Shelter Capacity of Artificial Reefs for Sea Cucumber Apostichopus japonicas Is Influenced by Water Flow and Food Resources in Laboratory Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Sea Cucumber and Maintenance
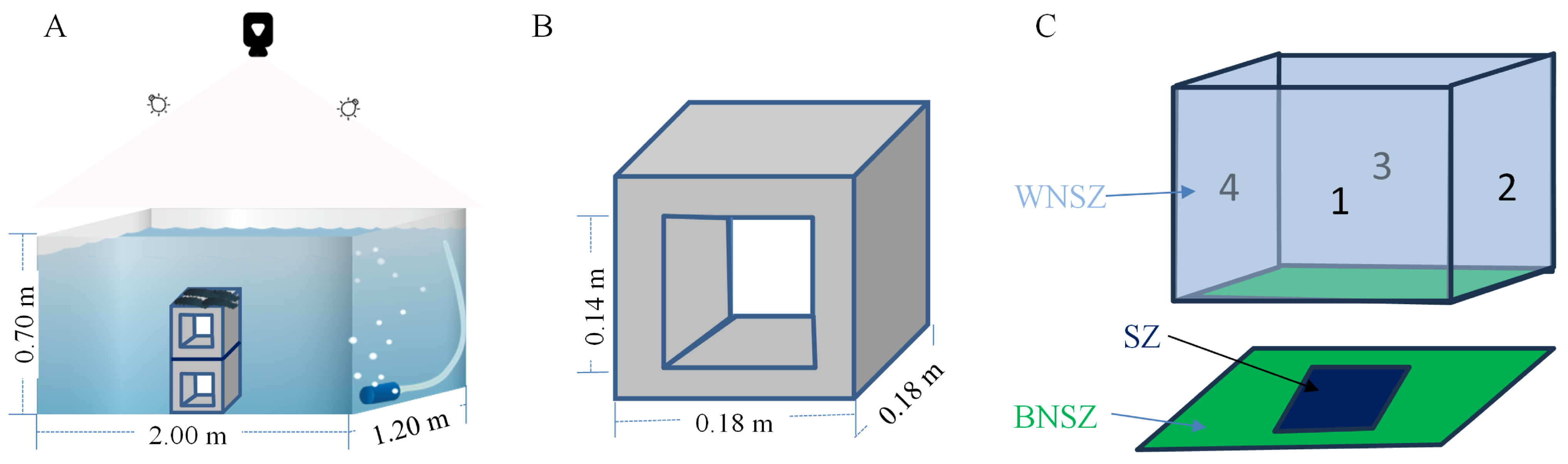
2.2. Construction of the Sheltering Behavior Observation System for Sea Cucumber
2.3. Experimental Design
2.3.1. Experiment 1: Effect of Water Flow on Shelter Capacity
2.3.2. Experiment 2: Effect of Food Availability on Shelter Capacity
2.3.3. Experiment 3: Density-Dependent Sheltering Behavior
2.3.4. Experiment 4: Relationship between Shelter Availability and Shelter Capacity
2.4. Data Collection and Data Analysis
3. Results
3.1. Distribution Dynamics of A. japonicas
3.2. Shelter Capacity under Different Water Flow Conditions
3.3. Shelter Capacity under Food Availability
3.4. Density-Dependent Sheltering Behavior
3.5. Relationship between Shelter Capacity and Shelter Availability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ru, X.; Zhang, L.; Li, X.; Liu, S.; Yang, H. Development strategies for the sea cucumber industry in China. J. Oceanol. Limnol. 2019, 37, 300–312. [Google Scholar] [CrossRef]
- Dong, G.; Dong, S.; Wang, F.; Tian, X. Effects of materials, incubation time and colors of artificial shelters on behavior of juvenile sea cucumber Apostichopus japonicus. Aquac. Eng. 2010, 43, 1–5. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Pan, Y.; Lin, C.; Wang, F.; Kan, R.; Yang, H. Feeding behavior and digestive physiology in sea cucumber Apostichopus japonicus. Physiol. Behav. 2015, 139, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Lee, K.; Kim, T. Shelter material and shape preferences of the sea cucumber, Apostichopus japonicus. Aquaculture 2019, 508, 206–213. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Xu, Q.; Qiu, T.; Yang, H.; Liu, S. An artificial oyster-shell reef for the culture and stock enhancement of sea cucumber, Apostichopus japonicus, in shallow seawater. Aquac. Res. 2014, 46, 2260–2269. [Google Scholar] [CrossRef]
- Chen, J. Overview of sea cucumber farming and sea ranching practices in China. SPC Beche-De-Mer Inf. Bull. 2003, 18, 18–23. [Google Scholar]
- Zhang, L.; Yang, H.; Xu, Q.; Xing, K.; Zhao, P.; Lin, C. A new system for the culture and stock enhancement of sea cucumber, Apostichopus japonicus (Selenka), in cofferdams. Aquac. Res. 2011, 42, 1431–1439. [Google Scholar] [CrossRef]
- Hu, F.; Ding, P.; Yu, Y.; Wen, B.; Cui, Z.; Yang, M.; Chi, X.; Sun, J.; Luo, J.; Sun, Z.; et al. Effects of artificial reefs on selectivity and behaviors of the sea cucumber Apostichopus japonicas: New insights into the pond culture. Aquac. Rep. 2021, 21, 100842. [Google Scholar] [CrossRef]
- Lin, C.G.; Ru, S.G.; Yang, H.S.; Zhang, L.B.; Liu, S.L.; Xu, Q. Selectivity of sea cucumber (Apostichopus japonicus, Selenka) for key indicators in artificial reef structure design. Mar. Sci. 2012, 36, 13–21. [Google Scholar]
- MacTavish, T.; Stenton-Dozey, J.; Vopel, K.; Savage, C. Deposit-Feeding Sea Cucumbers Enhance Mineralization and Nutrient Cycling in Organically-Enriched Coastal Sediments. PLoS ONE 2012, 7, e50031. [Google Scholar] [CrossRef]
- Xia, B.; Ren, Y.; Wang, J.; Sun, Y.; Zhang, Z. Effects of feeding frequency and density on growth, energy budget and physiological performance of sea cucumber Apostichopus japonicus (Selenka). Aquaculture 2017, 466, 26–32. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, L.; Lin, C.; Sun, J.; Kan, R.; Yang, H. Influence of flow velocity on motor behavior of sea cucumber Apostichopus japonicus. Physiol. Behav. 2015, 144, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Sun, L.; Liu, S.; Sun, J.; Zhang, L.; Yang, H. Effects of different flow velocities on behavior and TRPA1 expression in the sea cucumber Apostichopus japonicas. J. Oceanol. Limnol. 2020, 38, 1328–1340. [Google Scholar] [CrossRef]
- Chen, M.; Sun, S.; Xu, Q.; Gao, F.; Wang, H.; Wang, A. Influence of Water Temperature and Flow Velocity on Locomotion Behavior in Tropical Commercially Important Sea Cucumber Stichopus monotuberculatus. Front. Mar. Sci. 2022, 9, 931430. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, L.; Zhang, T.; Yang, H. Effects of mud substrate and water current on the behavioral characteristics and growth of the sea cucumber Apostichopus japonicus in the Yuehu lagoon of northern China. Aquac. Int. 2014, 22, 423–433. [Google Scholar] [CrossRef]
- Steneck, R.S. Possible Demographic Consequences of Intraspecific Shelter Competition among American Lobsters. J. Crustac. Biol. 2006, 26, 628–638. [Google Scholar] [CrossRef]
- Finstad, A.G.; Einum, S.; Forseth, T.; Ugedal, O. Shelter availability affects behaviour, size-dependent and mean growth of juvenile Atlantic salmon. Freshw. Biol. 2007, 52, 1710–1718. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B.; Lu, Y.; Yu, L.; Wang, F.; Liu, D. An evaluation of the preferences of the juvenile swimming crab, Portunus trituberculatus, for different structural properties of shelters. Aquaculture 2022, 557, 738316. [Google Scholar] [CrossRef]
- Finstad, A.G.; Einum, S.; Ugedal, O.; Forseth, T. Spatial distribution of limited resources and local density regulation in juvenile Atlantic salmon. J. Anim. Ecol. 2009, 78, 226–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Ding, G.; Yu, D.; Yang, W.; Sun, Q.; Wang, X.; Lin, H. Moderate relative size of covered and non-covered structures of artificial reef enhances the sheltering effect on reef fish. Front. Mar. Sci. 2023, 10, 1130626. [Google Scholar] [CrossRef]
- Larranaga, N.; Steingrímsson, S. Shelter availability alters diel activity and space use in a stream fish. Behav. Ecol. 2015, 26, 578–586. [Google Scholar] [CrossRef]
- Lachance, A.-A.; Dutil, J.-D.; Larocque, R.; Daigle, G. Shelter use and behaviour of juvenile Spotted Wolffish (Anarhichas minor) in an experimental context. Environ. Biol. Fishes 2010, 88, 207–215. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Sun, M. Behavior characteristics of Apostichopus japonicus and attractive effects of artificial reef models under different light intensities. J. Fish Sci. China 2006, 13, 20–27. [Google Scholar]
- Bohnsack, J.A.; Harper, D.E.; McClellan, D.B.; Hulsbeck, M. Effects of Reef Size on Colonization and Assemblage Structure of Fishes at Artificial Reefs Off Southeastern Florida, USA. Bull. Mar. Sci. 1994, 55, 796–823. [Google Scholar]
- Behringer, D.C.; Butler, M.J. Density-dependent population dynamics in juvenile Panulirus argus (Latreille): The impact of artificial density enhancement. J. Exp. Mar. Biol. Ecol. 2006, 334, 84–95. [Google Scholar] [CrossRef]
- Wocher, H.; Harsányi, A.; Schwarz, F.J. Husbandry conditions in burbot (Lota lota L.): Impact of shelter availability and stocking density on growth and behaviour. Aquaculture 2011, 315, 340–347. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Kim, D.; Na, W.-B. Estimation of effective usable and burial volumes of artificial reefs and the prediction of cost-effective management. Ocean Coast. Manag. 2016, 120, 135–147. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Yitnosumarto, S.; O’Neill, M.E. On Levene’s Tests of Variance Homogeneity. Aust. J. Stat. 1986, 28, 230–241. [Google Scholar] [CrossRef]
- Ostertagová, E.; Ostertag, O.; Kováč, J. Methodology and Application of the Kruskal-Wallis Test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Demšar, J. Statistical comparisons of classifiers over multiple data sets. J. Mach. Learn. Res. 2006, 7, 1–30. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Sadeh, A.; Mangel, M.; Blaustein, L. Context-dependent reproductive habitat selection: The interactive roles of structural complexity and cannibalistic conspecifics. Ecol. Lett. 2009, 12, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, L.; Yu, Z.; Yang, H. Effects of habitat distribution and intraspecific competition on aggregation features of sea cucumber Apostichopus japonicus. Mar. Sci. 2018, 42, 138–144. [Google Scholar]
- Hirsch, P.; Burkhardt-Holm, P.; Töpfer, I.; Fischer, P. Movement patterns and shelter choice of spiny-cheek crayfish (Orconectes limosus) in a large lake’s littoral zone. Aquat. Invasions 2016, 11, 55–65. [Google Scholar] [CrossRef]
- Folpp, H.R.; Schilling, H.T.; Clark, G.F.; Lowry, M.B.; Maslen, B.; Gregson, M.; Suthers, I.M. Artificial reefs increase fish abundance in habitat-limited estuaries. J. Appl. Ecol. 2020, 57, 1752–1761. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Zhang, T.; Zhang, X.; Yang, H. Functional groupings and food web of an artificial reef used for sea cucumber aquaculture in northern China. J. Sea Res. 2017, 119, 1–7. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Sun, T.; Yan, R.; Wang, X.; Liao, G.; Lei, W. Shelter Capacity of Artificial Reefs for Sea Cucumber Apostichopus japonicas Is Influenced by Water Flow and Food Resources in Laboratory Experiments. J. Mar. Sci. Eng. 2024, 12, 993. https://doi.org/10.3390/jmse12060993
Yan S, Sun T, Yan R, Wang X, Liao G, Lei W. Shelter Capacity of Artificial Reefs for Sea Cucumber Apostichopus japonicas Is Influenced by Water Flow and Food Resources in Laboratory Experiments. Journal of Marine Science and Engineering. 2024; 12(6):993. https://doi.org/10.3390/jmse12060993
Chicago/Turabian StyleYan, Shengjun, Tao Sun, Rui Yan, Xiaoling Wang, Guoxiang Liao, and Wei Lei. 2024. "Shelter Capacity of Artificial Reefs for Sea Cucumber Apostichopus japonicas Is Influenced by Water Flow and Food Resources in Laboratory Experiments" Journal of Marine Science and Engineering 12, no. 6: 993. https://doi.org/10.3390/jmse12060993



