Antioxidant Systems in Extremophile Marine Fish Species
Abstract
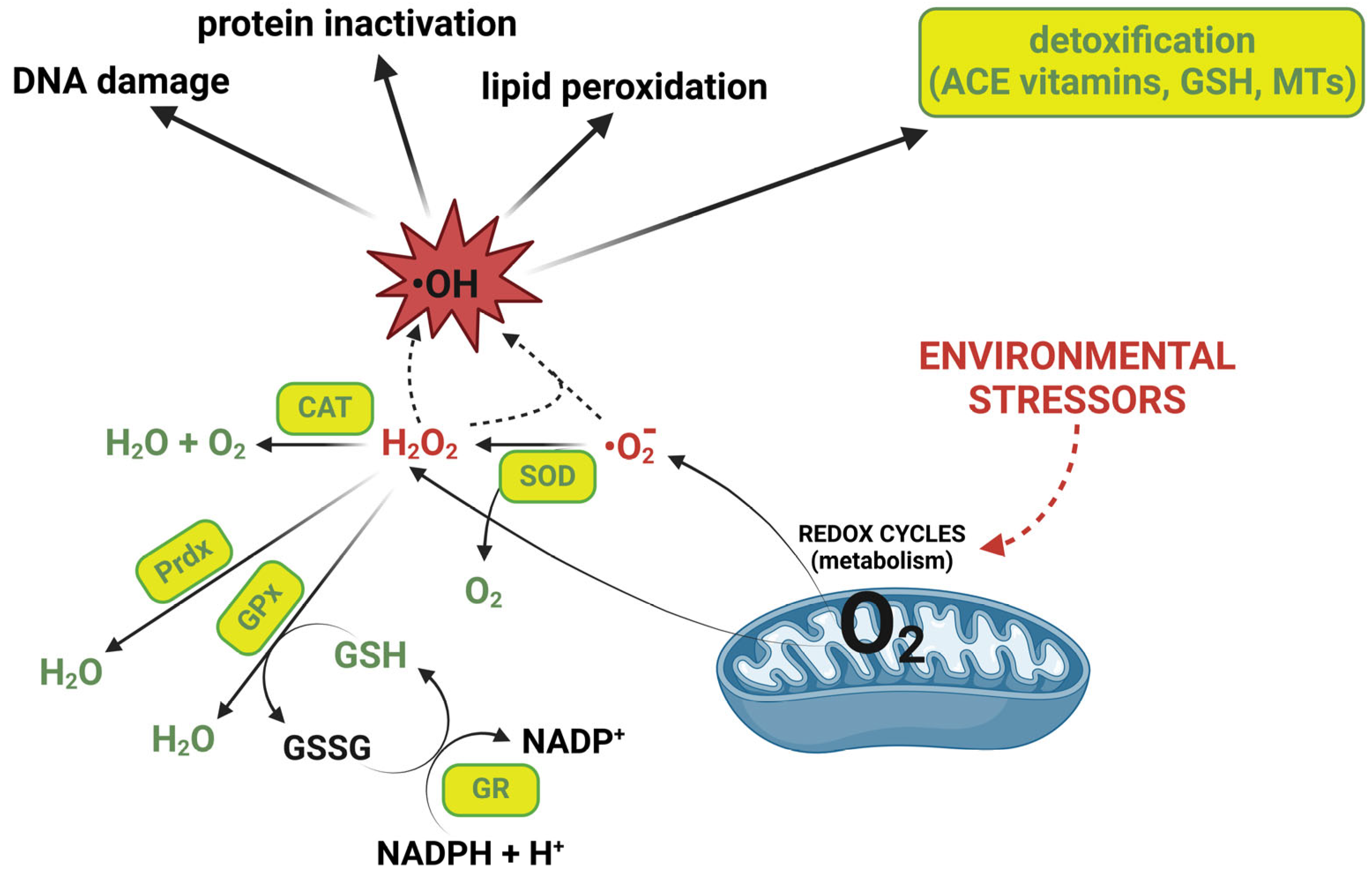
1. Introduction
2. Oxidative Stress and Antioxidant Defence
3. Antioxidants in Extreme Marine Environments
3.1. Antarctic Fish
3.1.1. Enzymatic Antioxidant Systems
3.1.2. Non-Enzymatic Antioxidant Systems
3.1.3. Environmental Stressors
Thermal Stress
Persistent Organic Pollutants (POPs)
3.2. Deep-Sea Fish
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Taenzer, L.; Wankel, S.D.; Kapit, J.; Pardis, W.A.; Herrera, S.; Auscavitch, S.; Grabb, K.C.; Cordes, E.; Hansel, C.M. Corals and Sponges Are Hotspots of Reactive Oxygen Species in the Deep Sea. PNAS Nexus 2023, 2, pgad398. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D. Bioactive Molecules from Extreme Environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D. Free Radical-scavenging Activity of Marine Proteins and Peptides. In Marine Proteins and Peptides; Wiley: New York, NY, USA, 2013; pp. 487–497. [Google Scholar]
- Campanyà-Llovet, N.; Snelgrove, P.V.R.; Parrish, C.C. Rethinking the Importance of Food Quality in Marine Benthic Food Webs. Prog. Oceanogr. 2017, 156, 240–251. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.M.; Hofmann, G.E. Expression of 70 kDa Heat Shock Proteins in Antarctic and New Zealand Notothenioid Fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 125, 229–238. [Google Scholar] [CrossRef]
- Bakiu, R.; Pacchini, S.; Piva, E.; Schumann, S.; Tolomeo, A.M.; Ferro, D.; Irato, P.; Santovito, G. Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus Hansoni. Int. J. Mol. Sci. 2022, 23, 12799. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Dettori, M.A.; Fabbri, D.; Alov, P.; Angelova, S.E.; Slavova-Kazakova, A.K.; Carta, P.; Menshov, V.A.; Yablonskaya, O.I.; Trofimov, A.V.; et al. Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants 2021, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Reactive Oxygen Species and Cellular Defense System. In Free Radicals in Human Health and Disease; Springer: New Delhi, India, 2015; pp. 17–29. [Google Scholar]
- Acworth, I.N.; McCabe, D.R.; Maher, T.J. The Analysis of Free Radicals, Their Reaction Products, and Antioxidants. In Oxidants, Antioxidants, and Free Radicals; Baskin, S.I., Salem, H., Eds.; Taylor and Francis: Washington, DC, USA, 1977; pp. 23–77. ISBN 978-0-203-74467-3. [Google Scholar]
- Franchi, N.; Ferro, D.; Ballarin, L.; Santovito, G. Transcription of Genes Involved in Glutathione Biosynthesis in the Solitary Tunicate Ciona Intestinalis Exposed to Metals. Aquat. Toxicol. 2012, 114–115, 14–22. [Google Scholar] [CrossRef]
- Tavassolifar, M.J.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Lesmana, R.; Parameswari, C.; Mandagi, G.F.; Wahyudi, J.F.; Permana, N.J.; Radhiyanti, P.T.; Gunadi, J.W. The Role of Exercise-Induced Reactive Oxygen Species (ROS) Hormesis in Aging: Friend or Foe. Cell. Physiol. Biochem. 2022, 56, 692–706. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide Anion Radical (O·2), Superoxide Dismutases, and Related Matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Schumann, S.; Dotteschini, S.; Brocca, G.; Radaelli, G.; Marion, A.; Irato, P.; Bertotto, D.; Santovito, G. Antioxidant Responses Induced by PFAS Exposure in Freshwater Fish in the Veneto Region. Antioxidants 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, E.; Bisaccia, P.; Corrà, F.; Bonato, M.; Irato, P.; Manuto, L.; Toppo, S.; Bakiu, R.; Santovito, G. Copper/Zinc Superoxide Dismutase from the Crocodile Icefish Chionodraco Hamatus: Antioxidant Defense at Constant Sub-Zero Temperature. Antioxidants 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E. Basic Mechanisms of Antioxidant Activity. BioFactors 1997, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Tissue-Specific Functions of Individual Glutathione Peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Bakiu, R.; Pucciarelli, S.; Miceli, C.; Vallesi, A.; Irato, P.; Santovito, G. Molecular Characterization, Protein-Protein Interaction Network, and Evolution of Four Glutathione Peroxidases from Tetrahymena Thermophila. Antioxidants 2020, 9, 949. [Google Scholar] [CrossRef]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C.E. Evolutionary and Structural Insights Into the Multifaceted Glutathione Peroxidase (Gpx) Superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1514. [Google Scholar] [CrossRef]
- Sattin, G.; Bakiu, R.; Tolomeo, A.M.; Carraro, A.; Coppola, D.; Ferro, D.; Patarnello, T.; Santovito, G. Characterization and Expression of a New Cytoplasmic Glutathione Peroxidase 1 Gene in the Antarctic Fish Trematomus Bernacchii. Hydrobiologia 2015, 761, 363–372. [Google Scholar] [CrossRef]
- Wood, Z.A.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, Mechanism and Regulation of Peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Knoops, B.; Loumaye, E.; Van Der Eecken, V. Evolution of the Peroxiredoxins. In Peroxiredoxin Systems. Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2007; pp. 27–40. [Google Scholar]
- Al-Asadi, S.; Malik, A.; Bakiu, R.; Santovito, G.; Menz, I.; Schuller, K. Characterization of the Peroxiredoxin 1 Subfamily from Tetrahymena Thermophila. Cell. Mol. Life Sci. 2019, 76, 4745–4768. [Google Scholar] [CrossRef] [PubMed]
- Formigari, A.; Boldrin, F.; Santovito, G.; Cassidy-Hanley, D.; Clark, T.G.; Piccinni, E. Functional Characterization of the 5′-Upstream Region of MTT5 Metallothionein Gene from Tetrahymena Thermophila. Protist 2010, 161, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Santovito, G.; Trentin, E.; Gobbi, I.; Bisaccia, P.; Tallandini, L.; Irato, P. Non-Enzymatic Antioxidant Responses of Mytilus Galloprovincialis: Insights into the Physiological Role against Metal-Induced Oxidative Stress. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2021, 240, 108909. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Webb, A.C.; Witter, D.; Burton, G.W.; Metcalfe, T.A.; Muller, D.P.R. Vitamin E Remains the Major Lipid-Soluble, Chain-Breaking Antioxidant in Human Plasma Even in Individuals Suffering Severe Vitamin E Deficiency. Arch. Biochem. Biophys. 1987, 259, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef] [PubMed]
- Sattin, G.; Santovito, G.; Cassini, A. Physiological Antioxidant Responses against High Environmental Oxygen Concentration: Glutathione Peroxidase from the Antarctic Teleost Trematomus Eulepidotus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, S27. [Google Scholar] [CrossRef]
- O’Quin, K.E.; Yoshizawa, M.; Doshi, P.; Jeffery, W.R. Quantitative Genetic Analysis of Retinal Degeneration in the Blind Cavefish Astyanax Mexicanus. PLoS ONE 2013, 8, e57281. [Google Scholar] [CrossRef]
- Terzibasi, E.; Valenzano, D.R.; Cellerino, A. The Short-Lived Fish Nothobranchius Furzeri as a New Model System for Aging Studies. Exp. Gerontol. 2007, 42, 81–89. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC Transporters in Fish Species: A Review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Andrade, D.C.; Gómez-Silva, B.; Batista-García, R.A.; Millet, G.P. Editorial: Adaptive Response of Living Beings to Extreme Environments: Integrative Approaches from Cellular and Molecular Biology, Biotechnology, Microbiology to Physiology. Front. Physiol. 2022, 13, 1068287. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.C.; Martinez, C.M.; Friedman, S.T.; Wainwright, P.C.; Price, S.A.; Tornabene, L. Alternating Regimes of Shallow and Deep-Sea Diversification Explain a Species-Richness Paradox in Marine Fishes. Proc. Natl. Acad. Sci. USA 2022, 119, e2123544119. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Longo, S.J. Functional Innovations and the Conquest of the Oceans by Acanthomorph Fishes. Curr. Biol. 2017, 27, R550–R557. [Google Scholar] [CrossRef] [PubMed]
- Hala, E.; Bakiu, R. Adriatic Sea Fishery Product Safety and Prospectives in Relation to Climate Change. Fishes 2024, 9, 160. [Google Scholar] [CrossRef]
- Daane, J.M.; Detrich, H.W. Adaptations and Diversity of Antarctic Fishes: A Genomic Perspective. Annu. Rev. Anim. Biosci. 2022, 10, 39–62. [Google Scholar] [CrossRef]
- Ruud, J.T. Vertebrates without Erythrocytes and Blood Pigment. Nature 1954, 173, 848–850. [Google Scholar] [CrossRef]
- Moylan, T.J.; Sidell, B.D. Concentrations of Myoglobin and Myoglobin mRNA in Heart Ventricles From Antarctic Fishes. J. Exp. Biol. 2000, 203, 1277–1286. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of Increasing Temperature on Antioxidant Defense System and Oxidative Stress Parameters in the Antarctic Fish Notothenia Coriiceps and Notothenia Rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Guillen, A.C.; Borges, M.E.; Herrerias, T.; Kandalski, P.K.; de Souza, M.R.D.P.; Donatti, L. Gradual Increase of Temperature Trigger Metabolic and Oxidative Responses in Plasma and Body Tissues in the Antarctic Fish Notothenia Rossii. Fish Physiol. Biochem. 2022, 48, 337–354. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of Reactive Species and Induction of Antioxidant Defence Systems in Polar and Temperate Marine Invertebrates and Fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, É.G.; Hermes-Lima, M. Role of Redox Metabolism for Adaptation of Aquatic Animals to Drastic Changes in Oxygen Availability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Carraro, A.; Bakiu, R.; Toppo, S.; Place, S.P.; Ferro, D.; Santovito, G. Peroxiredoxin 6 from the Antarctic Emerald Rockcod: Molecular Characterization of Its Response to Warming. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 59–71. [Google Scholar] [CrossRef]
- Santovito, G.; Piccinni, E.; Boldrin, F.; Irato, P. Comparative Study on Metal Homeostasis and Detoxification in Two Antarctic Teleosts. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2012, 155, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Colella, A.; Patamia, M.; Galtieri, A.; Giardina, B. Cold Adaptation and Oxidative Metabolism of Antarctic Fish. Ital. J. Zool. 2000, 67, 33–36. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative Pathways of Chemical Toxicity and Oxidative Stress Biomarkers in Marine Organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Cassini, A.; Favero, M.; Albergoni, V. Comparative Studies of Antioxidant Enzymes in Red-Blooded and White-Blooded Antarctic Teleost Fish. Pagothenia Bernacchii and Chionodraco Hamatus. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 333–336. [Google Scholar] [CrossRef]
- Witas, H.; Gabryelak, T.; Matkovics, B. Comparative Studies on Superoxide Dismutase and Catalase Activities in Livers of Fish and Other Antarctic Vertebrates. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1984, 77, 409–411. [Google Scholar] [CrossRef]
- Benedetti, M.; Nigro, M.; Regoli, F. Characterisation of Antioxidant Defences in Three Antarctic Notothenioid Species from Terra Nova Bay (Ross Sea). Chem. Ecol. 2010, 26, 305–314. [Google Scholar] [CrossRef]
- Enzor, L.A.; Place, S.P. Is Warmer Better? Decreased Oxidative Damage in Notothenioid Fish after Long-Term Acclimation to Multiple Stressors. J. Exp. Biol. 2014, 217, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, M.; Luquet, C.M.; Evelson, P.A.; Polo, J.M.; Llesuy, S. Antioxidant Levels from Different Antarctic Fish Caught around South Georgia Island and Shag Rocks. Polar Biol. 2000, 23, 160–165. [Google Scholar] [CrossRef]
- Tolomeo, A.M.; Carraro, A.; Bakiu, R.; Toppo, S.; Garofalo, F.; Pellegrino, D.; Gerdol, M.; Ferro, D.; Place, S.P.; Santovito, G. Molecular Characterization of Novel Mitochondrial Peroxiredoxins from the Antarctic Emerald Rockcod and Their Gene Expression in Response to Environmental Warming. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2019, 225, 108580. [Google Scholar] [CrossRef] [PubMed]
- Römisch, K.; Matheson, T. Cell Biology in the Antarctic: Studying Life in the Freezer. Nat. Cell Biol. 2003, 5, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-Adapted Enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Santovito, G.; Marino, S.M.; Sattin, G.; Cappellini, R.; Bubacco, L.; Beltramini, M. Cloning and Characterization of Cytoplasmic Carbonic Anhydrase from Gills of Four Antarctic Fish: Insights into the Evolution of Fish Carbonic Anhydrase and Cold Adaptation. Polar Biol. 2012, 35, 1587–1600. [Google Scholar] [CrossRef]
- Esterbauer, H.; Dieber-Rotheneder, M.; Striegl, G.; Waeg, G. Role of Vitamin E in Preventing the Oxidation of Low-Density Lipoprotein. Am. J. Clin. Nutr. 1991, 53, 314S–321S. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Buc Calderon, P. Free Radicals and Oxidation Phenomena in Biological Systems; M. Dekker: New York, NY, USA, 1995; ISBN 978-0-8247-9587-0. [Google Scholar]
- Kornbrust, D.J.; Mavis, R.D. Relative Susceptibility of Microsomes from Lung, Heart, Liver, Kidney, Brain and Testes to Lipid Peroxidation: Correlation with Vitamin E Content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Cuddihy, S.; Hill, J.V.; Davison, W. A Comparison of Plasma Vitamin C and E Levels in Two Antarctic and Two Temperate Water Fish Species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 371–378. [Google Scholar] [CrossRef]
- Johnston, I.A.; Calvo, J.; Guderley, H.; Fernandez, D.; Palmer, L. Latitudinal Variation in the Abundance and Oxidative Capacities of Muscle Mitochondria in Perciform Fishes. J. Exp. Biol. 1998, 201, 1–12. [Google Scholar] [CrossRef]
- Klein, R.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Antioxidant Defense System and Oxidative Status in Antarctic Fishes: The Sluggish Rockcod Notothenia Coriiceps versus the Active Marbled Notothen Notothenia Rossii. J. Therm. Biol. 2017, 68, 119–127. [Google Scholar] [CrossRef]
- Santovito, G.; Piccinni, E.; Irato, P. An Improved Method for Rapid Determination of the Reduced and Oxidized States of Metallothioneins in Biological Samples. In Environmental Research Summaries: Volume 2; Melekhin, D.S., Dolukhanov, M.F., Eds.; Nova Science Publisher Inc.: New York, NY, USA, 2012; pp. 287–288. [Google Scholar]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a First Line of Defense against Cadmium Toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The Role of Glutathione in Copper Metabolism and Toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Kägi, J.H.R. Overview of Metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar] [CrossRef]
- Scudiero, R.; De Prisco, P.P.; Camardella, L.; D’Avino, R.; di Prisco, G.; Parisi, E. Apparent Deficiency of Metallothionein in the Liver of the Antarctic Icefish Chionodraco Hamatus. Identification and Isolation of a Zinc-Containing Protein Unlike Metallothionein. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 201–207. [Google Scholar] [CrossRef]
- Scudiero, R.; Carginale, V.; Riggio, M.; Capasso, C.; Capasso, A.; Kille, P.; di Prisco, G.; Parisi, E. Difference in Hepatic Metallothionein Content in Antarctic Red-Blooded and Haemoglobinless Fish: Undetectable Metallothionein Levels in Haemoglobinless Fish Is Accompanied by Accumulation of Untranslated Metallothionein mRNA. Biochem. J. 1997, 322, 207–211. [Google Scholar] [CrossRef]
- Bargelloni, L.; Scudiero, R.; Parisi, E.; Carginale, V.; Capasso, C.; Patarnello, T. Metallothioneins in Antarctic Fish: Evidence for Independent Duplication and Gene Conversion. Mol. Biol. Evol. 1999, 16, 885–897. [Google Scholar] [CrossRef]
- Bakiu, R.; Boldrin, F.; Pacchini, S.; Schumann, S.; Piva, E.; Tolomeo, A.M.; Ferro, D.; Grapputo, A.; Santovito, G.; Irato, P. Molecular Evolution of Metallothioneins of Antarctic Fish: A Physiological Adaptation to Peculiar Seawater Chemical Characteristics. J. Mar. Sci. Eng. 2022, 10, 1592. [Google Scholar] [CrossRef]
- Westerlund, S.; Öhman, P. Cadmium, Copper, Cobalt, Nickel, Lead, and Zinc in the Water Column of the Weddell Sea, Antarctica. Geochim. Cosmochim. Acta 1991, 55, 2127–2146. [Google Scholar] [CrossRef]
- King, C.K.; Dowse, M.C.; Simpson, S.L.; Jolley, D.F. An Assessment of Five Australian Polychaetes and Bivalves for Use in Whole-Sediment Toxicity Tests: Toxicity and Accumulation of Copper and Zinc from Water and Sediment. Arch. Environ. Contam. Toxicol. 2004, 47, 314–323. [Google Scholar] [CrossRef]
- Vacchi, M.; La Mesa, M. The Diet of the Antarctic Fish Trematomus Newnesi Boulenger, 1902 (Nototheniidae) from Terra Nova Bay, Ross Sea. Antacrt. Sci. 1995, 7, 37–38. [Google Scholar] [CrossRef]
- Cerro-Gálvez, E.; Roscales, J.L.; Jiménez, B.; Sala, M.M.; Dachs, J.; Vila-Costa, M. Microbial Responses to Perfluoroalkyl Substances and Perfluorooctanesulfonate (PFOS) Desulfurization in the Antarctic Marine Environment. Water Res. 2020, 171, 115434. [Google Scholar] [CrossRef]
- Marrone, A.; La Russa, D.; Brunelli, E.; Santovito, G.; La Russa, M.F.; Barca, D.; Pellegrino, D. Antarctic Fish as a Global Pollution Sensor: Metals Biomonitoring in a Twelve-Year Period. Front. Mol. Biosci. 2021, 8, 794946. [Google Scholar] [CrossRef]
- Chapman, P.M.; Riddle, M.J. Missing and Needed: Polar Marine Ecotoxicology. Mar. Pollut. Bull. 2003, 46, 927–928. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Mark, F.C.; Bock, C. Oxygen Limited Thermal Tolerance in Fish? Respir. Physiol. Neurobiol. 2004, 141, 243–260. [Google Scholar] [CrossRef]
- Pörtner, H.-O. Oxygen- and Capacity-Limitation of Thermal Tolerance: A Matrix for Integrating Climate-Related Stressor Effects in Marine Ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Carney Almroth, B.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sundell, K.; Axelsson, M.; Sturve, J. Warmer Water Temperature Results in Oxidative Damage in an Antarctic Fish, the Bald Notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Roscales, J.L.; Vicente, A.; Ryan, P.G.; González-Solís, J.; Jiménez, B. Spatial and Interspecies Heterogeneity in Concentrations of Perfluoroalkyl Substances (PFASs) in Seabirds of the Southern Ocean. Environ. Sci. Technol. 2019, 53, 9855–9865. [Google Scholar] [CrossRef]
- Gao, K.; Miao, X.; Fu, J.; Chen, Y.; Li, H.; Pan, W.; Fu, J.; Zhang, Q.; Zhang, A.; Jiang, G. Occurrence and Trophic Transfer of Per- and Polyfluoroalkyl Substances in an Antarctic Ecosystem. Environ. Pollut. 2020, 257, 113383. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. Pfas Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef]
- Pacchini, S.; Piva, E.; Schumann, S.; Irato, P.; Pellegrino, D.; Santovito, G. An Experimental Study on Antioxidant Enzyme Gene Expression in Trematomus Newnesi (Boulenger, 1902) Experimentally Exposed to Perfluoro-Octanoic Acid. Antioxidants 2023, 12, 352. [Google Scholar] [CrossRef]
- Regoli, F.; Nigro, M.; Benedetti, M.; Gorbi, S.; Pretti, C.; Gervasi, P.G.; Fattorini, D. Interactions between Metabolism of Trace Metals and Xenobiotic Agonists of the Aryl Hydrocarbon Receptor in the Antarctic Fish Trematomus Bernacchii: Environmental Perspectives. Environ. Toxicol. Chem. 2005, 24, 1475–1482. [Google Scholar] [CrossRef]
- van Hurk, P.D.; Faisal, M.; Roberts, M.H., Jr. Interactive Effects of Cadmium and Benzo[a]Pyrene on Metallothionein Induction in Mummichog (Fundulus Heteroclitus). Mar. Environ. Res. 2000, 50, 83–87. [Google Scholar] [CrossRef]
- Stohs, S.; Bagchi, D. Oxidative Mechanisms in the Toxicity of Metal Ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Oliveira, M.; Santos, M.A.; Pacheco, M. Glutathione Protects Heavy Metal-Induced Inhibition of Hepatic Microsomal Ethoxyresorufin O-Deethylase Activity in Dicentrarchus labrax L. Ecotoxicol. Environ. Saf. 2004, 58, 379–385. [Google Scholar] [CrossRef]
- Sutton, T.T.; Milligan, R.J. Deep-Sea Ecology. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–45. [Google Scholar]
- de Busserolles, F.; Marshall, N.J. Seeing in the Deep-Sea: Visual Adaptations in Lanternfishes. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160070. [Google Scholar] [CrossRef]
- Horodysky, A.Z.; Brill, R.W.; Crawford, K.C.; Seagroves, E.S.; Johnson, A.K. Comparative Visual Ecophysiology of Mid-Atlantic Temperate Reef Fishes. Biol. Open 2013, 2, 1371–1381. [Google Scholar] [CrossRef][Green Version]
- McClain, C.R.; Webb, T.J.; Nunnally, C.C.; Dixon, S.R.; Finnegan, S.; Nelson, J.A. Metabolic Niches and Biodiversity: A Test Case in the Deep Sea Benthos. Front. Mar. Sci. 2020, 7, 216. [Google Scholar] [CrossRef]
- Lazo, J.P.; Holt, J.G.; Arnold, C.R. Towards the Development of Suitable Microdiets for Substitution of Live Prey in the Rearing of Red Drum (Sciaenops ocellatus) Larvae: Applications of Studies on Digestive Physiology. Fish. Sci. 2002, 68, 888–891. [Google Scholar] [CrossRef][Green Version]
- Maruska, K.P.; Gelsleichter, J. Hormones and Reproduction in Chondrichthyan Fishes. In Hormones and Reproduction of Vertebrates; Elsevier: Amsterdam, The Netherlands, 2011; pp. 209–237. [Google Scholar]
- Robison, B.H. Deep Pelagic Biology. J. Exp. Mar. Biol. Ecol. 2004, 300, 253–272. [Google Scholar] [CrossRef]
- Downie, A.T.; Lefevre, S.; Illing, B.; Harris, J.; Jarrold, M.D.; McCormick, M.I.; Nilsson, G.E.; Rummer, J.L. Rapid Physiological and Transcriptomic Changes Associated with Oxygen Delivery in Larval Anemonefish Suggest a Role in Adaptation to Life on Hypoxic Coral Reefs. PLoS Biol. 2023, 21, e3002102. [Google Scholar] [CrossRef]
- Pan, Y.K.; Ern, R.; Morrison, P.R.; Brauner, C.J.; Esbaugh, A.J. Acclimation to Prolonged Hypoxia Alters Hemoglobin Isoform Expression and Increases Hemoglobin Oxygen Affinity and Aerobic Performance in a Marine Fish. Sci. Rep. 2017, 7, 7834. [Google Scholar] [CrossRef]
- Lavado, R.; Garcia de la Parra, L.M.; Escartfn, E.; Porte, C. Antioxidant Defences in Coastal and Deep-Sea Fish: A Comparative Study. Rapp. Comm. Int. Méditerranée 2004, 37, 385. [Google Scholar]
- Canals, M.; Puig, P.; de Madron, X.D.; Heussner, S.; Palanques, A.; Fabres, J. Flushing Submarine Canyons. Nature 2006, 444, 354–357. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine Biotechnology for Production of Food Ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Company, J.B.; Puig, P.; Sardà, F.; Palanques, A.; Latasa, M.; Scharek, R. Climate Influence on Deep Sea Populations. PLoS ONE 2008, 3, e1431. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Tyler, P.A.; Baker, M.C.; Bergstad, O.A.; Clark, M.R.; Escobar, E.; Levin, L.A.; Menot, L.; Rowden, A.A.; Smith, C.R.; et al. Man and the Last Great Wilderness: Human Impact on the Deep Sea. PLoS ONE 2011, 6, e22588. [Google Scholar] [CrossRef]
- Borghi, V.; Porte, C. Organotin Pollution in Deep-Sea Fish from the Northwestern Mediterranean. Environ. Sci. Technol. 2002, 36, 4224–4228. [Google Scholar] [CrossRef]
- Storelli, M.M.; Losada, S.; Marcotrigiano, G.O.; Roosens, L.; Barone, G.; Neels, H.; Covaci, A. Polychlorinated Biphenyl and Organochlorine Pesticide Contamination Signatures in Deep-Sea Fish from the Mediterranean Sea. Environ. Res. 2009, 109, 851–856. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Rotllant, G.; Ábalos, M.; Parera, J.; Dachs, J.; Company, J.B.; Calafat, A.; Abad, E. Accumulation of Dioxins in Deep-Sea Crustaceans, Fish and Sediments from a Submarine Canyon (NW Mediterranean). Prog. Oceanogr. 2013, 118, 260–272. [Google Scholar] [CrossRef]
- Siscar, R.; Koenig, S.; Torreblanca, A.; Solé, M. The Role of Metallothionein and Selenium in Metal Detoxification in the Liver of Deep-Sea Fish from the NW Mediterranean Sea. Sci. Total Environ. 2014, 466–467, 898–905. [Google Scholar] [CrossRef]
- Ribalta, C.; Sanchez-Hernandez, J.C.; Sole, M. Hepatic Biotransformation and Antioxidant Enzyme Activities in Mediterranean Fish from Different Habitat Depths. Sci. Total Environ. 2015, 532, 176–183. [Google Scholar] [CrossRef]
- D’Onghia, G.; Politou, C.Y.; Bozzano, A.; Lloris, D.; Rotllant, G.; Sión, L.; Mastrototaro, F. Deep-Water Fish Assemblages in the Mediterranean Sea. Sci. Mar. 2004, 68, 87–99. [Google Scholar] [CrossRef]
- Fanelli, E.; Papiol, V.; Cartes, J.; Rumolo, P.; López-Pérez, C. Trophic Webs of Deep-Sea Megafauna on Mainland and Insular Slopes of the NW Mediterranean: A Comparison by Stable Isotope Analysis. Mar. Ecol. Prog. Ser. 2013, 490, 199–221. [Google Scholar] [CrossRef]
- Fernandez-Arcaya, U.; Rotllant, G.; Ramirez-Llodra, E.; Recasens, L.; Aguzzi, J.; Flexas, M.M.; Sanchez-Vidal, A.; López-Fernández, P.; García, J.A.; Company, J.B. Reproductive Biology and Recruitment of the Deep-Sea Fish Community from the NW Mediterranean Continental Margin. Prog. Oceanogr. 2013, 118, 222–234. [Google Scholar] [CrossRef]
- Godin, D.V.; Garnett, M.E. Species-Related Variations in Tissue Antioxidant Status—I. Differences in Antioxidant Enzyme Profiles. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 737–742. [Google Scholar] [CrossRef]
- Janssens, B.J.; Childress, J.J.; Baguet, F.; Rees, J.-F. Reduced Enzymatic Antioxidative Defense in Deep-Sea Fish. J. Exp. Biol. 2000, 203, 3717–3725. [Google Scholar] [CrossRef]
- Koenig, S.; Fernández, P.; Company, J.B.; Huertas, D.; Solé, M. Are Deep-Sea Organisms Dwelling within a Submarine Canyon More at Risk from Anthropogenic Contamination than Those from the Adjacent Open Slope? A Case Study of Blanes Canyon (NW Mediterranean). Prog. Oceanogr. 2013, 118, 249–259. [Google Scholar] [CrossRef]
- Lemaire, B.; Priede, I.; Collins, M.; Bailey, D.; Schtickzelle, N.; Thomé, J.; Rees, J. Effects of Organochlorines on Cytochrome P450 Activity and Antioxidant Enzymes in Liver of Roundnose Grenadier Coryphaenoides Rupestris. Aquat. Biol. 2010, 8, 161–168. [Google Scholar] [CrossRef]
- Koenig, S.; Porte, C.; Solé, M.; Sturve, J. Biliary PAH and Alkylphenol Metabolites, Biomarker Enzyme Activities, and Gene Expression Levels in the Deep-Sea Fish Alepocephalus rostratus. Environ. Sci. Technol. 2013, 47, 2854–2861. [Google Scholar] [CrossRef]
- Ferro, D.; Franchi, N.; Mangano, V.; Bakiu, R.; Cammarata, M.; Parrinello, N.; Santovito, G.; Ballarin, L. Characterization and Metal-Induced Gene Transcription of Two New Copper Zinc Superoxide Dismutases in the Solitary Ascidian Ciona Intestinalis. Aquat. Toxicol. 2013, 140–141, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Ferro, D.; Bakiu, R.; Ballarin, L.; Santovito, G. Typical 2-Cys Peroxiredoxins as a Defense Mechanism against Metal-Induced Oxidative Stress in the Solitary Ascidian Ciona Robusta. Antioxidants 2022, 11, 93. [Google Scholar] [CrossRef]
- Lavut, A.; Raveh, D. Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability. PLoS Genet. 2012, 8, e1002527. [Google Scholar] [CrossRef] [PubMed]
- Nicorelli, E.; Gerdol, M.; Buonocore, F.; Pallavicini, A.; Scapigliati, G.; Guidolin, L.; Irato, P.; Corrà, F.; Santovito, G. First Evidence of T Cell Restricted Intracellular Antigen (TIA) Protein Gene Expression in Antarctic Fish. Invertebr. Surviv. J. 2018, 15, 127. [Google Scholar]
- Drago, L.; Peronato, A.; Franchi, N.; Ballarin, L.; Bakiu, R.; Santovito, G. Stress Granules in Ciona Robusta: First Evidences of TIA-1-Related Nucleolysin and Tristetraprolin Gene Expression under Metal Exposure. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2021, 243, 108977. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Ji, Q.; Song, J.; Wang, L.; Liu, B.; Wang, J.; Li, C. The Function of Apostichopus Japonicas Catalase in Sea Cucumber Intestinal Immunity. Aquaculture 2020, 521, 735103. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Zhang, H.; Liu, R.; Chen, S.; Lin, L. Analysis of Environmental Selection Pressure of Superoxide Dismutase in Deep-Sea Sea Cucumber. J. Oceanol. Limnol. 2024, 42, 893–904. [Google Scholar] [CrossRef]
- Andriashev, A.P.; Stein, D.L. Review of the Snailfish Genus Careproctus (Liparidae, Scorpaeniformes) in Antarctic and Adjacent Waters. Contrib. Sci. 1998, 470, 1–63. [Google Scholar] [CrossRef]
- Eastman, J.T.; Lannoo, M.J. Morphology of the Brain and Sense Organs in the Snailfish Paraliparis Devriesi: Neural Convergence and Sensory Compensation on the Antarctic Shelf. J. Morphol. 1998, 237, 213–236. [Google Scholar] [CrossRef]
- Jung, A.; Johnson, P.; Eastman, J.T.; DeVries, A.L. Protein Content and Freezing Avoidance Properties of the Subdermal Extracellular Matrix and Serum of the Antarctic Snailfish, Paraliparis Devriesi. Fish Physiol. Biochem. 1995, 14, 71–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakiu, R.; Piva, E.; Pacchini, S.; Santovito, G. Antioxidant Systems in Extremophile Marine Fish Species. J. Mar. Sci. Eng. 2024, 12, 1280. https://doi.org/10.3390/jmse12081280
Bakiu R, Piva E, Pacchini S, Santovito G. Antioxidant Systems in Extremophile Marine Fish Species. Journal of Marine Science and Engineering. 2024; 12(8):1280. https://doi.org/10.3390/jmse12081280
Chicago/Turabian StyleBakiu, Rigers, Elisabetta Piva, Sara Pacchini, and Gianfranco Santovito. 2024. "Antioxidant Systems in Extremophile Marine Fish Species" Journal of Marine Science and Engineering 12, no. 8: 1280. https://doi.org/10.3390/jmse12081280
APA StyleBakiu, R., Piva, E., Pacchini, S., & Santovito, G. (2024). Antioxidant Systems in Extremophile Marine Fish Species. Journal of Marine Science and Engineering, 12(8), 1280. https://doi.org/10.3390/jmse12081280






