The Proteome Profile of Halimeda macroloba under Elevated Temperature: A Case Study from Thailand
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Comparative Proteomic Analysis and Classification of Temperature-Responsive Proteins in Halimeda macroloba
3.2. Comparison of Significant Differentially Abundant Proteins and the Associated Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 423–552. [Google Scholar] [CrossRef]
- Lobban, C.S.; Harrison, P.J. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Wilson, K.L.; Kay, L.M.; Schmidt, A.L.; Lotze, H.K. Effects of increasing water temperatures on survival and growth of ecologically and economically important seaweeds in Atlantic Canada: Implications for climate change. Mar. Biol. 2015, 162, 2431–2444. [Google Scholar] [CrossRef]
- Graba-Landry, A.C.; Loffler, Z.; McClure, E.C.; Pratchett, M.S.; Hoey, A.S. Impaired growth and survival of tropical macroalgae (Sargassum spp.) at elevated temperatures. Coral Reefs 2020, 39, 475–486. [Google Scholar] [CrossRef]
- Lee, J.E.; Kang, J.W. The interactive effects of elevated temperature and nutrient concentrations on the physiological responses of Ulva linza Linnaeus (Ulvales, Chlorophyta). J. Appl. Phycol. 2020, 32, 2459–2467. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Wang, S.; Chen, X.; Liao, J.; Guo, Y.; Xin, R.; Huang, B.; Xie, E. Transcriptome analysis reveals the molecular mechanisms of adaptation to high temperatures in Gracilaria bailinae. Front. Plant Sci. 2023, 14, 1125324. [Google Scholar] [CrossRef]
- Mayakun, J.; Liao, C.-P.; Liu, S.-L. The standing stock and CaCO3 contribution of Halimeda macroloba in the tropical seagrass-dominated ecosystem in Dongsha Island, the main island of Dongsha Atoll, South China Sea. J. Mar. Biol. Assoc. U.K. 2020, 100, 1219–1227. [Google Scholar] [CrossRef]
- Schubert, N.; Alvarez-Filip, L.; Hofmann, L.C. Systematic review and meta-analysis of ocean acidification effects in Halimeda: Implications for algal carbonate production. Clim. Chang. Ecol. 2023, 4, 100059. [Google Scholar] [CrossRef]
- Mayakun, J.; Prathep, A. Calcium carbonate productivity by Halimeda macroloba in the tropical intertidal ecosystem: The significant contributor to global carbonate budgets. Phycol. Res. 2019, 67, 94–101. [Google Scholar] [CrossRef]
- Prathep, A.; Kaewsrikhaw, R.; Mayakun, J.; Darakrai, A. The effects of light intensity and temperature on the calcification rate of Halimeda macroloba. J. Appl. Phycol. 2018, 30, 3405–3412. [Google Scholar] [CrossRef]
- Wei, Z.; Mo, J.; Huang, R.; Hu, Q.; Long, C.; Ding, D.; Yang, F.; Long, L. Physiological performance of three calcifying green macroalgae Halimeda species in response to altered seawater temperatures. Acta Oceanol. Sin. 2020, 39, 89–100. [Google Scholar] [CrossRef]
- Sinutok, S.; Hill, R.; Doblin, M.A.; Kühl, M.; Ralph, P.J. Microenvironmental changes support evidence of photosynthesis and calcification inhibition in Halimeda under ocean acidification and warming. Coral Reefs 2012, 31, 1201–1213. [Google Scholar] [CrossRef]
- Kurdrid, P.; Senachak, J.; Sirijuntarut, M.; Yutthanasirikul, R.; Phuengcharoen, P.; Jeamton, W.; Roytrakul, S.; Cheevadhanarak, S.; Hongsthong, A. Comparative analysis of the Spirulina platensis subcellular proteome in response to low- and high-temperature stresses: Uncovering cross-talk of signaling components. Proteome Sci. 2011, 9, 39. [Google Scholar] [CrossRef]
- Samutrtai, P.; Krobthong, S.; Roytrakul, S. Proteomics for toxicological pathways screening: A case comparison of low-concentration ionic and nanoparticulate silver. Anal. Sci. 2020, 36, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Sithtisarn, S.; Yokthongwattana, K.; Mahong, B.; Roytrakul, S.; Paemanee, A.; Phaonakrop, N.; Yokthongwattana, C. Comparative proteomic analysis of Chlamydomonas reinhardtii control and a salinity-tolerant strain revealed a differential protein expression pattern. Planta 2017, 246, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Chamnanmanoontham, N.; Pongprayoon, W.; Pichyangkura, R.; Roytrakul, S.; Chadchawan, S. Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regul. 2014, 75, 101–114. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Sun, P.; Mao, Y.; Li, G.; Cao, M.; Kong, F.; Wang, L.; Bi, G. Comparative transcriptome profiling of Pyropia yezoensis (Ueda) M.S. Hwang & H.G. Choi in response to temperature stresses. BMC Genom. 2015, 16, 463. [Google Scholar] [CrossRef]
- Calegario, G.; Freitas, L.; Santos, E.; Silva, B.; Oliveira, L.; Garcia, G.; Omachi, C.; Pereira, R.; Thompson, C.; Thompson, F. Environmental modulation of the proteomic profiles from closely phylogenetically related populations of the red seaweed Plocamium brasiliense. PeerJ 2019, 7, e6469. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, Y.; Ji, D.; Xie, J.; Chen, C.; Xie, C. Proteomic analysis of the economic seaweed Pyropia haitanensis in response to desiccation. Algal Res. 2016, 19, 198–206. [Google Scholar] [CrossRef]
- Park, H.S.; Jeong, W.J.; Kim, E.; Jung, Y.; Lim, J.M.; Hwang, M.S.; Park, E.J.; Ha, D.S.; Choi, D.W. Heat shock protein gene family of the Porphyra seriata and enhancement of heat stress tolerance by PsHSP70 in Chlamydomonas. Mar. Biotechnol. 2012, 14, 332–342. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Li, J.; Fu, W.; Yao, J.; Duan, D. Characterization and expression analysis of hsp70 gene from Ulva prolifera J. Agardh (Chlorophycophyta, Chlorophyceae). Mar Genom. 2012, 5, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Li, B.; Xu, Y.; Chen, C.; Xie, C. Cloning and quantitative analysis of five heat shock protein 70 genes from Pyropia haitanensis. J. Appl. Phycol. 2015, 27, 499–509. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Harada, N.; Nishimura, Y.; Saito, T.; Nakamura, M.; Fujiwara, T.; Kuroiwa, T.; Misumi, O. Algae sense exact temperatures: Small heat shock proteins are expressed at the survival threshold temperature in Cyanidioschyzon merolae and Chlamydomonas reinhardtii. Genome. Biol. Evol. 2014, 6, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jiang, Y.; Bu, Y.; Wang, S.; Zhang, H.; Wang, R. Growth, photosynthetic pigment proteins responses and transcriptome analysis provide insights into survival strategies against short-term cold stress in the blue-green algae, Arthrospira. Aquac. Rep. 2022, 27, 101403. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Novosel, N.; Mišić Radić, T.; Zemla, J.; Lekka, M.; Čačković, A.; Kasum, D.; Legović, T.; Žutinić, P.; Gligora Udovič, M.; Ivošević DeNardis, N. Temperature-induced response in algal cell surface properties and behaviour: An experimental approach. J. Appl. Phycol. 2022, 34, 243–259. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Fernando, D.; Gentili, F.G. Morphological and cellular organization of green microalgae to cope with cold stress in subarctic environment. Algal Res. 2023, 75, 103254. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Yuan, H.; Yang, J. Physiological and transcription level responses of microalgae Auxenochlorella protothecoides to cold and heat induced oxidative stress. Environ. Res. 2022, 211, 113023. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.N.; Strauss, J.; Thomas, D.N.; Mock, T.; Underwood, G.J.C. Identifying metabolic pathways for production of extracellular polymeric substances by the diatom Fragilariopsis cylindrus inhabiting sea ice. ISME J. 2018, 12, 1237–1251. [Google Scholar] [CrossRef]
- Chardon, F.; Bedu, M.; Calenge, F.; Klemens, P.A.W.; Spinner, L.; Clement, G.; Chietera, G.; Léran, S.; Ferrand, M.; Lacombe, B.; et al. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr. Biol. 2013, 23, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 205–224. [Google Scholar]
- Stein, O.; Granot, D. Plant fructokinases: Evolutionary, developmental, and metabolic aspects in sink tissues. Front. Plant Sci. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Green, B.R.; Durnford, D.G. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 685–714. [Google Scholar] [CrossRef] [PubMed]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-Y.; Chi, Y.-H.; Wang, J.-Z.; Zhou, J.-X.; Cheng, Y.-S.; Zhang, B.-L.; Ma, A.; Vanitha, J.; Ramachandran, S. Sucrose metabolism gene families and their biological functions. Sci. Rep. 2015, 5, 17583. [Google Scholar] [CrossRef]
- Müller, W.; Wegmann, K. Sucrose biosynthesis in Dunaliella. Planta 1978, 141, 155–158. [Google Scholar] [CrossRef]
- Nagao, M.; Uemura, M. Sucrose phosphate phosphatase in the green alga Klebsormidium flaccidum (Streptophyta) lacks an extensive C-terminal domain and differs from that of land plants. Planta 2012, 235, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Soto, C.; Cheng, L.; Caesar, L.; Schmidtko, S.; Jewett, E.B.; Cheripka, A.; Rigor, I.; Caballero, A.; Chiba, S.; Báez, J.C.; et al. An Overview of Ocean Climate Change Indicators: Sea Surface Temperature, Ocean Heat Content, Ocean pH, Dissolved Oxygen Concentration, Arctic Sea Ice Extent, Thickness and Volume, Sea Level and Strength of the AMOC (Atlantic Meridional Overturning Circulation). Front. Mar. Sci. 2021, 8, 642372. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Tapken, D.; Hollmann, M. Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation. J. Mol. Biol. 2008, 383, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Turano, F.J. The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6872–6877. [Google Scholar] [CrossRef] [PubMed]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. 2014, 111, E4560–E4567. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.C.; Pinto-Pacheco, B.; Pitteloud, J.-P.; Buccella, D. Formation of ternary complexes with MgATP: Effects on the detection of Mg2+ in biological samples by bidentate fluorescent sensors. Inorg. Chem. 2014, 53, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Chesinta, V.; Andrea, M.P.R. Role of magnesium in the regulation of hepatic glucose homeostasis. In Glucose Homeostasis; Leszek, S., Ed.; IntechOpen: Rijeka, Croatia, 2014; p. Ch. 4. [Google Scholar]
- Kleczkowski, L.A.; Igamberdiev, A.U. Magnesium and cell energetics: At the junction of metabolism of adenylate and non-adenylate nucleotides. J. Plant Physiol. 2023, 280, 153901. [Google Scholar] [CrossRef] [PubMed]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxid. Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef]
- Run, C.; Yang, Q.; Liu, Z.; OuYang, B.; Chou, J.J. Molecular basis of MgATP selectivity of the mitochondrial SCaMC carrier. Structure 2015, 23, 1394–1403. [Google Scholar] [CrossRef][Green Version]
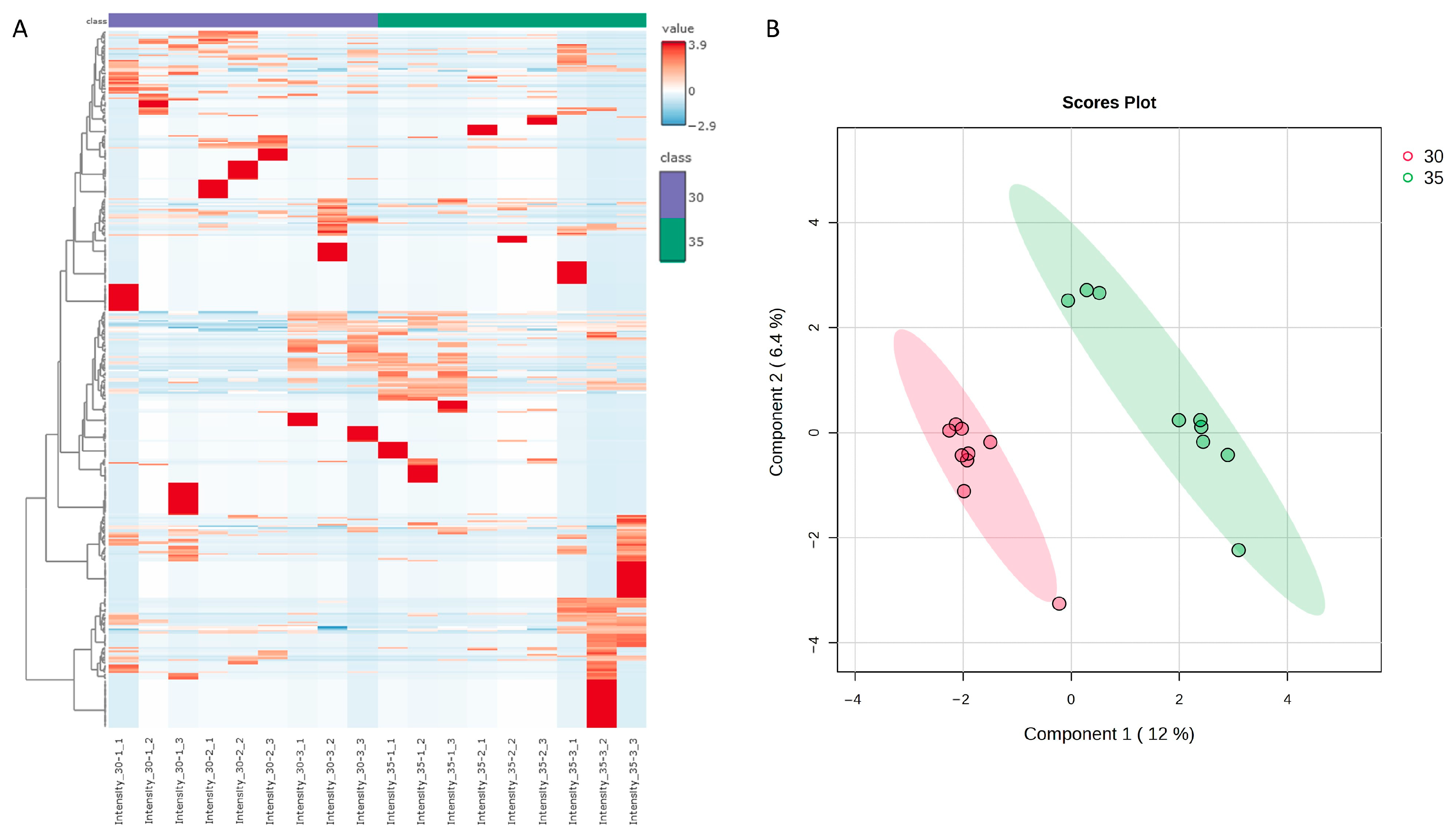
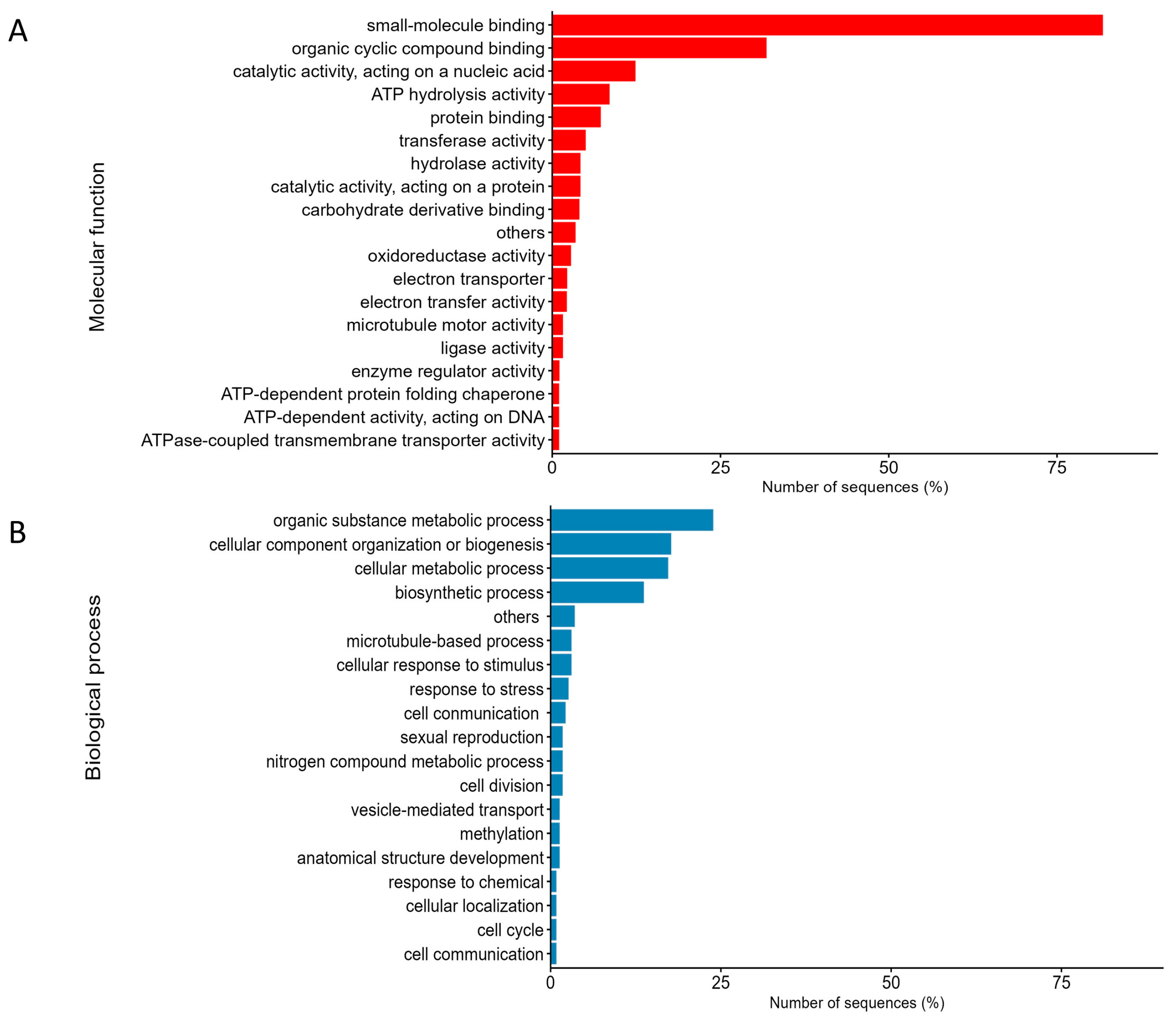

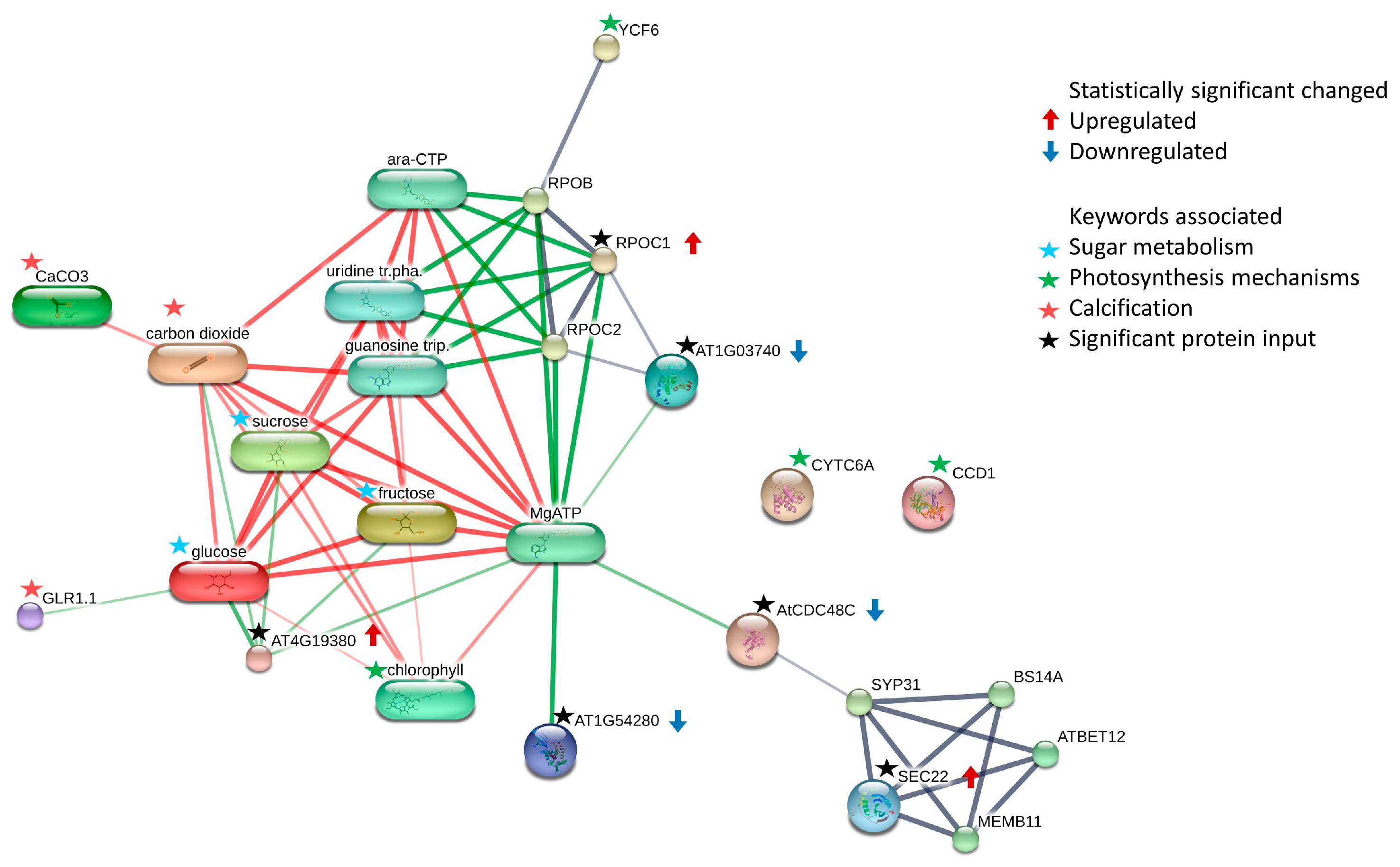
| Protein IDs | Protein Names | Gene Ontology (Biological Process) | Gene Ontology (Molecular Function) | FC | log2(FC) |
|---|---|---|---|---|---|
| A0A0D6E2P6 | Cell division protein | Cell division | Small-molecule binding | 0.10701 | −3.2242 |
| A0A8S1IWT5 | Protein kinase domain-containing protein | Cellular metabolic process | Small-molecule binding | 0.18418 | −2.4408 |
| A0A8S1J0X3 | Phospholipid-transporting ATPase | Macromolecule localization | Small-molecule binding | 0.249 | −2.0058 |
| A0A8S1J4V3 | TPM domain-containing protein | - | - | 0.27397 | −1.8679 |
| A0A386B1A2 | Small ribosomal subunit protein uS3c | Biosynthetic process | Organic cyclic compound binding | 0.29894 | −1.7421 |
| A0A8S1J867 | Uncharacterized protein | - | - | 0.48295 | −1.05 |
| A0A8S1J8Q1 | Uncharacterized protein | - | - | 3.1415 | 1.6515 |
| A0A8S1J7D8 | Uncharacterized protein | - | - | 3.6946 | 1.8854 |
| A0A8S1ILY7 | Secreted protein | Establishment of localization | Transport | 4.8054 | 2.2647 |
| A0A3S5X2Q5 | DNA-directed RNA polymerase subunit beta | Biosynthetic process | Organic cyclic compound binding | 7.9662 | 2.9939 |
| A0A8S1J7E9 | Long-chain alcohol oxidase | Oxidoreductase activity | Small-molecule binding | 19.498 | 4.2853 |
| A0A8S1IXJ6 | Fungal lipase-like domain-containing protein | Organic substance metabolic process | Gene Ontology (molecular function) | 29.472 | 4.8813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chintakovid, N.; Phaonakrop, N.; Surachat, K.; Phetcharat, S.; Wutiruk, T.; Roytrakul, S.; Mayakun, J. The Proteome Profile of Halimeda macroloba under Elevated Temperature: A Case Study from Thailand. J. Mar. Sci. Eng. 2024, 12, 1073. https://doi.org/10.3390/jmse12071073
Chintakovid N, Phaonakrop N, Surachat K, Phetcharat S, Wutiruk T, Roytrakul S, Mayakun J. The Proteome Profile of Halimeda macroloba under Elevated Temperature: A Case Study from Thailand. Journal of Marine Science and Engineering. 2024; 12(7):1073. https://doi.org/10.3390/jmse12071073
Chicago/Turabian StyleChintakovid, Nutwadee, Narumon Phaonakrop, Komwit Surachat, Sinjai Phetcharat, Tarawit Wutiruk, Sittiruk Roytrakul, and Jaruwan Mayakun. 2024. "The Proteome Profile of Halimeda macroloba under Elevated Temperature: A Case Study from Thailand" Journal of Marine Science and Engineering 12, no. 7: 1073. https://doi.org/10.3390/jmse12071073
APA StyleChintakovid, N., Phaonakrop, N., Surachat, K., Phetcharat, S., Wutiruk, T., Roytrakul, S., & Mayakun, J. (2024). The Proteome Profile of Halimeda macroloba under Elevated Temperature: A Case Study from Thailand. Journal of Marine Science and Engineering, 12(7), 1073. https://doi.org/10.3390/jmse12071073








