A Parallelized Climatological Drifter-Based Model of Sargassum Biomass Dynamics in the Tropical Atlantic
Abstract
:1. Introduction
2. Materials and Methods
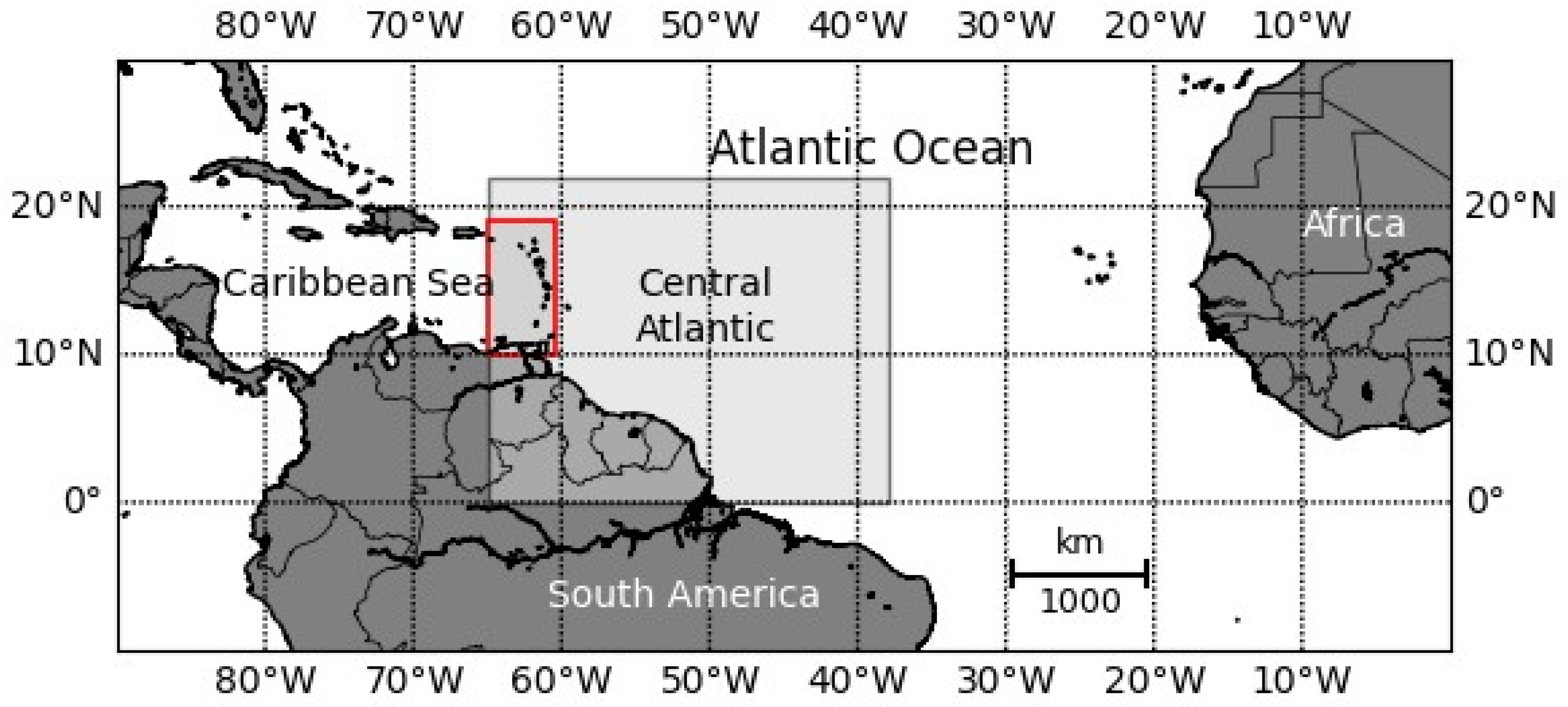
2.1. Study Area
2.2. Data Sources and Processing
2.2.1. Satellite Data for Initializing Lagrangian Drift Model
2.2.2. Current and Wind Climatological Data
2.2.3. Sea Surface Temperature Data Used in Biomass Dynamics Model
2.3. Parallel Implementation of Lagrangian Drift Model for Sargassum Advection
| Algorithm 1 ForwardTrack |
| Inputs: Iterable data structure containing tuples of latitude and longitude values representing Lagrangian particles. Output: Data structure containing latitude and longitude tracks for each particle.
|
2.4. Modelling Sargassum Biomass Dynamics
2.5. Quantification of Sargassum Biomass
3. Results and Discussion
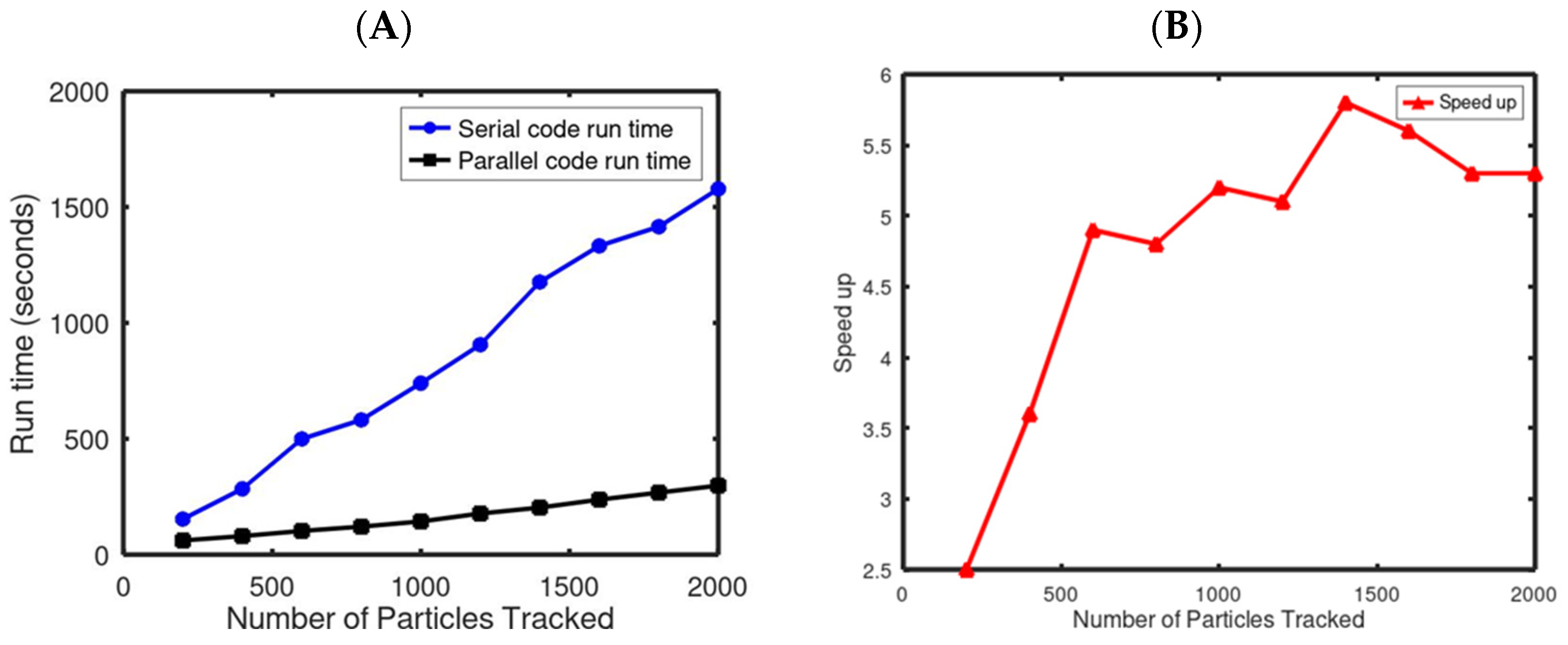
3.1. Benchmarking the Parallel Climatological Drifter-Based Sargassum Model
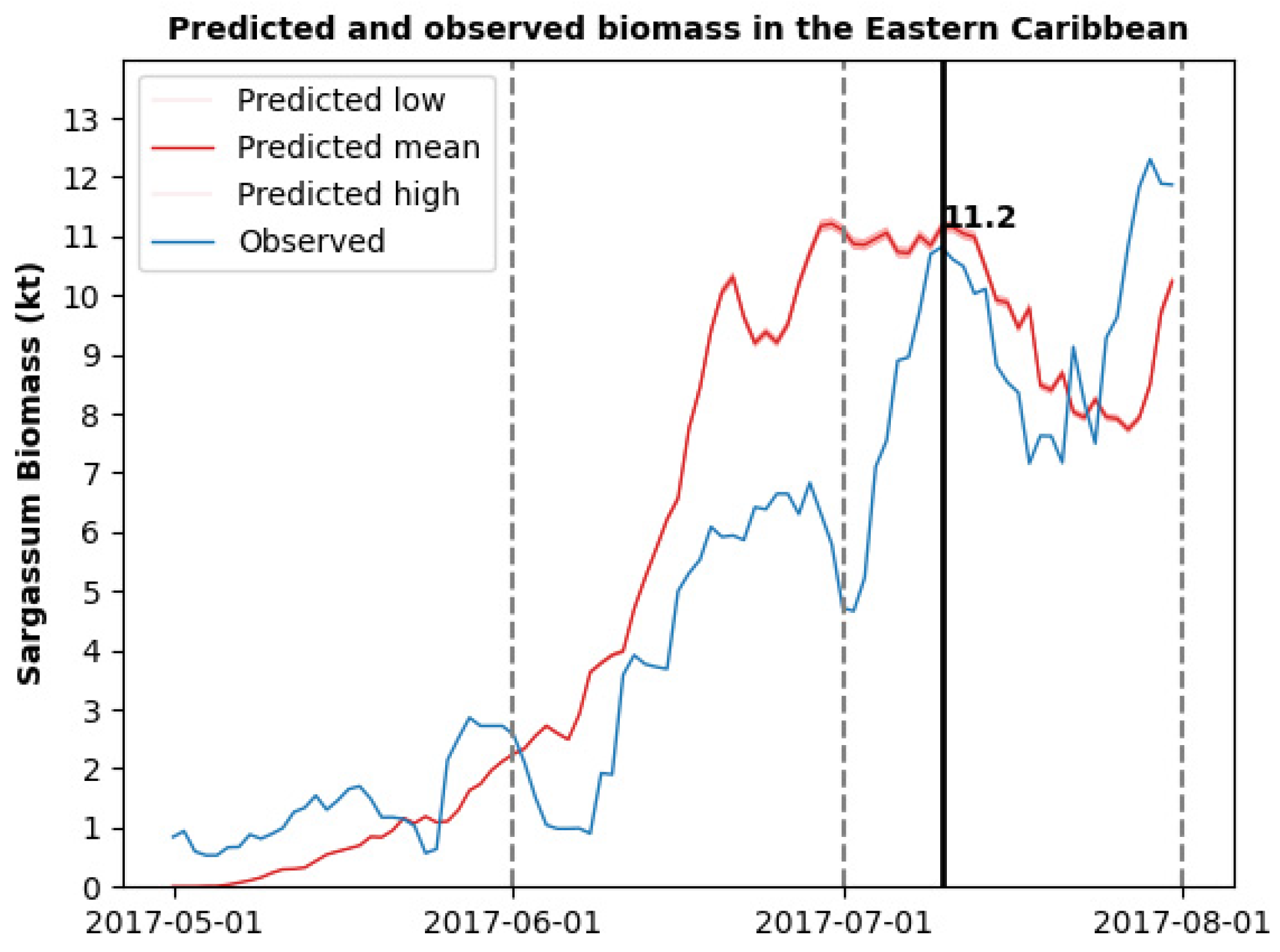
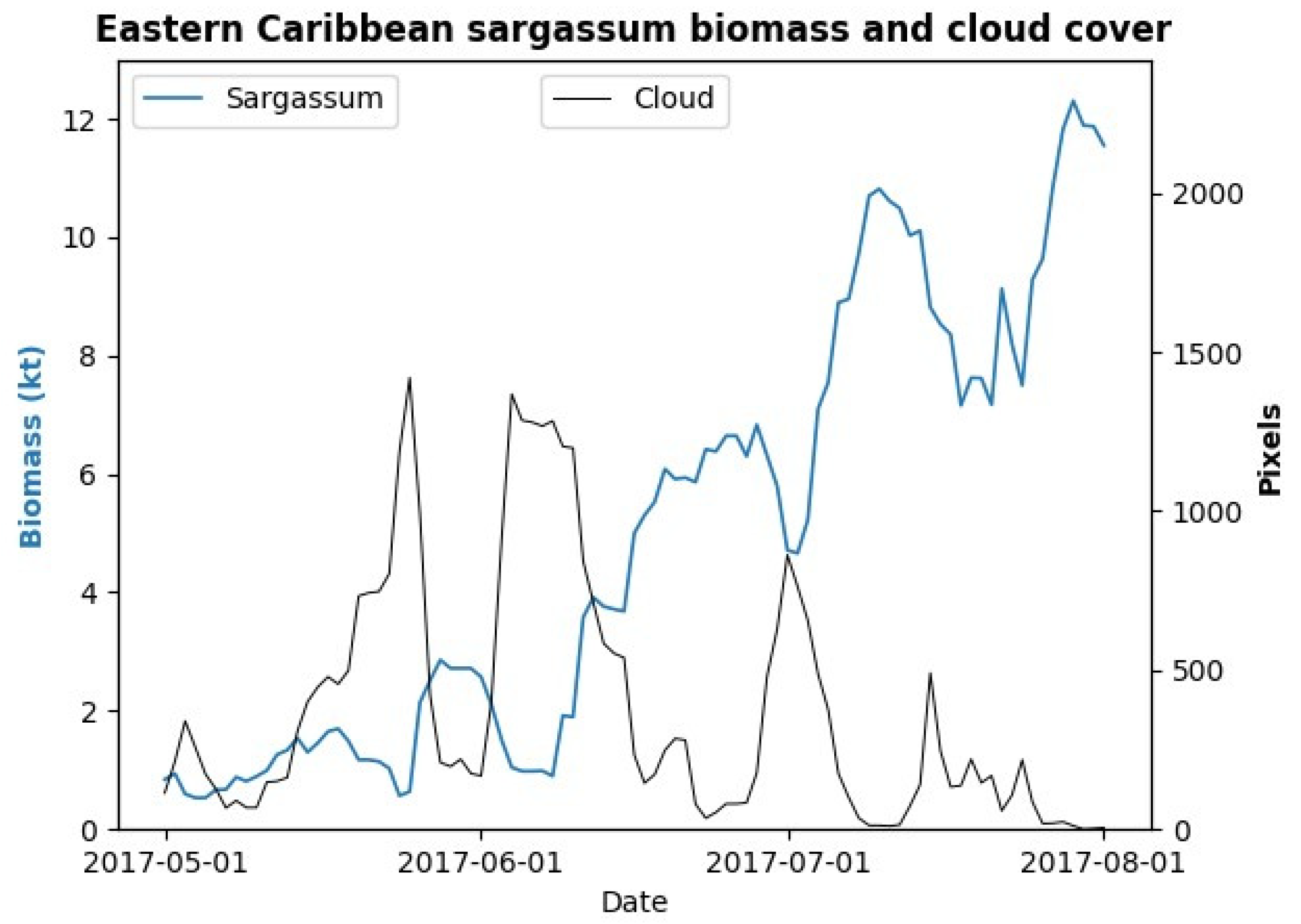
3.2. Sargassum Biomass Dynamics for a Single Morphotype
3.3. Sargassum Biomass Dynamics for Multiple Morphotypes
3.4. Limitations and Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme-Caribbean Environment Programme. Sargassum White Paper—Turning the crisis into an opportunity. In Proceedings of the Ninth Meeting of the Scientific and Technical Advisory Committee (STAC) to the Protocol Concerning Specially Protected Areas and Wildlife (SPAW) in the Wider Caribbean Region, Kingston, Jamaica, 17–19 March 2021.
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Jordán-Dahlgren, E. Pelagic Sargassum cleanup cost in Mexico. Ocean. Coast. Manag. 2023, 237, 106542. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Skliris, N.; Marsh, R.; Addo, K.A.; Oxenford, H. Physical drivers of pelagic sargassum bloom interannual variability in the Central West Atlantic over 2010–2020. Ocean Dyn. 2022, 72, 383–404. [Google Scholar] [CrossRef]
- Maréchal, J.-P.; Hellio, C.; Hu, C. A simple, fast, and reliable method to predict Sargassum washing ashore in the Lesser Antilles. Remote Sens. Appl. Soc. Environ. 2017, 5, 54–63. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.A.; Oxenford, H.A.; Van Tussenbroek, B. Pelagic Sargassum—A Guide to Current and Potential Uses in the Caribbean; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of turning the sargassum crisis into gold: Current constraints and implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of pelagic sargassum biomass into sustainable applications: Current trends and challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.; Oxenford, H.A.; Cox, S.-A.L.; Johnson, D.R.; Bellamy, J. Forecasting seasonal sargassum events across the Tropical Atlantic: Overview and challenges. Front. Mar. Sci. 2022, 9, 914501. [Google Scholar] [CrossRef]
- de la Barreda-Bautista, B.; Metcalfe, S.E.; Smith, G.; Sjögersten, S.; Boyd, D.S.; Cerdeira-Estrada, S.; Lopez-Ramırez, P.; Magaldi, A.; Ressl, R.; Perera-Valderrama, S.; et al. Monitoring holopelagic Sargassum spp. along the Mexican Caribbean coast: Understanding and addressing user requirements for satellite remote sensing. Front. Mar. Sci. 2023, 10, 1166000. [Google Scholar] [CrossRef]
- Putman, N.F.; Beyea, R.T.; Iporac, L.A.R.; Triñanes, J.; Ackerman, E.G.; Olascoaga, M.J.; Appendini, C.M.; Arriaga, J.; Collado-Vides, L.; Lumpkin, R.; et al. Improving satellite monitoring of coastal inundations of pelagic Sargassum algae with wind and citizen science data. Aquat. Bot. 2023, 188, 103672. [Google Scholar] [CrossRef]
- Chassignet, E.P.; Hurlburt, H.E.; Smedstad, O.M.; Halliwell, G.R.; Hogan, P.J.; Wallcraft, A.J.; Baraille, R.; Bleck, R. The HYCOM (hybrid coordinate ocean model) data assimilative system. J. Mar. Syst. 2007, 65, 60–83. [Google Scholar] [CrossRef]
- Madec, G. NEMO Ocean Engine. Note du Pôle de Modélisation; Institut Pierre-Simon Laplace (IPSL): Guyancourt, France, 2016; Volume 27, pp. 1288–1619. [Google Scholar]
- Johnson, D.; Franks, J.; Oxenford, H.A.; Cox, S.-A. Pelagic sargassum prediction and marine connectivity in the Tropical Atlantic. Gulf Caribb. Res. 2020, 31, 20–30. [Google Scholar] [CrossRef]
- Podlejski, W.; Berline, L.; Nerini, D.; Doglioli, A.; Lett, C. A new Sargassum drift model derived from features tracking in MODIS images. Mar. Pollut. Bull. 2023, 188, 114629. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, S.; Barnes, B.B.; Xie, Y.; Wang, M.; Cannizzaro, J.P.; English, D.C. Mapping and quantifying pelagic Sargassum in the Atlantic Ocean using multi-band medium-resolution satellite data and deep learning. Remote Sens. Environ. 2023, 289, 113515. [Google Scholar] [CrossRef]
- Jouanno, J.; Benshila, R.; Berline, L.; Soulié, A.; Radenac, M.H.; Morvan, G.; Diaz, F.; Sheinbaum, J.; Chevalier, C.; Thibaut, T.; et al. A NEMO-based model of Sargassum distribution in the Tropical Atlantic: Description of the model and sensitivity analysis (NEMO-Sarg1.0). Geosci. Model Dev. 2021, 14, 4069–4086. [Google Scholar] [CrossRef]
- Marsh, R.; Addo, K.A.; Jayson-Quashigah, P.N.; Oxenford, H.A.; Maxam, A.; Anderson, R.; Skliris, N.; Dash, J.; Tompkins, E.L. Seasonal predictions of holopelagic Sargassum across the Tropical Atlantic accounting for uncertainty in drivers and processes: The SARTRAC ensemble forecast system. Front. Mar. Sci. 2021, 8, 722524. [Google Scholar] [CrossRef]
- Magaña-Gallegos, E.; García-Sánchez, M.; Graham, C.; Olivos-Ortiz, A.; Siuda, A.N.; van Tussenbroek, B.I. Growth rates of pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2023, 185, 103614. [Google Scholar] [CrossRef]
- Siuda, A.N.; Blanfuné, A.; Dibner, S.; Verlaque, M.; Boudouresque, C.F.; Connan, S.; Goodwin, D.S.; Stiger-Pouvreau, V.; Viard, F.; Rousseau, F.; et al. Morphological and Molecular Characters Differentiate Common Morphotypes of Atlantic Holopelagic Sargassum. Phycology 2024, 4, 256–275. [Google Scholar] [CrossRef]
- Corbin, M.; Oxenford, H.A. Assessing growth of pelagic sargassum in the Tropical Atlantic. Aquat. Bot. 2023, 187, 103654. [Google Scholar] [CrossRef]
- Singh, N.; Browne, L.M.; Butler, R. Parallel astronomical data processing with Python: Recipes for multicore machines. Astron. Comput. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Abeykoon, S.K.; Lin, M.; Van Dam, K.K. Parallelizing X-ray photon correlation spectroscopy software tools using python multiprocessing. In Proceedings of the 2017 New York Scientific Data Summit (NYSDS), New York, NY, USA, 6–9 August 2017; pp. 1–10. [Google Scholar]
- Parr, A.E. Quantitative observations on the pelagic Sargassum vegetation of the western North Atlantic. Bull. Bingham Oceanogr. Coll. 1939, 6, 1–94. [Google Scholar]
- Godinez-Ortega, J.L.; Cuatlan-Cortes, J.V.; Lopez-Bautista, J.M.; van Tussenbroek, B.I. A natural history of floating Sargassum species (Sargasso) from Mexico. In Natural History and Ecology of Mexico and Central America; Hufnagel, L., Ed.; IntechOpen: London, UK, 2021; pp. 59–94. [Google Scholar] [CrossRef]
- Ody, A.; Thibaut, T.; Berline, L.; Changeux, T.; André, J.M.; Chevalier, C.; Blanfuné, A.; Blanchot, J.; Ruitton, S.; Stiger-Pouvreau, V.; et al. From in situ to satellite observations of pelagic Sargassum distribution and aggregation in the Tropical North Atlantic Ocean. PLoS ONE 2019, 14, e0222584. [Google Scholar] [CrossRef] [PubMed]
- Laval, M.; Belmouhcine, A.; Courtrai, L.; Descloitres, J.; Salazar-Garibay, A.; Schamberger, L.; Chevalier, C. Detection of sargassum from sentinel satellite sensors using deep learning approach. Remote Sens. 2023, 15, 1104. [Google Scholar] [CrossRef]
- Cailleau, S.; Bessières, L.; Chiendje, L.; Dubost, F.; Reffray, G.; Lellouche, J.M.; van Gennip, S.; Régnier, C.; Drevillon, M.; Tressol, M.; et al. CAR36, a regional high-resolution ocean forecasting system for improving drift and beaching of Sargassum in the Caribbean archipelago. Geosci. Model Dev. 2024, 17, 3157–3173. [Google Scholar] [CrossRef]
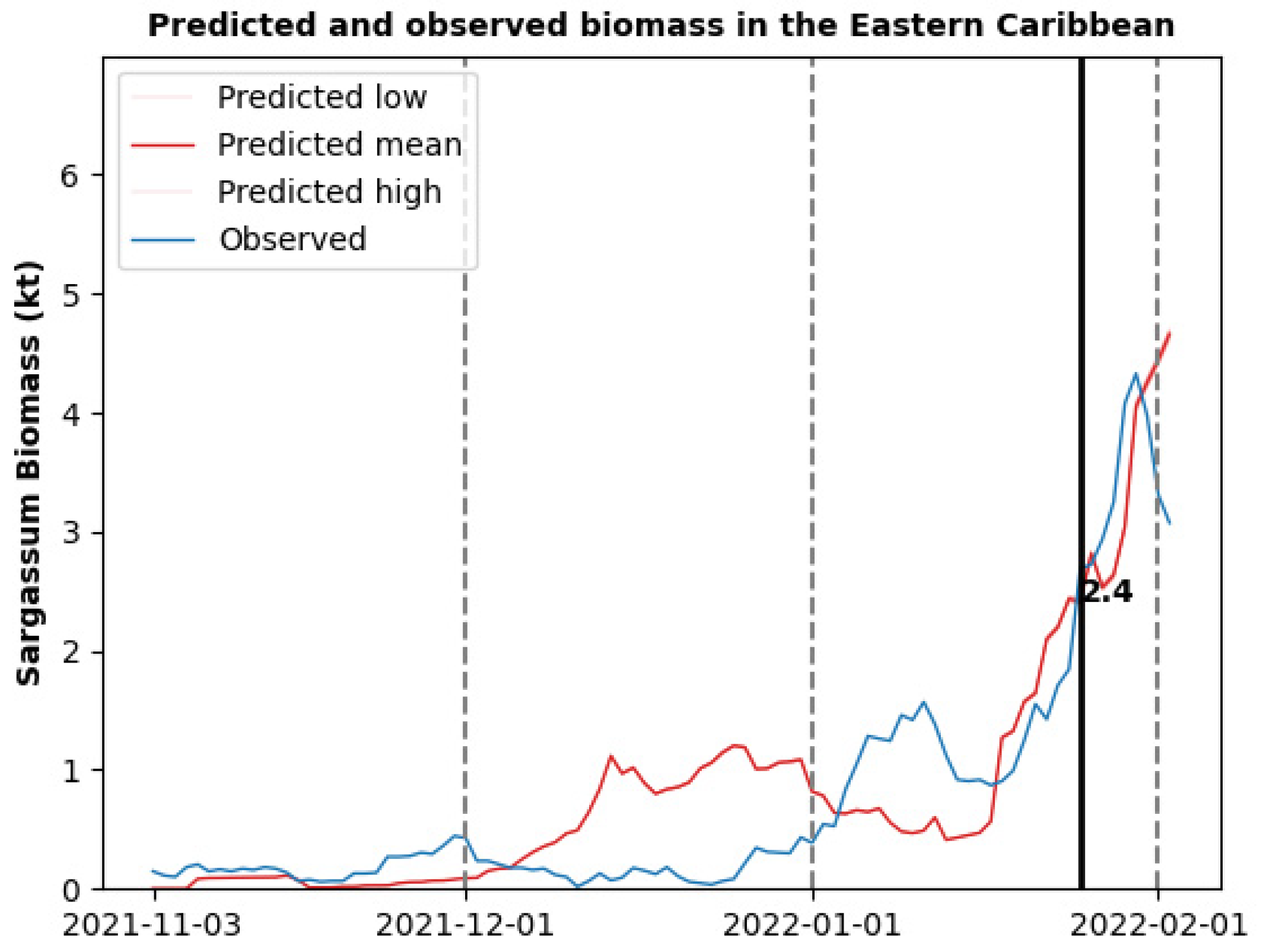
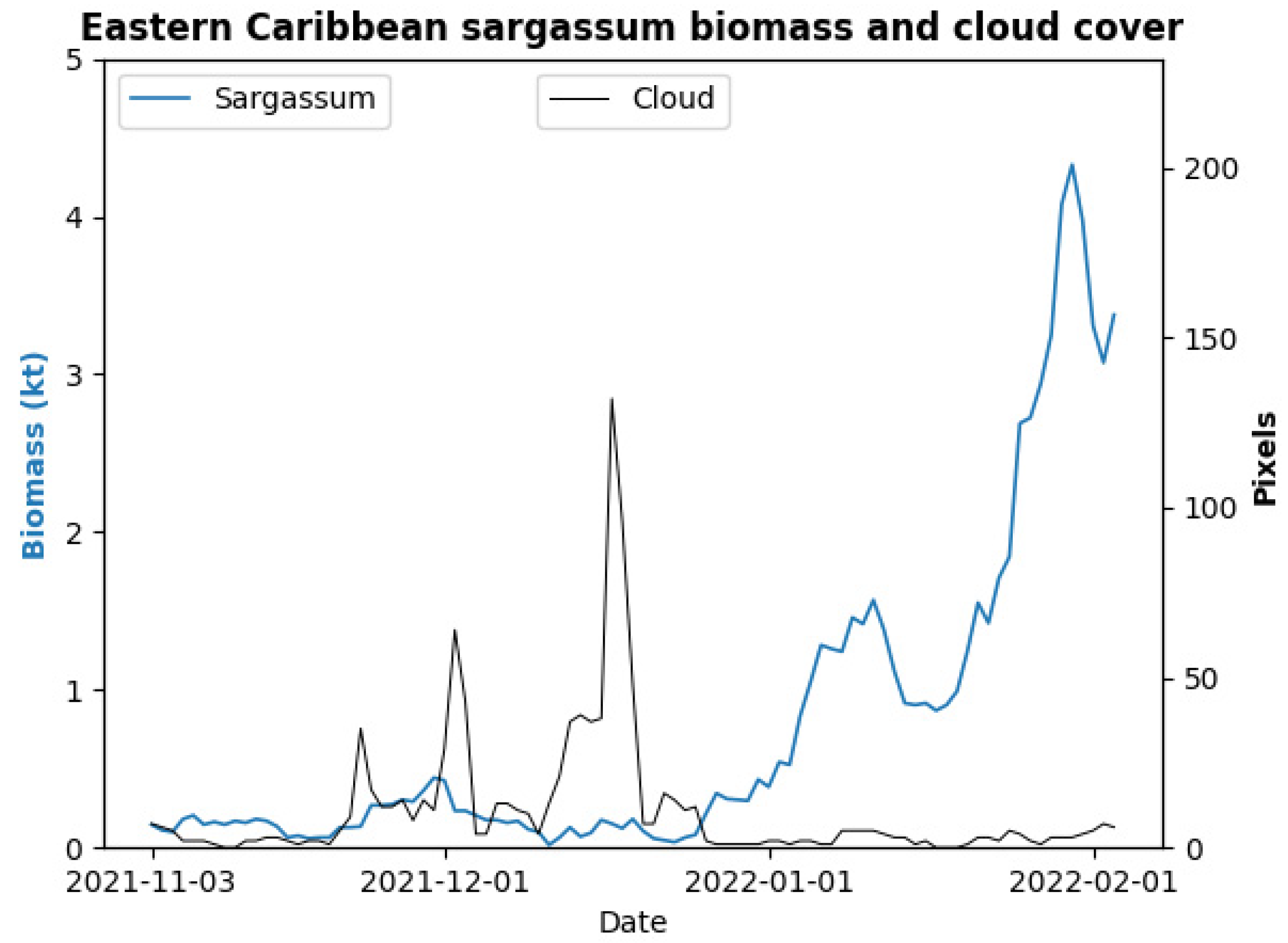
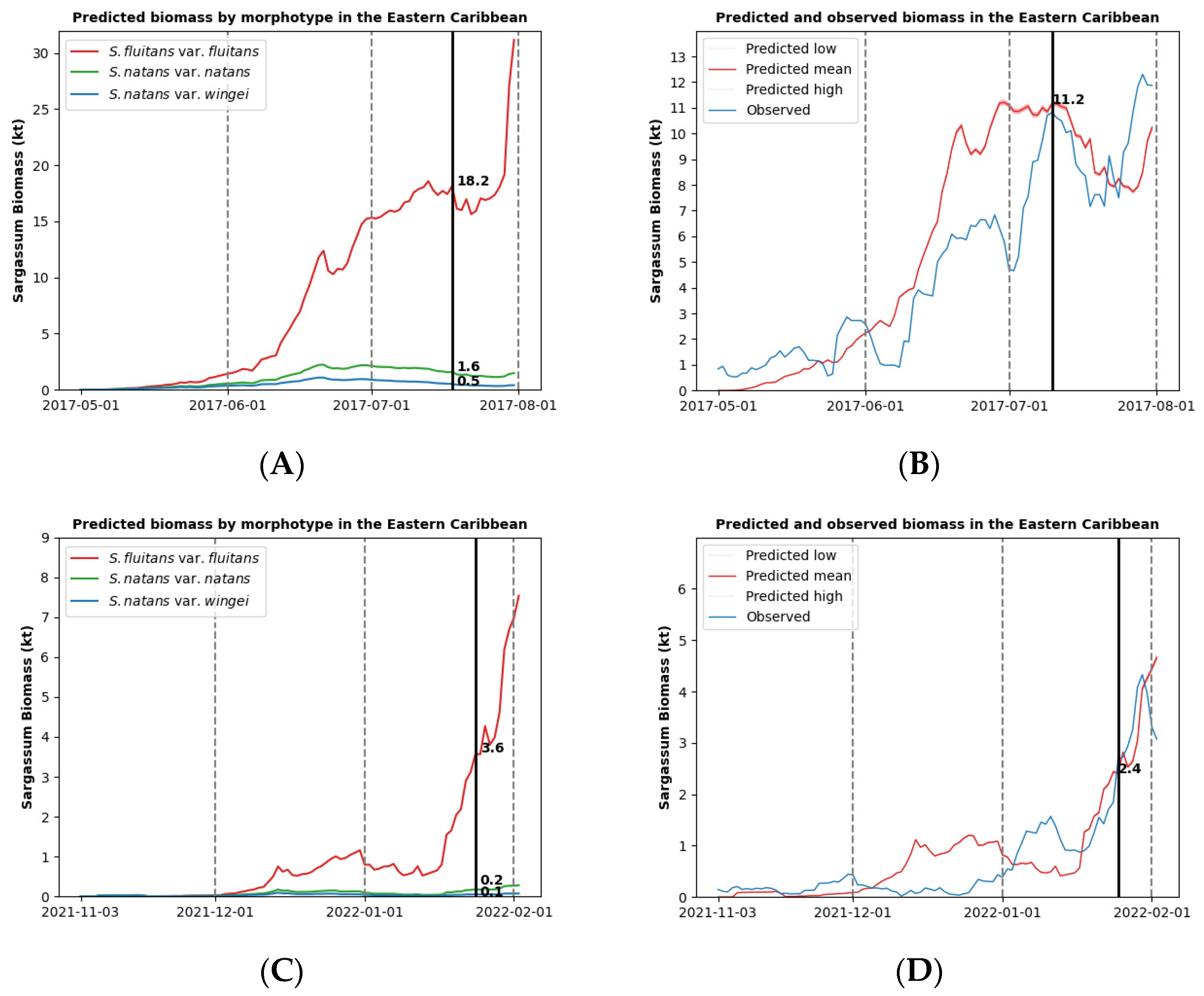
| Parameter | Description | Range | Unit |
|---|---|---|---|
| Tmin | Growth ceases below this temperature | 10–14 | °C |
| Tmax | Growth ceases above this temperature | 40–50 | °C |
| Topt | Optimum temperature for growth | 22–27 | °C |
| µmax | Maximum carbon uptake rate | 0.029–0.080 | d−1 |
| m | Maximum mortality rate | 0.06–0.10 | d−1 |
| λm | Temperature-dependent mortality coefficient | 0.25–0.65 |
| Parameter | S. fluitans var. fluitans | S. natans var. natans | S. natans var. wingei |
|---|---|---|---|
| Initial biomass fraction | 0.3 | 0.3 | 0.3 |
| Warm growth rate | 0.060 | 0.042 | 0.029 |
| Cool growth rate | 0.098 | 0.051 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payne, K.; Greene, K.; Oxenford, H.A. A Parallelized Climatological Drifter-Based Model of Sargassum Biomass Dynamics in the Tropical Atlantic. J. Mar. Sci. Eng. 2024, 12, 1214. https://doi.org/10.3390/jmse12071214
Payne K, Greene K, Oxenford HA. A Parallelized Climatological Drifter-Based Model of Sargassum Biomass Dynamics in the Tropical Atlantic. Journal of Marine Science and Engineering. 2024; 12(7):1214. https://doi.org/10.3390/jmse12071214
Chicago/Turabian StylePayne, Karl, Khalil Greene, and Hazel A. Oxenford. 2024. "A Parallelized Climatological Drifter-Based Model of Sargassum Biomass Dynamics in the Tropical Atlantic" Journal of Marine Science and Engineering 12, no. 7: 1214. https://doi.org/10.3390/jmse12071214






