A Proposed Methodology for the Dynamic Standard Evaluation of Water Quality in Estuaries: A Case Study of the Pearl River Estuary
Abstract
1. Introduction
2. Method Construction
2.1. Classification of Seawater Quality in the Seawater Quality Standards
2.2. Surface Water Function and Standard Classification in Surface Water Environmental Quality Standards
2.3. Construction of Dynamic Standard Methods for Water Quality in Estuarine Areas
3. Application Example: Evaluation of Water Quality in the PRE (Pb, pH, PI, DO, Cu, Zn, Cd, and Hg)
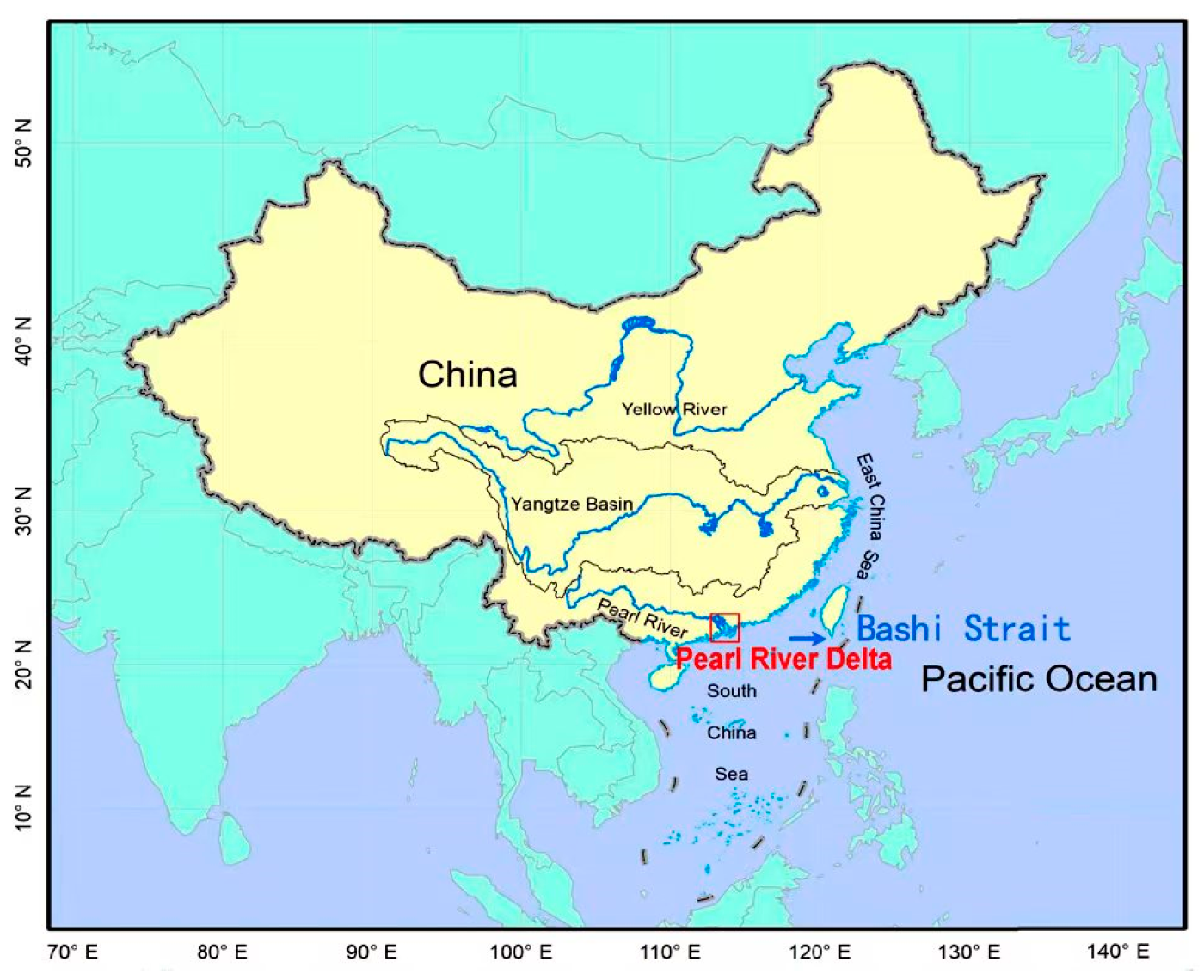
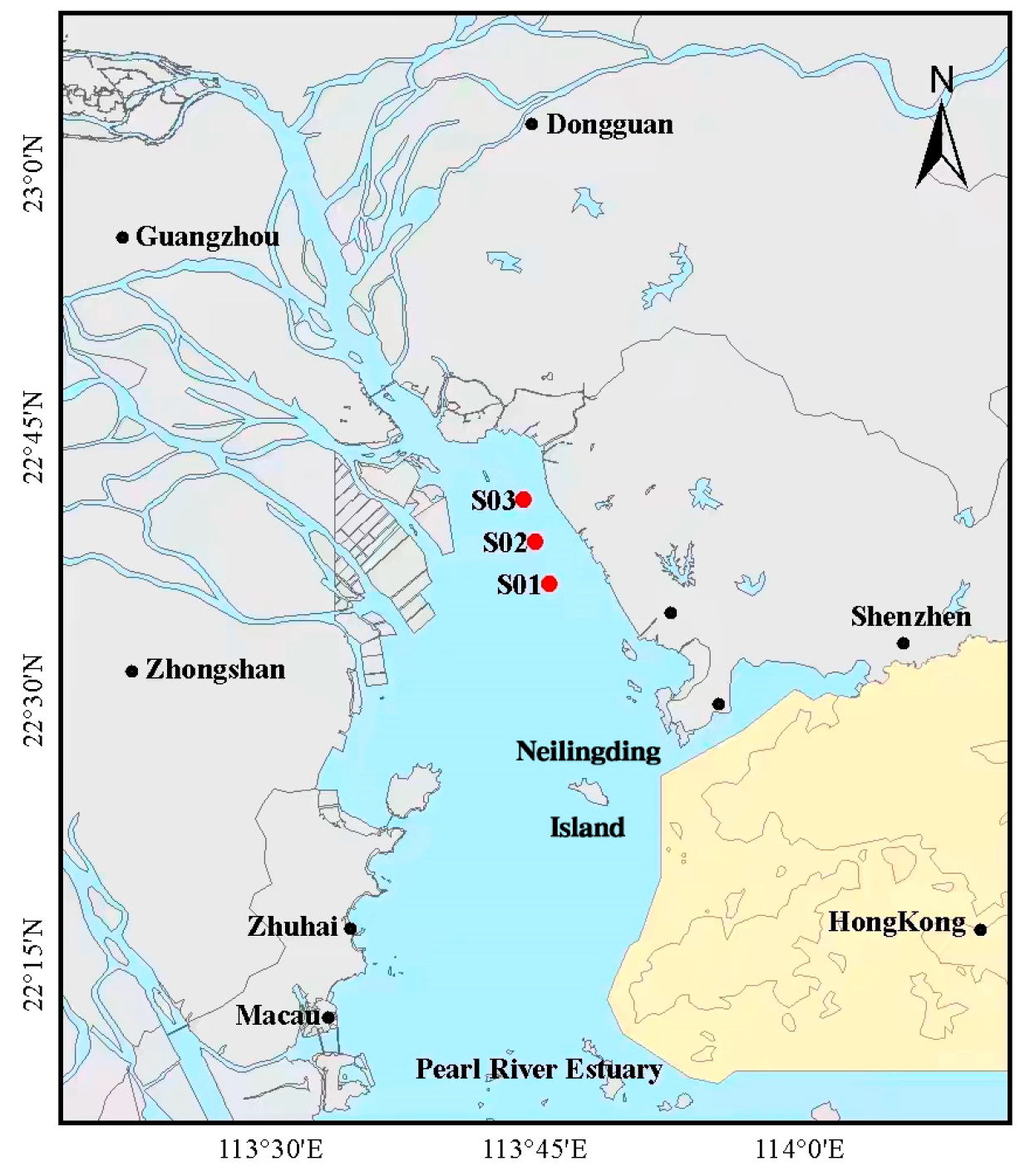
3.1. Description of the Monitoring Area
3.2. Sample Collection and Analysis
3.3. Analytical Quality Assurance
3.4. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Li, M.; Pang, B.; Lan, W.; Fu, J. Delineation of estuarine mixed zone and establishment of nutrient standard limits. China Environ. Monit. 2020, 36, 74–82. [Google Scholar] [CrossRef]
- Alber, M. A conceptual model of estuarine freshwater inflow management. Estuaries 2002, 25, 1246–1261. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Wang, Y.; Bao, M.; Li, X. Analysis of water quality evaluation standards for estuaries. Jiangsu Water Resour. 2022, 4, 62–65. [Google Scholar] [CrossRef]
- Wang, J.; Han, G.; Zhang, Z. The Latest Progress in International Marine Environmental Monitoring and Evaluation; Ocean Press: Beijing, China, 2010. [Google Scholar]
- Meng, W. Research on China’s Ocean Engineering and Technology Development Strategy: Ocean Environment and Ecology Volume; Ocean Press: Beijing, China, 2014. [Google Scholar]
- Chen, J. Estuarine Water Eco-Health Assessment Technical Method and Application; Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Yang, F.; Lin, Z.; Zhang, Z.; Wang, L.; Yu, L.; Wang, J. Analysis on the problems of environmental quality standards for surface water and seawater in China. Mar. Dev. Manag. 2018, 35, 36–41. [Google Scholar]
- GB3097-1997; National Seawater Quality Standard of the People’s Republic of China. National Environmental Protection Administration: Beijing, China, 1997.
- GB3838-2002; General Administration of Quality Supervision of Inspection and Quarantine of China. National Standard of the People’s Republic of China Environmental Quality Standard for Surface Water. State Environmental Protection Administration: Beijing, China, 2002.
- Quan, Y.; Ji, X.; Wang, L.; Shi, B. Water quality analysis and evaluation of main estuaries in Qinhuangdao. Bull. Oceanol. Limnol. 2016, 38, 24–28. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zheng, B. Problems and countermeasures of water environmental management in estuaries. Res. Environ. Sci. 2017, 30, 645–653. [Google Scholar] [CrossRef]
- Bricker, S.B.; Ferreira, J.G.; Simas, T. An integrated methodology for assessment of estuarine trophic status. Ecol. Model. 2003, 169, 39–60. [Google Scholar] [CrossRef]
- Ireland Environmental Protection Agency. Water Quality in Ireland 2001–2003; Environmental Protection Agency: Wexford, Ireland, 2005. [Google Scholar]
- OSPAR Commission. OSPAR Integrated Report 2003 on the Eutrophication Status of the OSPAR Maritime Area Based upon the First Application of the Comprehensive Procedure; The Convention for the Protection of the Marine Environment of the North-East Atlantic: Paris, France, 2003. [Google Scholar]
- Office of Environment and Heritage. Assessing Estuary Ecosystem Health: Sampling, Data Analysis and Reporting Protocols; State of NSW and Office of Environment and Heritage: Parramatta, NSW, Australia, 2013. [Google Scholar]
- Peng, T.; Wang, Z.; Zhao, Q. Ecosystem health assessment for Huangbai river based on PSR model. Water Resour. Prot. 2016, 32, 141–153. [Google Scholar]
- Ye, S.F.; Liu, X.; Ding, D.W. Ecosystem health assessment of the Changjiang river estuary: Indicator system and its primarily assessment. Acta Oceanol. Sin. 2007, 29, 128–136. [Google Scholar]
- Australian and New Zealand Environment and Conservation Council, Agriculture and Resource Management Council of Australia and New Zealand. Australia and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conberra Council: Canberra, ACT, Australia, 2000. [Google Scholar]
- European Commission. Guidance on Typology, Reference Conditions and Classification Systems for Transitional and Coastal Water; European Commission: Copenhagen, Denmark, 2002. [Google Scholar]
- US Environmental Protection Agency. Estuarine and Coastal Marine Waters: Bioassessment and Biocriteria Technical Guidance; Office of Water: Washington, DC, USA, 2000. [Google Scholar]
- European Commission. Common Implementation Stategy for the Water Framework Directive (2000/30/EC-Technical Guidance for Deriving Environmental Quality Standards; European Commission: Copenhagen, Denmark, 2000. [Google Scholar]
- US Environmental Protection Agency. National Estuary Program Coastal Condition Report. Chapter 5: Gulf of Mexico National Estuary Program Coastal Condition, Sarasota Bay Estuary Program; US Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Schuwirth, N. Towards an integrated surface water quality assessment: Aggregation over multiple pollutants and time. Water Res. 2020, 186, 761–774. [Google Scholar] [CrossRef]
- Reichert, J.; Schellenberg, J.; Schubert, P.; Wilke, T. Responses of reef building corals to microplastic exposure. Environ. Pollut. 2018, 237, 955–960. [Google Scholar] [CrossRef]
- Bawiec, A.; Paweska, K.; Jarzab, A. Changes in the Microbial Composition of Municipal Wastewater Treated in Biological Processes. J. Eco. Eng. 2016, 17, 41–46. [Google Scholar] [CrossRef]
- Lal, P.P.; Juste-Poinapen Ms, N.; Poinapen, J. Assessing the water quality of Suva foreshore for the establishment of estuary and marine recreational water guidelines in the Fiji Islands. Water Sci. Technol. 2021, 84, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Singh, V.P.; He, Y.; Bai, X. An improved index for water quality evaluation in an estuary region: A case study in the Eastern Pearl River Delta, China. Water Policy 2019, 21, 310–325. [Google Scholar] [CrossRef]
- Tri, D.Q.; Linh, N.T.M.; Thai, T.H.; Kandasamy, J. Application of 1D–2D coupled modeling in water quality assessment: A case study in Ca Mau Peninsula, Vietnam. Phys. Chem. Earth Parts A/B/C 2019, 113, 83–99. [Google Scholar]
- Tao, W.; Niu, L.; Dong, Y.; Fu, T.; Lou, Q. Nutrient pollution and its dynamic source-sink pattern in the Pearl River Estuary (South China). Front. Mar. Sci. 2021, 8, 713907. [Google Scholar] [CrossRef]
- Li, X.Q.; Lu, C.Q.; Zhang, Y.F.; Zhao, H.D.; Wang, J.Y.; Liu, H.B. Low dissolved oxygen in the Pearl River estuary in summer: Long-term spatio-temporal patterns, trends, and regulating factors. J. Mar. Sci. Eng. 2020, 151, 110814. [Google Scholar] [CrossRef]
- Tao, W.; Niu, L.X.; Liu, F.; Cai, H.Y.; Ou, S.Y.; Zeng, D.N.; Lou, Q.S.; Yang, Q.S. Influence of river-tide dynamics on phytoplankton variability and their ecological implications in two Chinese tropical estuaries. Ecol. Indic. 2020, 115, 106458. [Google Scholar] [CrossRef]
- Niu, L.X.; Cai, H.Y.; Jia, L.W.; Luo, X.X.; Tao, W.; Dong, Y.H.; Yang, Q.S. Metal pollution in the Pearl River Estuary and implications for estuary management: The influence of hydrological connectivity associated with estuarine mixing. EES 2021, 225, 112747. [Google Scholar]
- Tian, F.G.; Ni, Z.X.; Huang, B.B.; Lou, Q.S.; Zhong, Y.H.; Deng, W. Distribution characters of heavy metals in seawater and surface sediments in Daya Bay. J. Appl. Oceanogr. 2022, 41, 462–474. [Google Scholar]
- Tao, W.; Li, H.D.; Zhang, W.P.; Lou, Q.S.; Gong, J.; Ye, J.J. Characteristics of Heavy Metals in Seawater and Sediments from Daya Bay (South China): Environmental Fates, Source Apportionment and Ecological Risks. Sustainability 2021, 13, 10237. [Google Scholar] [CrossRef]
| Similarities and Differences | Environmental Quality Standards for Surface Water (GB3838-2002) | Seawater Quality Standard (GB3097-1997) | ||
|---|---|---|---|---|
| Functional Categories Correspondence Relationship | Class I | Source water and nature reserves | N/A | |
| Class II | First-grade protection area of centralized surface drinking water sources, habitat of rare aquatic organisms, and feeding ground for young fish | Marine nature reserves, rare and endangered marine life reserves, and marine fishery waters | Class I | |
| Class III | Class II protection zone of centralized surface water sources for drinking water, fishery waters such as aquaculture, and swimming areas | Aquaculture areas, bathing beaches, and industrial water areas directly related to human consumption | Class II | |
| Class IV | General industrial water areas and entertainment water areas where the human body is not directly in contact | General industrial water area, coastal scenic tourist area | Class III | |
| Class V | Agricultural water use areas and general landscape requirements for water areas | Marine port waters and marine development operation areas | Class IV | |
| Element | AI | AII | AIII | AIV |
|---|---|---|---|---|
| Pb (mg/L) | ≤0.001 | ≤0.005 | ≤0.010 | ≤0.050 |
| pH | 7.8~8.5 | 6.8~8.8 | ||
| PI (mg/LO2) | ≤2 | ≤3 | ≤4 | ≤5 |
| DO (mg/L) | >6 | >5 | >4 | >3 |
| Cu (mg/L) | ≤0.005 | ≤0.010 | ≤0.050 | |
| Zn (mg/L) | ≤0.020 | ≤0.050 | ≤0.10 | ≤0.50 |
| Cd (mg/L) | ≤0.001 | ≤0.005 | ≤0.010 | |
| Hg (mg/L) | ≤0.00005 | ≤0.0002 | ≤0.0005 | |
| Element | BII | BIII | BIV | BV |
|---|---|---|---|---|
| Pb (mg/L) | ≤0.01 | ≤0.05 | ≤0.05 | ≤0.1 |
| pH | 6~9 | |||
| PI (mg/LO2) | ≤4 | ≤6 | ≤10 | ≤15 |
| DO (mg/L) | ≥6 | ≥5 | ≥3 | ≥2 |
| Cu (mg/L) | ≤1.0 | ≤1.0 | ≤1.0 | ≤1.0 |
| Zn (mg/L) | ≤1.0 | ≤1.0 | ≤2.0 | ≤2.0 |
| Cd (mg/L) | ≤0.005 | ≤0.005 | ≤0.005 | ≤0.01 |
| Hg (mg/L) | ≤0.00005 | ≤0.0001 | ≤0.001 | ≤0.001 |
| Sites | S01 | S02 | S03 | ||||
|---|---|---|---|---|---|---|---|
| Ebb tide | Water depth (m) | 0.5 | 8.0 | 0.5 | 8.0 | 0.5 | 7.0 |
| Salinity (S) | 16.453 | 17.641 | 17.565 | 17.643 | 15.405 | 16.439 | |
| Pb (mg/L) | 0.0006 | 0.0006 | 0.0010 | 0.0013 | 0.0007 | 0.0006 | |
| pH | 7.62 | 7.62 | 7.64 | 7.62 | 7.57 | 7.58 | |
| PI (mg/LO2) | 1.61 | 1.90 | 1.42 | 2.00 | 1.53 | 1.92 | |
| DO (mg/L) | 5.38 | 5.32 | 5.82 | 5.42 | 5.27 | 5.33 | |
| Cu (mg/L) | 0.0020 | 0.0016 | 0.0020 | 0.0022 | 0.0026 | 0.0022 | |
| Zn (mg/L) | 0.0069 | 0.0072 | 0.0109 | 0.0076 | 0.0071 | 0.0071 | |
| Cd (mg/L) | 0.00037 | 0.00038 | 0.00044 | 0.00042 | 0.0004 | 0.00043 | |
| Hg (mg/L) | 0.000028 | 0.000042 | 0.000030 | 0.000038 | 0.000028 | 0.000042 | |
| Rising tide | Water depth (m) | 0.5 | 7.0 | 0.5 | 7.0 | 0.5 | 8.0 |
| Salinity (S) | 15.660 | 15.663 | 15.435 | 15.743 | 13.551 | 14.529 | |
| Pb (mg/L) | 0.0008 | 0.0006 | 0.0005 | 0.0006 | 0.0005 | 0.0005 | |
| pH | 7.58 | 7.43 | 7.51 | 7.51 | 7.38 | 7.38 | |
| PI (mg/LO2) | 2.56 | 2.15 | 3.02 | 3.15 | 3.49 | 3.12 | |
| DO (mg/L) | 5.24 | 5.24 | 4.93 | 4.76 | 4.39 | 4.58 | |
| Cu (mg/L) | 0.0016 | 0.0018 | 0.0017 | 0.0015 | 0.0015 | 0.0015 | |
| Zn (mg/L) | 0.0079 | 0.0079 | 0.0078 | 0.0066 | 0.0073 | 0.0068 | |
| Cd (mg/L) | 0.00037 | 0.0004 | 0.00033 | 0.00033 | 0.0004 | 0.0004 | |
| Hg (mg/L) | 0.000024 | 0.000034 | 0.000031 | 0.000034 | 0.000028 | 0.000037 | |
| Sites | S01 | S02 | S03 | ||||
|---|---|---|---|---|---|---|---|
| Ebb tide | Depth (m) | 0.5 | 8.0 | 0.5 | 8.0 | 0.5 | 7.0 |
| Pb | Class I | Class I | Class I | Class II | Class I | Class I | |
| pH | Class III | Class III | Class III | Class III | Class III | Class III | |
| PI | Class I | Class I | Class I | Class I | Class I | Class I | |
| DO | Class II | Class II | Class II | Class II | Class II | Class II | |
| Cu | Class I | Class I | Class I | Class I | Class I | Class I | |
| Zn | Class I | Class I | Class I | Class I | Class I | Class I | |
| Cd | Class I | Class I | Class I | Class I | Class I | Class I | |
| Hg | Class I | Class I | Class I | Class I | Class I | Class I | |
| Rising tide | Depth (m) | 0.5 | 7.0 | 0.5 | 7.0 | 0.5 | 8.0 |
| Pb | Class I | Class I | Class I | Class I | Class I | Class I | |
| pH | Class III | Class III | Class III | Class III | Class III | Class III | |
| PI | Class II | Class II | Class III | Class III | Class III | Class III | |
| DO | Class II | Class II | Class III | Class III | Class III | Class III | |
| Cu | Class I | Class I | Class I | Class I | Class I | Class I | |
| Zn | Class I | Class I | Class I | Class I | Class I | Class I | |
| Cd | Class I | Class I | Class I | Class I | Class I | Class I | |
| Hg | Class I | Class I | Class I | Class I | Class I | Class I | |
| Sites | Depth (m) | Salinity (S) | CI (mg/L) | CII (mg/L) | CIII (mg/L) | CIV (mg/L) | |
|---|---|---|---|---|---|---|---|
| Ebb tide | S01 | 0.5 | 16.453 | 0.0058 | 0.0288 | 0.0312 | 0.0765 |
| 8.0 | 17.641 | 0.0055 | 0.0273 | 0.0298 | 0.0748 | ||
| S02 | 0.5 | 17.565 | 0.0055 | 0.0274 | 0.0299 | 0.0749 | |
| 8.0 | 17.643 | 0.0055 | 0.0273 | 0.0298 | 0.0748 | ||
| S03 | 0.5 | 15.405 | 0.0060 | 0.0302 | 0.0324 | 0.0780 | |
| 7.0 | 16.439 | 0.0058 | 0.0289 | 0.0312 | 0.0765 | ||
| Rising tide | S01 | 0.5 | 15.660 | 0.0060 | 0.0299 | 0.0321 | 0.0776 |
| 7.0 | 15.663 | 0.0060 | 0.0299 | 0.0321 | 0.0776 | ||
| S02 | 0.5 | 15.435 | 0.0060 | 0.0302 | 0.0324 | 0.0780 | |
| 7.0 | 15.743 | 0.0060 | 0.0298 | 0.0320 | 0.0775 | ||
| S03 | 0.5 | 13.551 | 0.0065 | 0.0326 | 0.0345 | 0.0806 | |
| 8.0 | 14.529 | 0.0063 | 0.0313 | 0.0334 | 0.0792 |
| Sites | Depth (m) | Salinity (S) | CI | CII | CIII | CIV | |
|---|---|---|---|---|---|---|---|
| Ebb tide | S01 | 0.5 | 16.453 | 6.85~8.76 | 6.85~8.76 | 6.38~8.91 | 6.38~8.91 |
| 8.0 | 17.641 | 6.91~8.75 | 6.91~8.75 | 6.40~8.90 | 6.40~8.90 | ||
| S02 | 0.5 | 17.565 | 6.90~8.75 | 6.90~8.75 | 6.40~8.90 | 6.40~8.90 | |
| 8.0 | 17.643 | 6.91~8.75 | 6.91~8.75 | 6.40~8.90 | 6.40~8.90 | ||
| S03 | 0.5 | 15.405 | 6.79~8.78 | 6.79~8.78 | 6.35~8.91 | 6.35~8.91 | |
| 7.0 | 16.439 | 6.85~8.77 | 6.85~8.77 | 6.38~8.91 | 6.38~8.91 | ||
| Rising tide | S01 | 0.5 | 15.660 | 6.81~8.78 | 6.81~8.78 | 6.36~8.91 | 6.36~8.91 |
| 7.0 | 15.663 | 6.81~8.78 | 6.81~8.78 | 6.36~8.91 | 6.36~8.91 | ||
| S02 | 0.5 | 15.435 | 6.79~8.78 | 6.79~8.78 | 6.35~8.91 | 6.35~8.91 | |
| 7.0 | 15.743 | 6.81~8.78 | 6.81~8.78 | 6.36~8.91 | 6.36~8.91 | ||
| S03 | 0.5 | 13.551 | 6.70~8.81 | 6.70~8.81 | 6.31~8.92 | 6.31~8.92 | |
| 8.0 | 14.529 | 6.75~8.79 | 6.75~8.79 | 6.33~8.92 | 6.33~8.92 |
| Sites | Depth (m) | Salinity (S) | CI (mg/LO2) | CII (mg/LO2) | CIII (mg/LO2) | CIV (mg/LO2) | |
|---|---|---|---|---|---|---|---|
| Ebb tide | S01 | 0.5 | 16.453 | 3.060 | 4.590 | 7.180 | 10.299 |
| 8.0 | 17.641 | 2.992 | 4.488 | 6.976 | 9.960 | ||
| S02 | 0.5 | 17.565 | 2.996 | 4.494 | 6.989 | 9.981 | |
| 8.0 | 17.643 | 2.992 | 4.488 | 6.976 | 9.959 | ||
| S03 | 0.5 | 15.405 | 3.120 | 4.680 | 7.359 | 10.599 | |
| 7.0 | 16.439 | 3.061 | 4.591 | 7.182 | 10.303 | ||
| Rising tide | S01 | 0.5 | 15.660 | 3.105 | 4.658 | 7.315 | 10.526 |
| 7.0 | 15.663 | 3.105 | 4.658 | 7.315 | 10.525 | ||
| S02 | 0.5 | 15.435 | 3.118 | 4.677 | 7.354 | 10.590 | |
| 7.0 | 15.743 | 3.100 | 4.651 | 7.301 | 10.502 | ||
| S03 | 0.5 | 13.551 | 3.226 | 4.839 | 7.677 | 11.128 | |
| 8.0 | 14.529 | 3.170 | 4.755 | 7.509 | 10.849 |
| Sites | Depth (m) | Salinity (S) | CI (mg/L) | CII (mg/L) | CIII (mg/L) | CIV (mg/L) | |
|---|---|---|---|---|---|---|---|
| Ebb tide | S01 | 0.5 | 16.453 | 6.000 | 5.000 | 3.470 | 2.470 |
| 8.0 | 17.641 | 6.000 | 5.000 | 3.504 | 2.504 | ||
| S02 | 0.5 | 17.565 | 6.000 | 5.000 | 3.502 | 2.502 | |
| 8.0 | 17.643 | 6.000 | 5.000 | 3.504 | 2.504 | ||
| S03 | 0.5 | 15.405 | 6.000 | 5.000 | 3.440 | 2.440 | |
| 7.0 | 16.439 | 6.000 | 5.000 | 3.470 | 2.470 | ||
| Rising tide | S01 | 0.5 | 15.660 | 6.000 | 5.000 | 3.447 | 2.447 |
| 7.0 | 15.663 | 6.000 | 5.000 | 3.448 | 2.448 | ||
| S02 | 0.5 | 15.435 | 6.000 | 5.000 | 3.441 | 2.441 | |
| 7.0 | 15.743 | 6.000 | 5.000 | 3.450 | 2.450 | ||
| S03 | 0.5 | 13.551 | 6.000 | 5.000 | 3.387 | 2.387 | |
| 8.0 | 14.529 | 6.000 | 5.000 | 3.415 | 2.415 |
| Sites | S01 | S02 | S03 | ||||
|---|---|---|---|---|---|---|---|
| Ebb tide | Depth (m) | 0.5 | 8.0 | 0.5 | 8.0 | 0.5 | 7.0 |
| salinity | 16.453 | 17.641 | 17.565 | 17.643 | 15.405 | 16.439 | |
| Pb | Class I | Class I | Class I | Class I | Class I | Class I | |
| pH | Class I | Class I | Class I | Class I | Class I | Class I | |
| PI | Class I | Class I | Class I | Class I | Class I | Class I | |
| DO | Class II | Class II | Class II | Class II | Class II | Class II | |
| Rising tide | Depth (m) | 0.5 | 7.0 | 0.5 | 7.0 | 0.5 | 8.0 |
| Salinity | 15.660 | 15.663 | 15.435 | 15.743 | 13.551 | 14.529 | |
| Pb | Class I | Class I | Class I | Class I | ClassI | Class I | |
| pH | Class I | Class I | Class I | Class I | Class I | Class I | |
| PI | Class I | Class I | Class I | Class II | Class II | Class I | |
| DO | Class II | Class II | Class III | Class III | Class III | Class III | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, H. A Proposed Methodology for the Dynamic Standard Evaluation of Water Quality in Estuaries: A Case Study of the Pearl River Estuary. J. Mar. Sci. Eng. 2024, 12, 1039. https://doi.org/10.3390/jmse12071039
Wang Z, Zhang H. A Proposed Methodology for the Dynamic Standard Evaluation of Water Quality in Estuaries: A Case Study of the Pearl River Estuary. Journal of Marine Science and Engineering. 2024; 12(7):1039. https://doi.org/10.3390/jmse12071039
Chicago/Turabian StyleWang, Zhongyuan, and Hongkang Zhang. 2024. "A Proposed Methodology for the Dynamic Standard Evaluation of Water Quality in Estuaries: A Case Study of the Pearl River Estuary" Journal of Marine Science and Engineering 12, no. 7: 1039. https://doi.org/10.3390/jmse12071039
APA StyleWang, Z., & Zhang, H. (2024). A Proposed Methodology for the Dynamic Standard Evaluation of Water Quality in Estuaries: A Case Study of the Pearl River Estuary. Journal of Marine Science and Engineering, 12(7), 1039. https://doi.org/10.3390/jmse12071039





