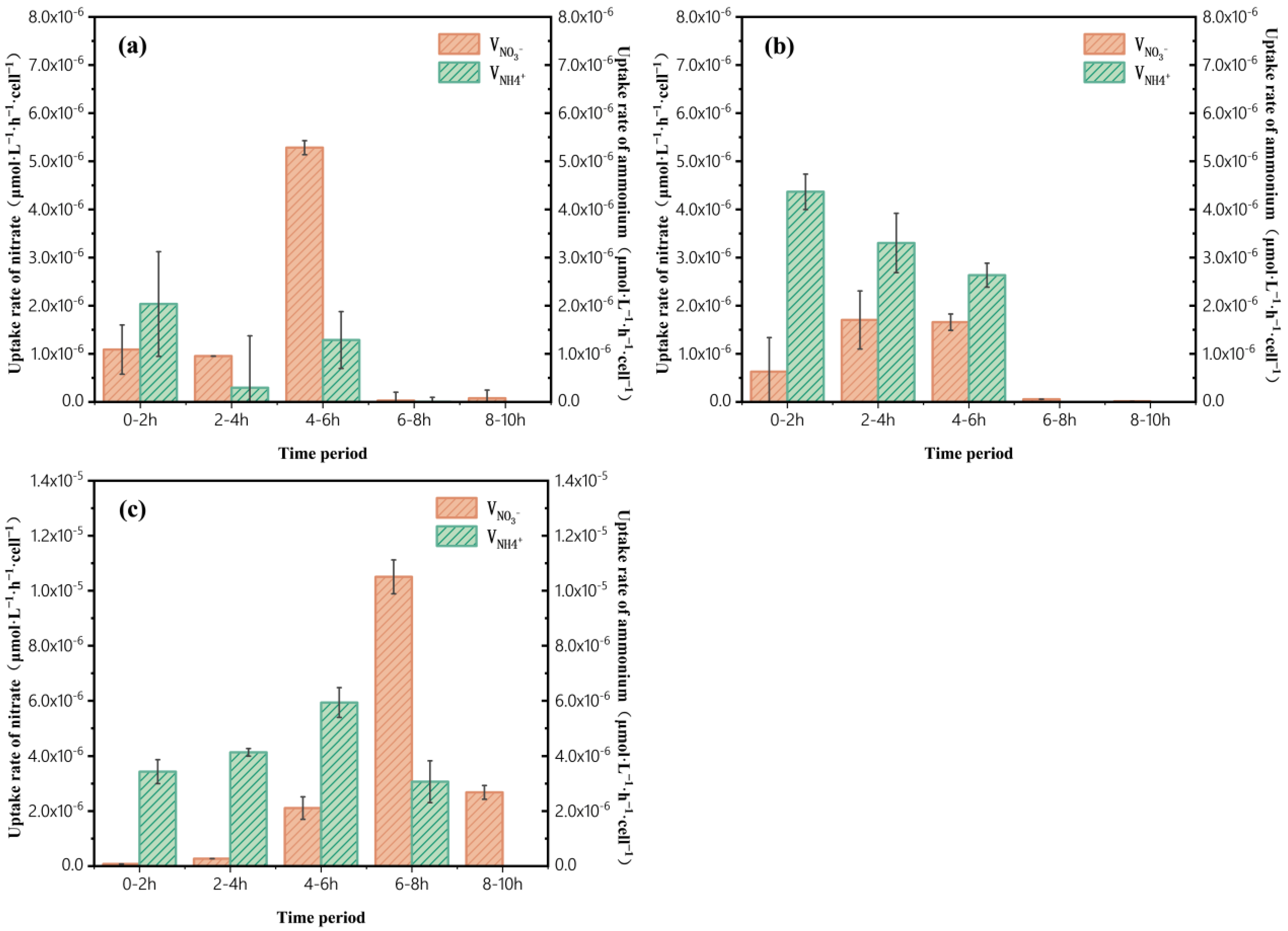
3.3. Variation in Uptake Rates
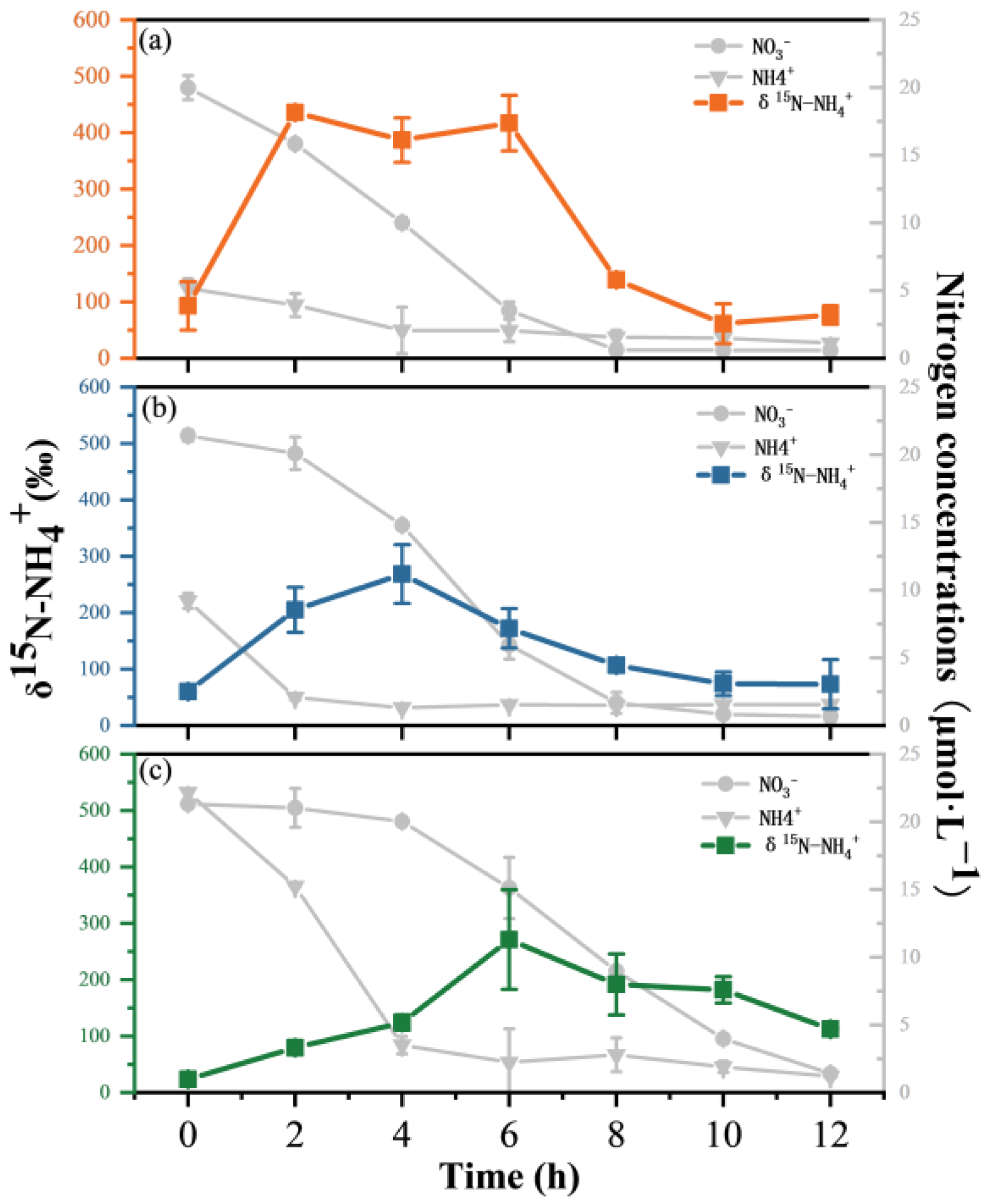
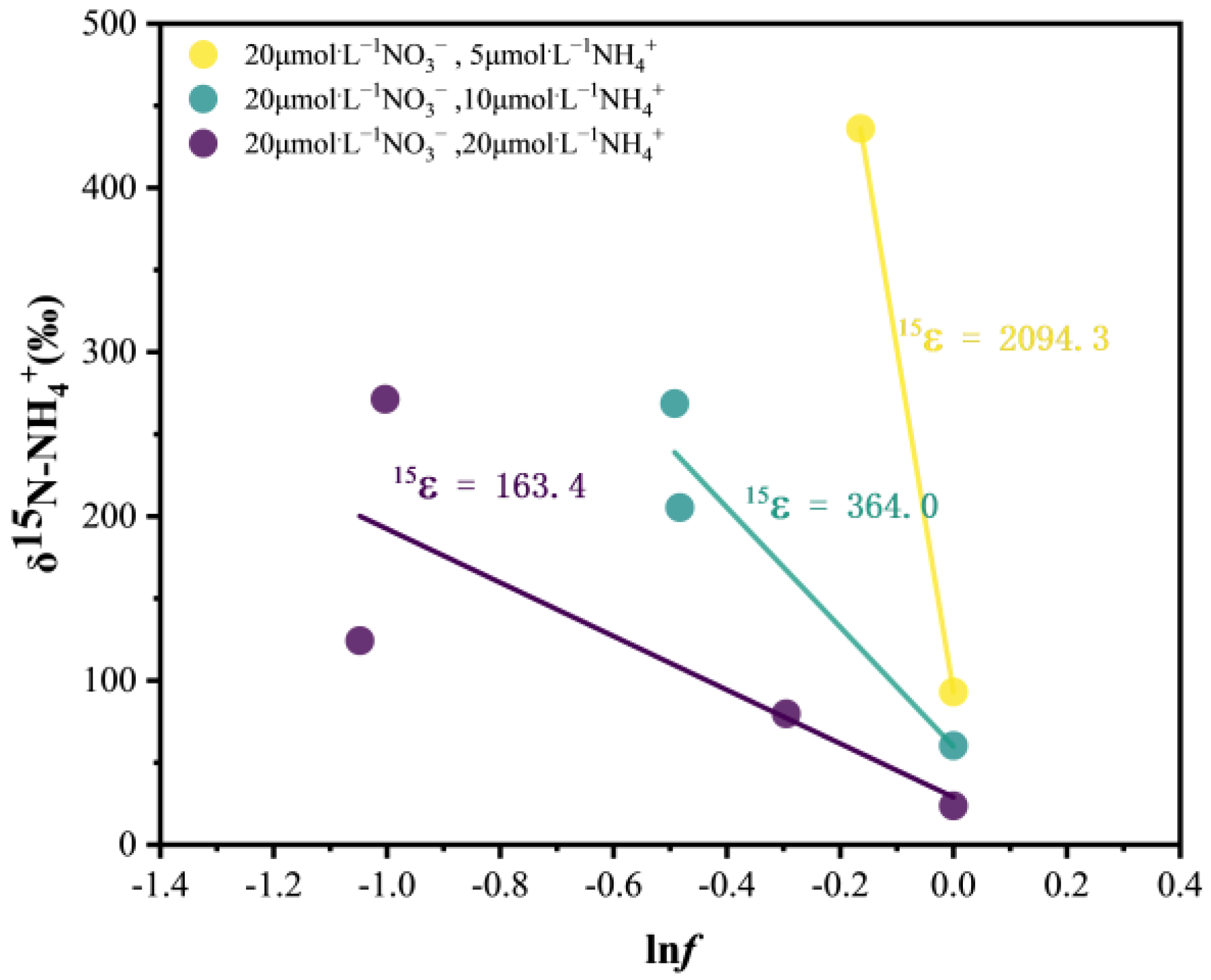
3.4. Variations in N Isotopes and Isotopic Fractionation (ε)
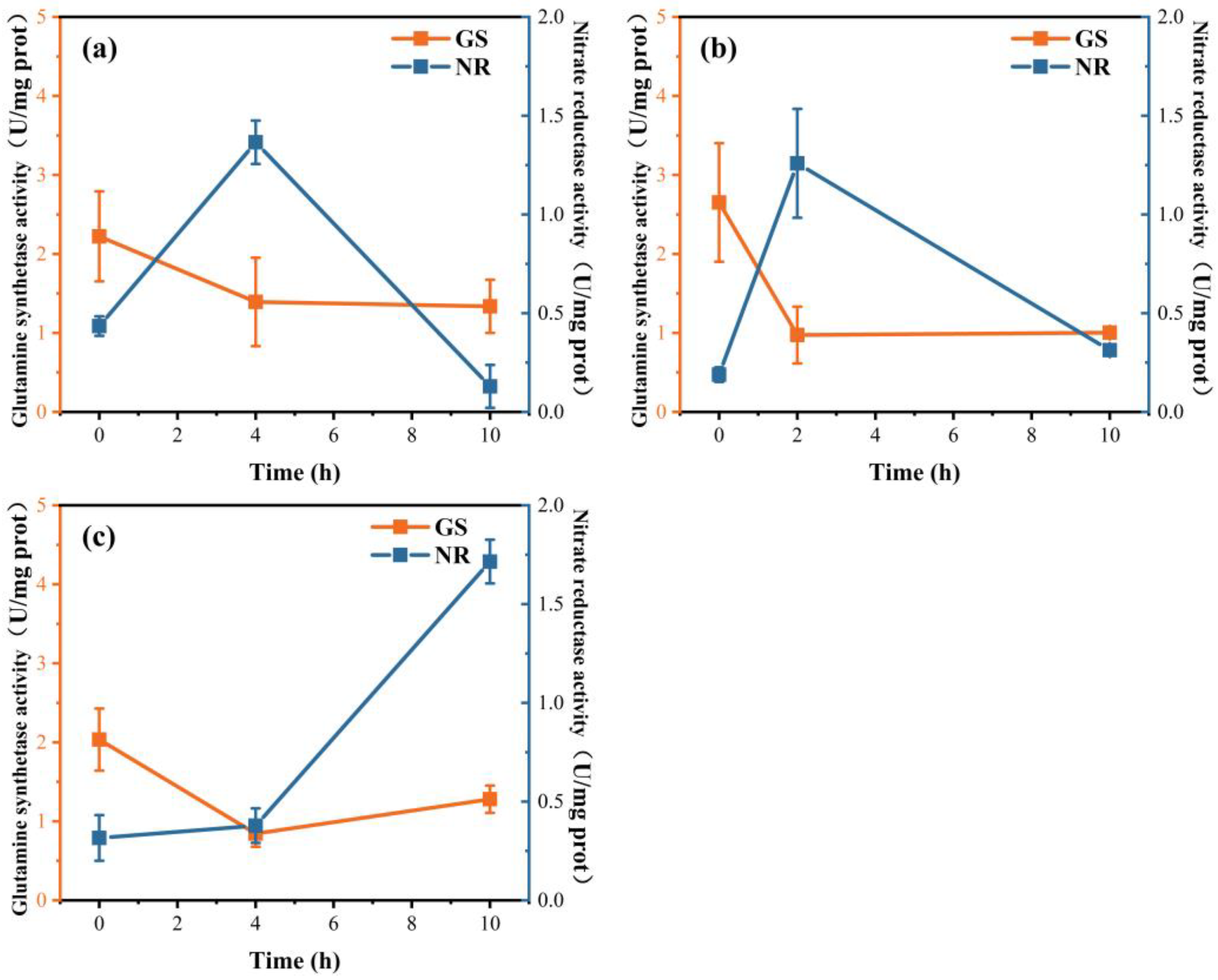
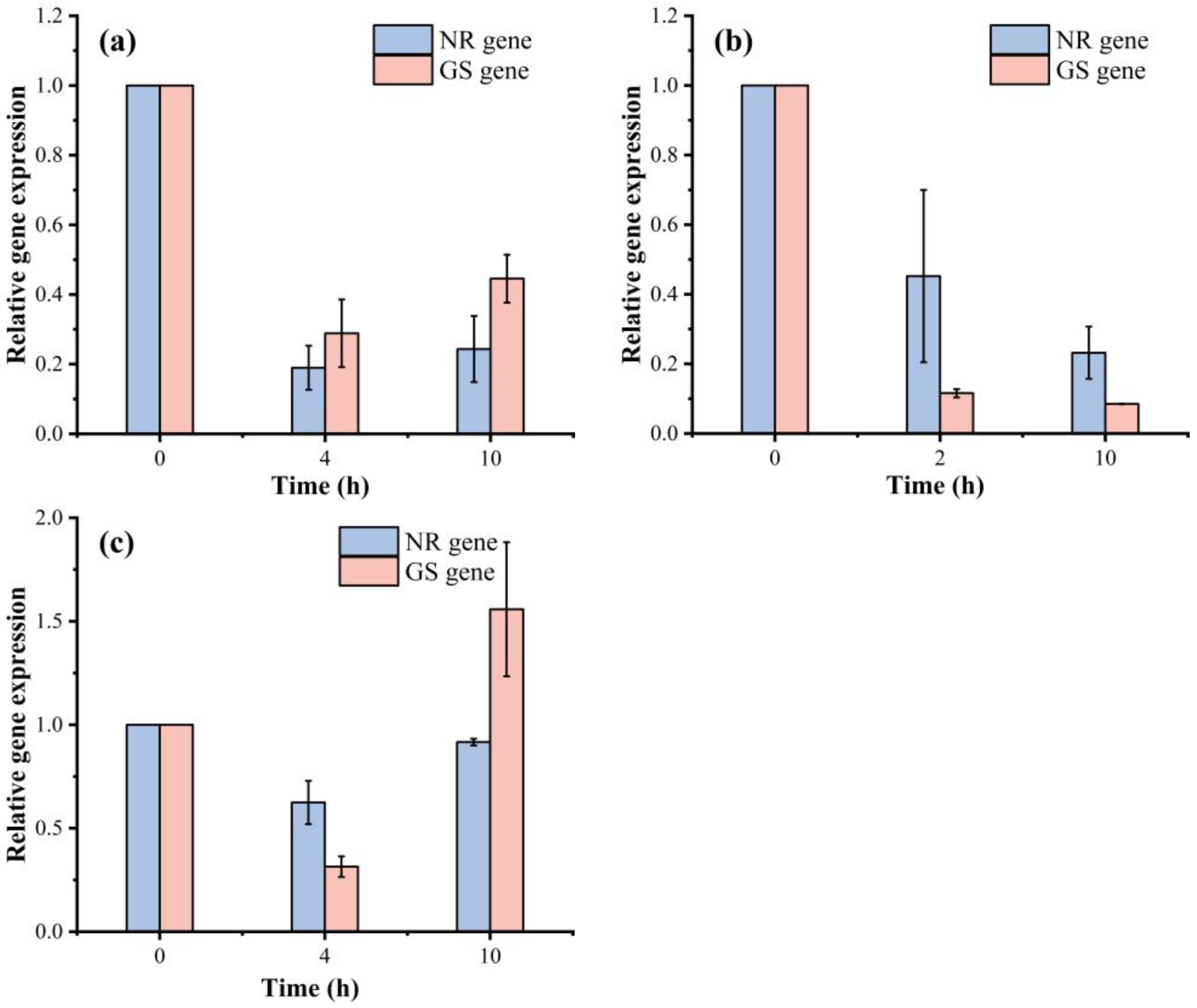
3.5. The Activities of NR and GS and the Expression of NR and GS Genes
4. Discussion
4.1. The Characteristics of NO3− and NH4+ Assimilation by P. globosa
4.1.1. Comparison of the Assimilation Rates of NO3− and NH4+
4.1.2. The Inhibitory Effect of Ambient Concentrations of NH4+ on the Assimilation of NO3−
4.2. Regulation of Nitrogen-Metabolizing Enzymes
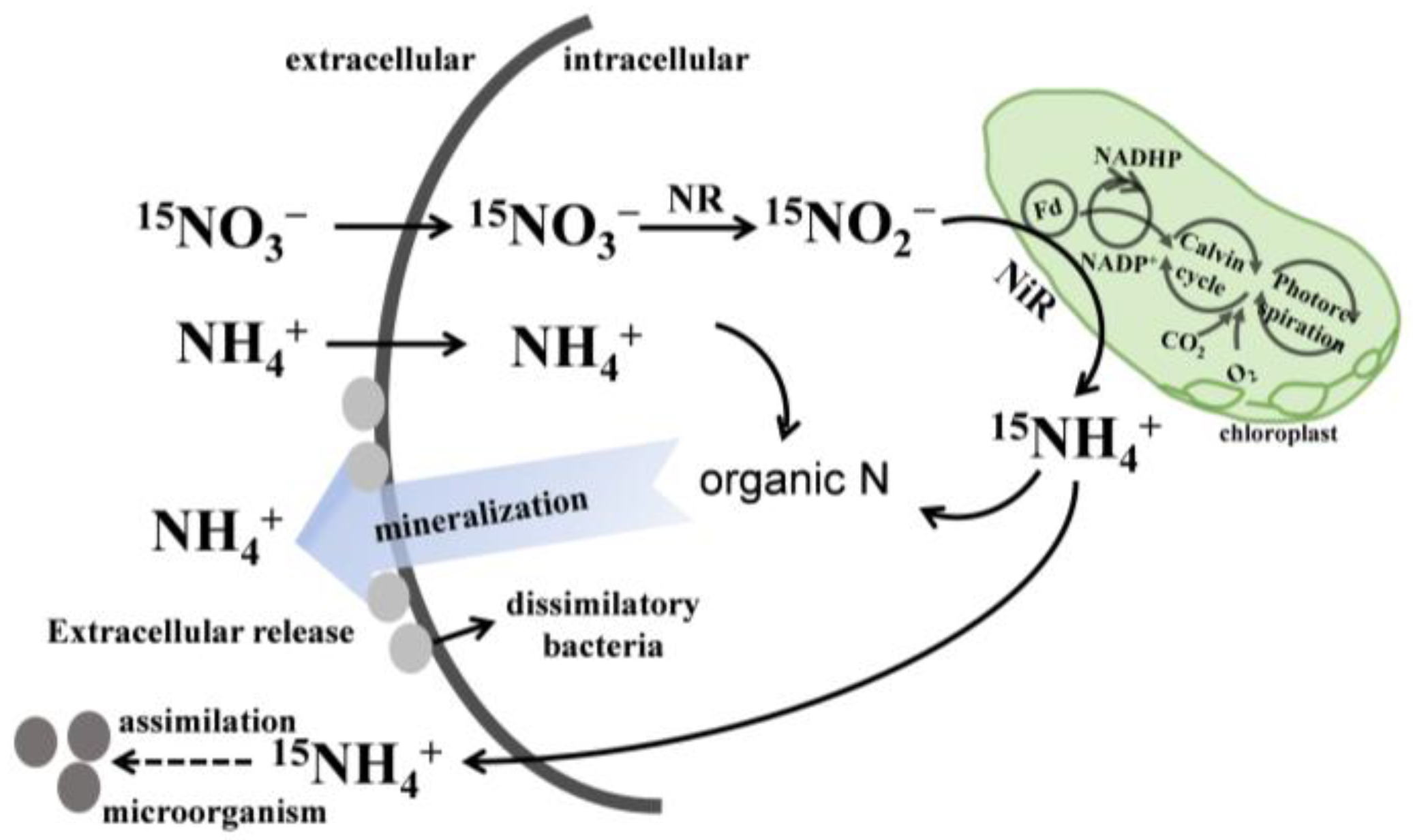
4.3. Mechanism of NH4+ Production in P. globosa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoemann, V.; Becquevort, S.; Stefels, J.; Rousseau, V.; Lancelot, C. Phaeocystis blooms in the global ocean and their controlling mechanisms: A review. J. Sea Res. 2005, 53, 43–66. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, J.; Wang, Z.; Xu, N.; Wang, Y.; Shen, P.; Lu, S.; Hodgkiss, I.J. Some observations on harmful algal bloom (HAB) events along the coast of Guangdong, southern China in 1998. Hydrobiologia 2004, 512, 209–214. [Google Scholar] [CrossRef]
- Mars Brisbin, M.; Mitarai, S. Differential Gene Expression Supports a Resource-Intensive, Defensive Role for Colony Production in the Bloom-Forming Haptophyte, Phaeocystis globosa. J. Eukaryot. Microbiol. 2019, 66, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.D.; Mari, X.; Bleijswijk, J.D.L.V.; Veldhuis, M.J.W. A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics: II. Significance for the microbial community. Harmful Algae 2005, 4, 875–893. [Google Scholar] [CrossRef]
- Tang, D.L.; Kawamura, H.; Doan-Nhu, H.; Takahashi, W. Remote sensing oceanography of a harmful algal bloom off the coast of southeastern Vietnam. J. Geophys. Res.-Ocean. 2004, 109, C03014. [Google Scholar] [CrossRef]
- Larsen, A.; Flaten, G.A.F.; Sandaa, R.-A.; Castberg, T.; Thyrhaug, R.; Erga, S.R.; Jacquet, S.; Bratbak, G. Spring phytoplankton bloom dynamics in Norwegian coastal waters: Microbial community succession and diversity. Limnol. Oceanogr. 2004, 49, 180–190. [Google Scholar] [CrossRef]
- Spilmont, N.; Denis, L.; Artigas, L.F.; Caloin, F.; Courcot, L.; Créach, A.; Desroy, N.; Gevaert, F.; Hacquebart, P.; Hubas, C.; et al. Impact of the Phaeocystis globosa spring bloom on the intertidal benthic compartment in the eastern English Channel: A synthesis. Mar. Pollut. Bull. 2009, 58, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, C. Factors affecting phytoplankton extracellular release in the Southern Bight of the North Sea. Mar. Ecol. Prog. Ser. 1983, 12, 115–121. [Google Scholar] [CrossRef]
- He, L.; Song, X.; Yu, F.; Wang, K.; Song, S.Q.; Yu, Z. Potential risk and prevention of phytoplankton outbreak to water-cooling system in nuclear power plant in Fangchenggang, Guangxi. J. Oceanol. Limnol. 2019, 50, 700–706. [Google Scholar] [CrossRef]
- Raven, J.A.; Wollenweber, B.; Handley, L.L. A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New. Phytol. 1992, 121, 19–32. [Google Scholar] [CrossRef]
- MacIsaac, J.J.; Dugdale, R.C. The kinetics of nitrate and ammonia uptake by natural populations of marine phytoplankton. Deep. Sea Res. Oceanogr. Abstr. 1969, 16, 45–57. [Google Scholar] [CrossRef]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Parker, A.E.; Burkholder, J.M.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- Ludwig, C.A. The availability of different forms of nitrogen to a green alga. Am. J. Bot. 1938, 25, 448–458. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Taylor, W.R.; Taft, J.L. Nitrogenous nutrition of the plankton in the Chesapeake Bay. 1. Nutrient availability and phytoplankton preferences. Limnol. Oceanogr. 1977, 22, 996–1011. [Google Scholar] [CrossRef]
- Eppley, R.W.; Coatsworth, J.L.; Solórzano, L. Studies of Nitrate Reductase In Marine Phytoplankton1. Limnol. Oceanogr. 1969, 14, 194–205. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Kokkinakis, S.A. Ammonium recycling limits nitrate use in the oceanic subarctic Pacific. Limnol. Oceanogr. 1990, 35, 1267–1278. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Smith, W.O., Jr. The role of nitrogen on the growth and colony development of Phaeocystis globosa (Prymnesiophyceae). Eur. J. Phycol. 2011, 46, 305–314. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Y.; Li, S. Nutritional requirements for the growth of Phaeocystis globosa scherffel. Acta Hydrobiol. Sin. 2007, 31, 24–29. [Google Scholar] [CrossRef]
- Lancelot, C.; Gypens, N.; Billen, G.; Garnier, J.; Roubeix, V. Testing an integrated river–ocean mathematical tool for linking marine eutrophication to land use: The Phaeocystis-dominated Belgian coastal zone (Southern North Sea) over the past 50 years. J. Mar. Syst. 2007, 64, 216–228. [Google Scholar] [CrossRef]
- Tungaraza, C.; Rousseau, V.; Brion, N.; Lancelot, C.; Gichuki, J.; Baeyens, W.; Goeyens, L. Contrasting nitrogen uptake by diatom and Phaeocystis-dominated phytoplankton assemblages in the North Sea. J. Exp. Mar. Biol. Ecol. 2003, 292, 19–41. [Google Scholar] [CrossRef]
- Smith, W.O.; Carlson, C.A.; Ducklow, H.W.; Hansell, D.A. Growth dynamics of Phaeocystis antarctica-dominated plankton assemblages from the Ross Sea. Mar. Ecol. Prog. Ser. 1998, 168, 229–244. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Robinson, D.H.; Worthen, D.L.; Dunbar, R.B.; DiTullio, G.R.; VanWoert, M.; Lizotte, M.P. Phytoplankton Community Structure and the Drawdown of Nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Z.; Song, X.; Yuan, Y.; Cao, X.; Yu, Z. Nutritional strategy for the preferential uptake of NO3--N by Phaeocystis globosa. Hydrobiologia 2019, 846, 109–122. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Mulholland, M.R.; Bernhardt, P.W.; Heil, C.A.; Bronk, D.A.; O’Neil, J.M. Nitrogen fixation and release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico. Limnol. Oceanogr. 2006, 51. [Google Scholar] [CrossRef]
- Malone, T.C.; Garside, C.; Haines, K.C.; Roels, O.A. Nitrate uptake and growth of Chaetoceros sp. in large outdoor continuous cultures1. Limnol. Oceanogr. 1975, 20, 9–19. [Google Scholar] [CrossRef]
- Auro, M.E.; Cochlan, W.P. Nitrogen Utilization and Toxin Production by Two Diatoms of the Pseudo-nitzschia pseudodelicatissima Complex: P. cuspidata and P. fryxelliana. J. Phycol. 2013, 49, 156–169. [Google Scholar] [CrossRef]
- Goering, J.J.; Dugdale, R.C.; Menzel, D.W. Estimates of In Situ Rates of Nitrogen Uptake by Trichodesmium sp. in the Tropical Atlantic Ocean1. Limnol. Oceanogr. 1966, 11, 614–620. [Google Scholar] [CrossRef]
- Kwon, H.K.; Oh, S.J.; Yang, H.S. Growth and uptake kinetics of nitrate and phosphate by benthic microalgae for phytoremediation of eutrophic coastal sediments. Bioresour. Technol. 2013, 129, 387–395. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P.; Hogue, V.E.; Marchi, A. The role of ammonium and nitrate in spring bloom development in San Francisco Bay. Estuar. Coast. Shelf Sci. 2007, 73, 17–29. [Google Scholar] [CrossRef]
- Ohmori, M.; Ohmori, K.; Strotmann, H. Inhibition of nitrate uptake by ammonia in a blue-green alga, Anabaena cylindrica. Arch. Microbiol. 1977, 114, 225–229. [Google Scholar] [CrossRef]
- L’Helguen, S.; Maguer, J.-F.; Caradec, J. Inhibition kinetics of nitrate uptake by ammonium in size-fractionated oceanic phytoplankton communities: Implications for new production and f-ratio estimates. J. Plankton Res. 2008, 30, 1179–1188. [Google Scholar] [CrossRef]
- Karsh, K.L.; Granger, J.; Kritee, K.; Sigman, D.M. Eukaryotic Assimilatory Nitrate Reductase Fractionates N and O Isotopes with a Ratio near Unity. Environ. Sci. Technol. 2012, 46, 5727–5735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Lou, Y.; Liu, Y.; Zhao, X.; Wang, G. Effect of water accommodated fractions of fuel oil on fixed carbon and nitrogen by microalgae: Implication by stable isotope analysis. Ecotoxicol. Environ. Saf. 2020, 195, 110488. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, C.; Spitz, Y.; Gypens, N.; Ruddick, K.G.; Becquevort, S.; Rousseau, V.; Lacroix, G.; Billen, G. Modelling diatom and Phaeocystis blooms and nutrient cycles in the Southern Bight of the North Sea: The MIRO model. Mar. Ecol. Prog. Ser. 2005, 289, 63–78. [Google Scholar] [CrossRef]
- Riegman, R.; Noordeloos, A.A.M.; Cadée, G.C. Phaeocystis blooms and eutrophication of the continental coastal zones of the North Sea. Mar. Biol. 1992, 112, 479–484. [Google Scholar] [CrossRef]
- Liang, D.Y.; Wang, X.D.; Huo, Y.P.; Wang, Y.; Li, S.S. Differences Between Solitary Cells and Colonial Cells in the Heteromorphic Life Cycle of Phaeocystis globosa: Morphology, Physiology, and Transcriptome. J. Ocean. Univ. China 2021, 20, 939–948. [Google Scholar] [CrossRef]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Ammonium assimilation in cyanobacteria. Photosynth. Res. 2005, 83, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant. Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 Functions as a Nitrate Sensor in Plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Malerba, M.E.; Connolly, S.R.; Heimann, K. An experimentally validated nitrate–ammonium–phytoplankton model including effects of starvation length and ammonium inhibition on nitrate uptake. Ecol. Model. 2015, 317, 30–40. [Google Scholar] [CrossRef]
- Wang, D.S.; Xu, D.; Wang, Y.T.; Fan, X.; Ye, N.H.; Wang, W.Q.; Zhang, X.W.; Mou, S.L.; Guan, Z. Adaptation involved in nitrogen metabolism in sea ice alga Chlamydomonas sp. ICE-L to Antarctic extreme environments. J. Appl. Phycol. 2015, 27, 787–796. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol. Oceanogr. 1999, 44, 556–572. [Google Scholar] [CrossRef]
- Lomas, M.; Rumbley, C.J.; Glibert, P. Ammonium release by nitrogen sufficient diatoms in response to rapid increases in irradiance. J. Plankton. Res. 2000, 22, 2351–2366. [Google Scholar] [CrossRef]
- Kamp, A.; de Beer, D.; Nitsch, J.L.; Lavik, G.; Stief, P. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl. Acad. Sci. USA 2011, 108, 5649–5654. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Buma, A.G.J.; van Rijssel, M. The Carbohydrates of Phaeocystis and Their Degradation in the Microbial Food Web. Biogeochemistry 2007, 83, 99–118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Wang, W.; Han, J.; Zhu, J.; Wang, Z.; He, L.; Song, X.; Yu, Z. Study on the Characteristics and Mechanism of Inorganic Nitrogen Nutrients Preferential Assimilation by Phaeocystis globosa. J. Mar. Sci. Eng. 2024, 12, 989. https://doi.org/10.3390/jmse12060989
Guo H, Wang W, Han J, Zhu J, Wang Z, He L, Song X, Yu Z. Study on the Characteristics and Mechanism of Inorganic Nitrogen Nutrients Preferential Assimilation by Phaeocystis globosa. Journal of Marine Science and Engineering. 2024; 12(6):989. https://doi.org/10.3390/jmse12060989
Chicago/Turabian StyleGuo, Honghong, Wentao Wang, Jishu Han, Jianan Zhu, Zhongshi Wang, Liyan He, Xiuxian Song, and Zhiming Yu. 2024. "Study on the Characteristics and Mechanism of Inorganic Nitrogen Nutrients Preferential Assimilation by Phaeocystis globosa" Journal of Marine Science and Engineering 12, no. 6: 989. https://doi.org/10.3390/jmse12060989
APA StyleGuo, H., Wang, W., Han, J., Zhu, J., Wang, Z., He, L., Song, X., & Yu, Z. (2024). Study on the Characteristics and Mechanism of Inorganic Nitrogen Nutrients Preferential Assimilation by Phaeocystis globosa. Journal of Marine Science and Engineering, 12(6), 989. https://doi.org/10.3390/jmse12060989





