Effects of Perforations on Internal Cathodic Protection and Recruitment of Marine Organisms to Steel Pipes
Abstract
:1. Introduction
Background
2. Materials and Methods
2.1. Overview
2.2. Pipe Configuration
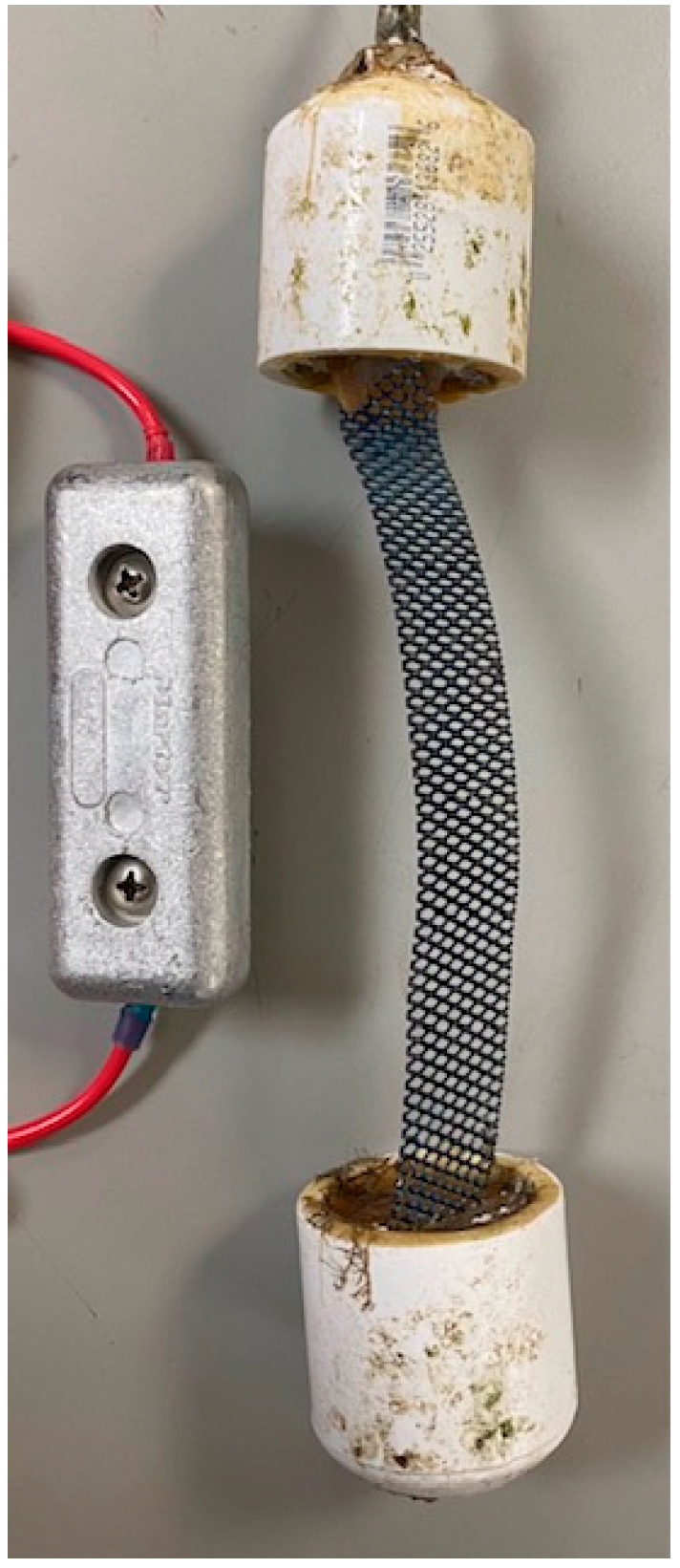
2.3. Arrangement of Cathodic Protection Systems
2.4. Data Collection
3. Results
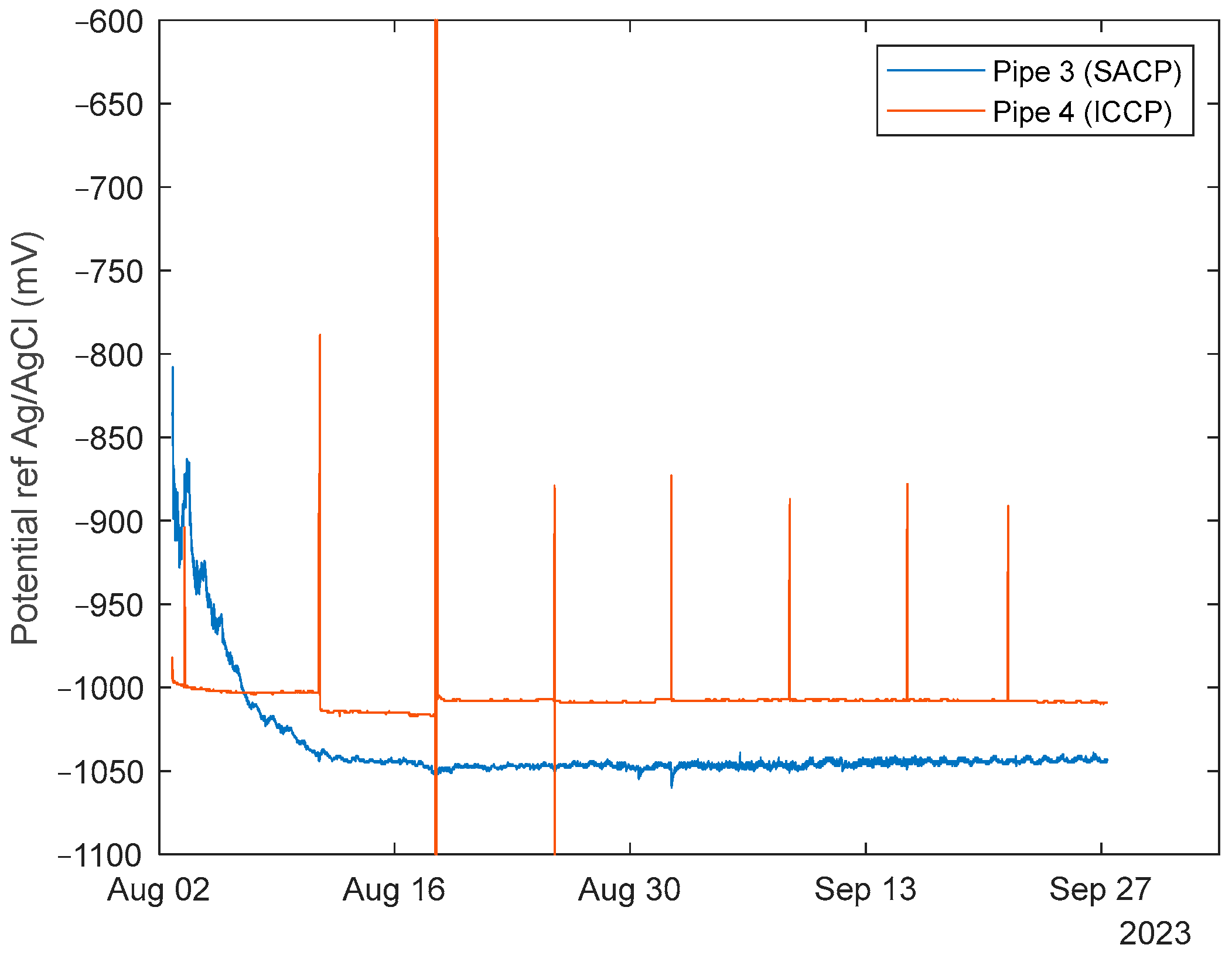
3.1. Internal Cathodic Protection Potentials
3.1.1. Sealed Pipes
3.1.2. Perforated Pipes
3.2. Internal Cathodic Protection Current Demand
3.2.1. Sealed Pipes
3.2.2. Perforated Pipes
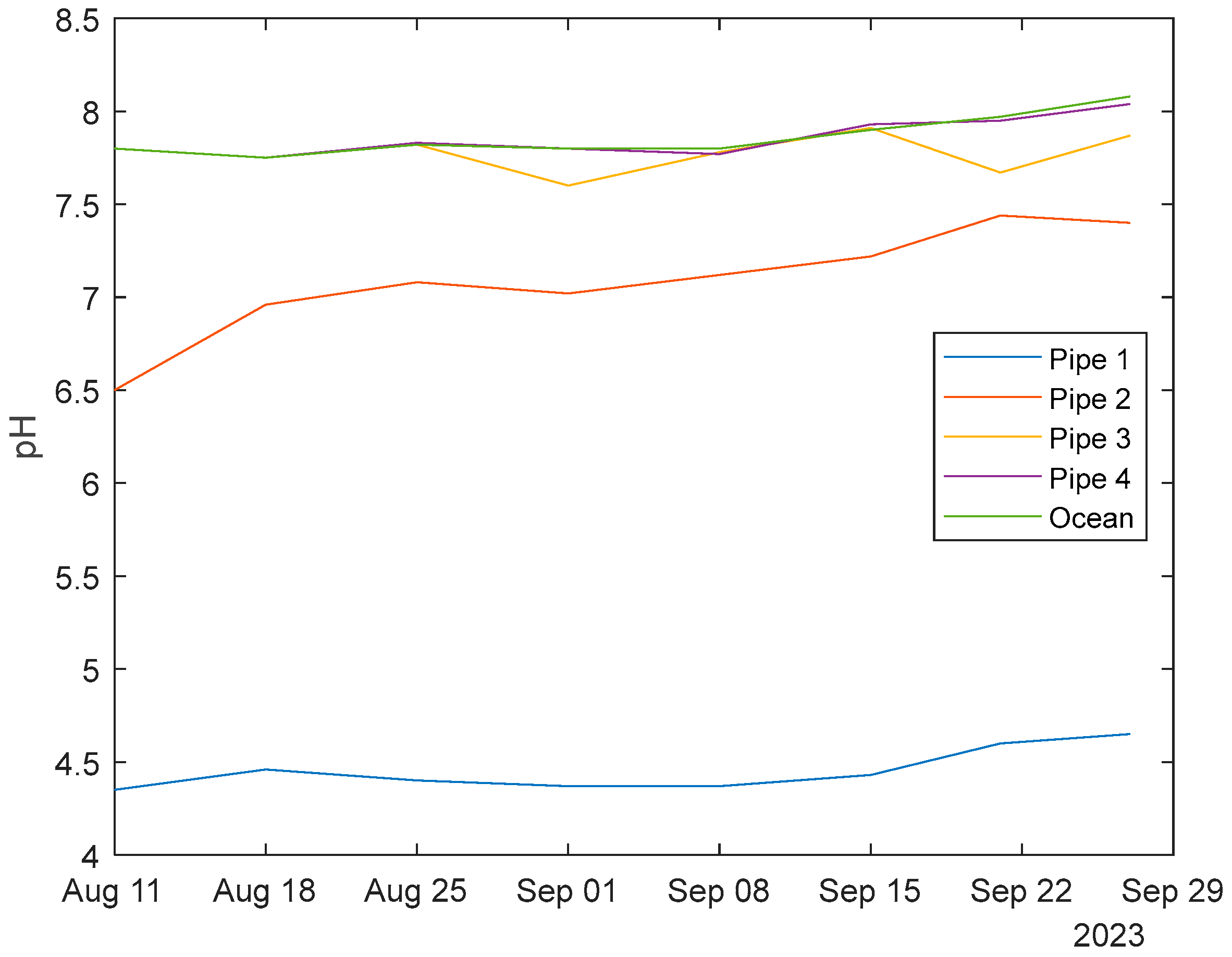
3.3. pH Readings
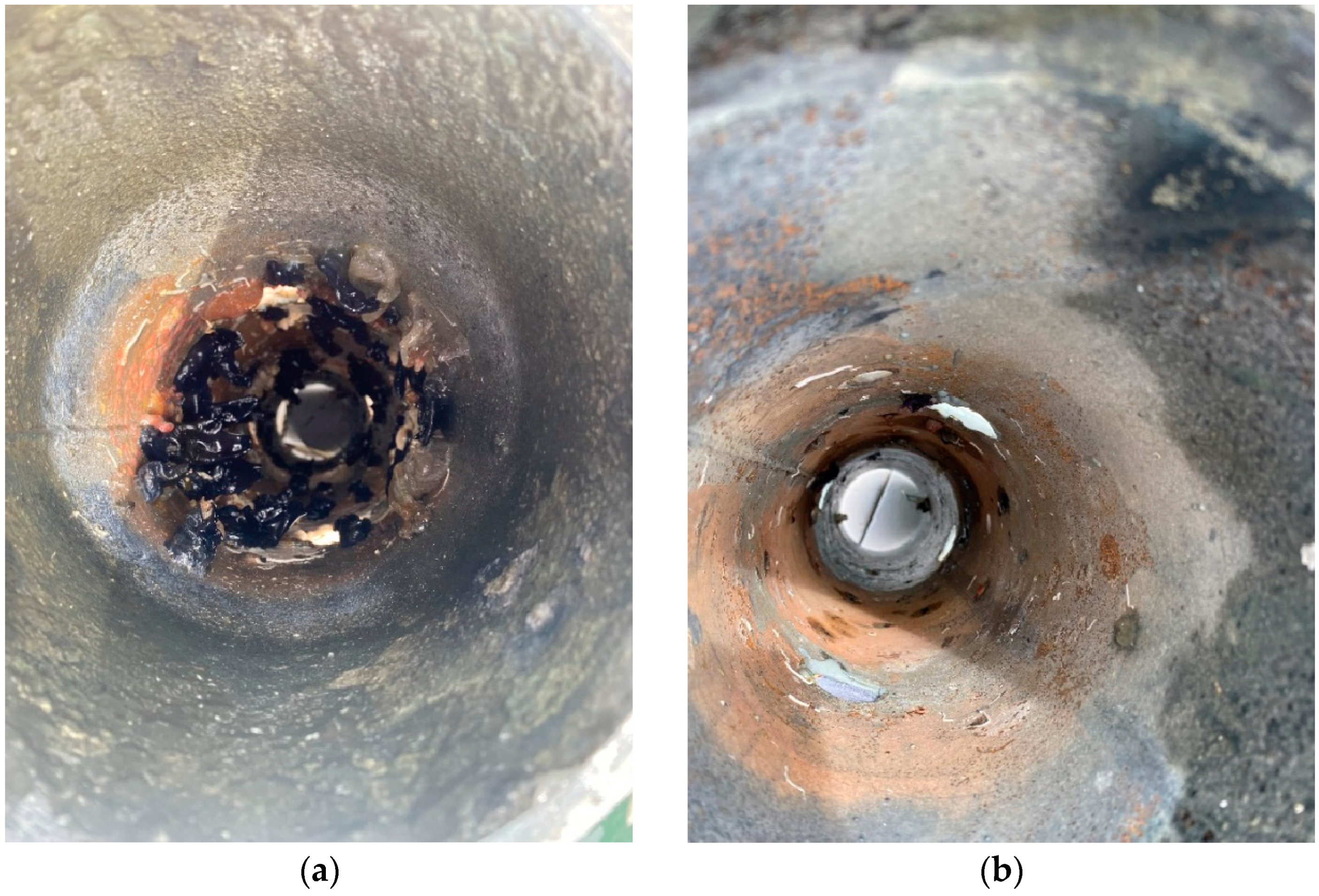
3.4. Corrosion Products and Elemental Analysis
3.4.1. Sealed Pipes
3.4.2. Perforated Pipes
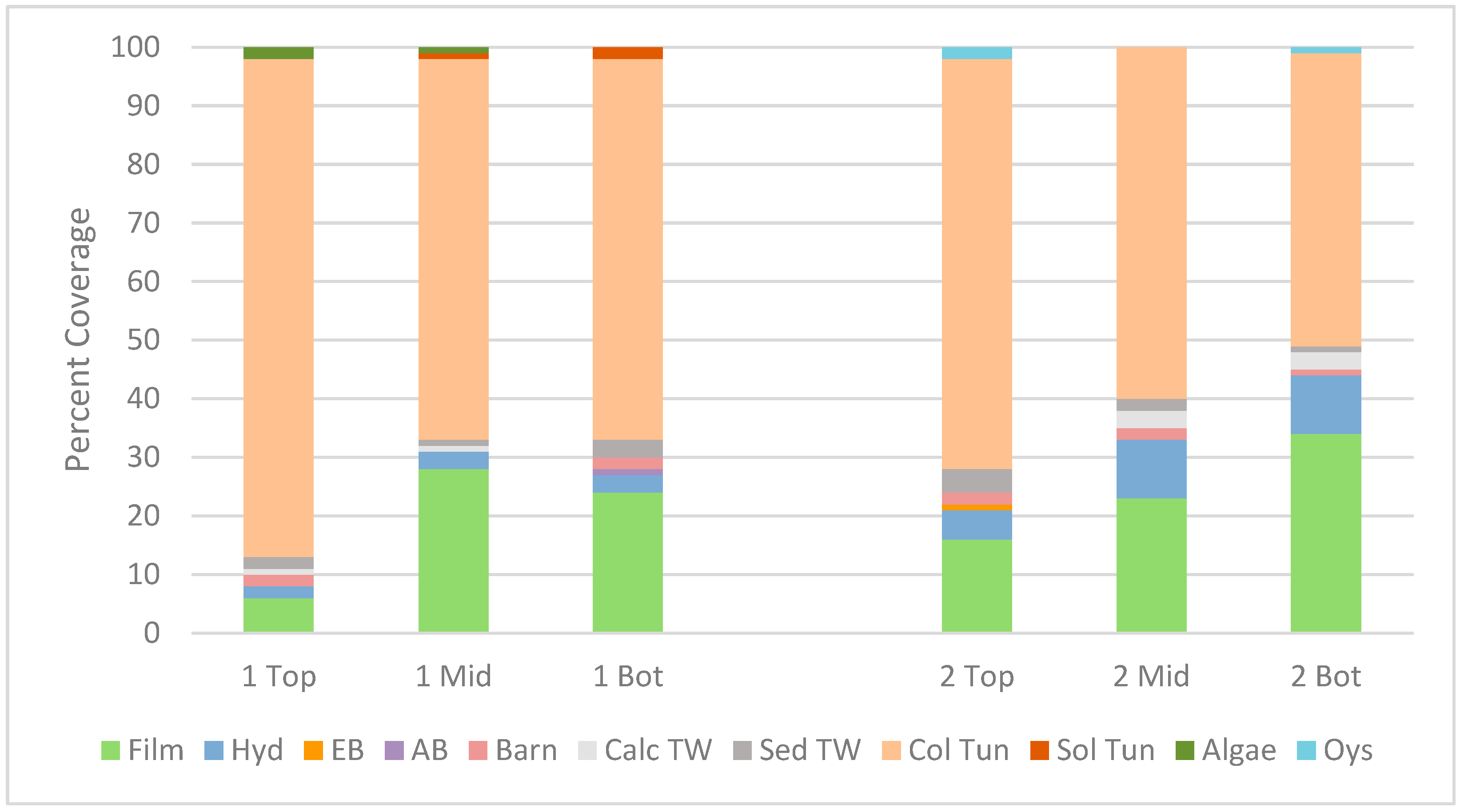
3.5. Marine Organism Recruitment
3.5.1. Internal Recruitment to Perforated Pipes
3.5.2. External Recruitment
4. Discussion
4.1. Internal Cathodic Protection and Water Chemistry
4.2. Internal Recruitment of Marine Organisms to the Perforated Pipes
4.3. Other Considerations of Perforation Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DNVGL-RP-B401; Cathodic Protection Design Recommended Practice. DNVGL: Bærum, Norway, 2017.
- Erdogan, C.; Swain, G. Conceptual sacrificial anode cathodic protection design for offshore wind monopiles. Ocn. Eng. 2021, 235, 109339. [Google Scholar] [CrossRef]
- Swain, G. A Guide to Developing a Biofouling Management Plan. Mar. Tech. Soc. J. 2017, 51, 105–110. [Google Scholar] [CrossRef]
- Maher, M. The Corrosion and Biofouling Characteristics of Sealed vs. Perforated Offshore Monopile Interiors: Experiment Design Comparing Corrosion and Environment Inside Steel Pipe. Master’s Thesis, Florida Institute of Technology, Melbourne, FL, USA, December 2018. [Google Scholar]
- Wassick, A.; Hunsucker, K.Z.; Swain, G. Measuring the recruitment and growth of biofouling communities using clear recruitment panels. Biofouling 2023, 39, 643–660. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Offshore Wind Market Report, 2023rd ed.; U.S. Department of Energy: Washington, DC, USA, 2023. [Google Scholar]
- Haselibozchaloee, D.; Correia, J.; Mendes, P.; de Jesus, A.; Berto, F. A review of fatigue damage assessment in offshore wind turbine structure. Int. J. Fatigue 2022, 164, 107145. [Google Scholar] [CrossRef]
- Price, S.J.; Figueira, R.B. Corrosion Protection Systems and Fatigue Corrosion in Offshore Wind Structures: Current Status and Future Perspectives. Coatings 2017, 7, 25. [Google Scholar] [CrossRef]
- Chaves, I.A.; Peterson, R.; Melchers, R.E.; Jeffrey, R. Corrosion of the interior steel surfaces of offshore monopiles. Ships Offshore Struct. 2024, 19, 125–133. [Google Scholar] [CrossRef]
- Black, A.R.; Mathiesen, T.; Hilbert, L.R. Corrosion Protection of Offshore Wind Foundations. In Proceedings of the CORROSION 2015, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Lomholt, T.N.; Mathiesen, T.; Egelund, S.; Bangsgaard, D.B. Unification of Corrosion Protection for Offshore Wind Farms—Collaboration in Partnerships. In Proceedings of the CORROSION 2018, Pheonix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Cherif, O. Impressed Current System for Internal Corrosion Protection in Monopiles for Offshore Windmills. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, NO, USA, 2022. [Google Scholar]
- Hilbert, L.R.; Black, A.R.; Andersen, F.; Mathiesen, T. Inspection and monitoring of corrosion inside monopile foundations for offshore wind turbines. In Proceedings of the EUROCORR 2011, Stockholm, Sweden, 4–8 September 2011. [Google Scholar]
- Krebs, D.I.T. ICCP System for Internal Protection of Monopiles for Offshore Wind Farms. In Proceedings of the CORROSION 2018, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Sunday, K.; Brennan, F. A review of offshore wind monopiles structural design achievements and challenges. Ocn. Eng. 2021, 235, 109409. [Google Scholar] [CrossRef]
- Jensen, K.S.; Petersen, S.J.; Pedersen, R.R. European offshore wind engineering—Past, present and future. Proc. Inst. Civ. Eng. 2018, 171, 159–165. [Google Scholar] [CrossRef]
- VGBE-S-021-01-2023-05-EN; Corrosion Protection for Offshore Wind Structures. 4th ed. vgbe energy e.V.: Essen, Germany, 2023.
- Kirchgeorg, T.; Weinberg, I.; Hornig, M.; Baier, R.; Schmid, M.J.; Brockmeyer, B. Emissions from corrosion protection systems of offshore wind farms: Evaluation of the potential impact on the marine environment. Mar. Poll. Bull. 2018, 136, 257–268. [Google Scholar] [CrossRef]
- Veronelli, L.A. Empirical and Computer Aided Design of Cathodic Protection Systems. Master’s Thesis, Pelitecnico di Milano, Milan, Italy, 5 October 2012. [Google Scholar]
- Delwiche, A.; Tavares, I. Retrofit Strategy using Aluminum Anodes for the Internal Section of Windturbine Monopiles. In Proceedings of the CORROSION 2017, New Orleans, LA, USA, 26–30 March 2017. [Google Scholar]
- Andersen, J.; Abrahamsen, R.; Andersen, T.L.; Andersen, M.T.; Baun, T.L.; Neubauer, J.L. Wave Load Mitigation by Perforation of Monopiles. J. Mar. Sci. Eng. 2020, 8, 352. [Google Scholar] [CrossRef]
- Elnikhely, E.A. Minimizing scour around bridge pile using holes. Ain Shams Eng. J. 2017, 8, 499–506. [Google Scholar] [CrossRef]
- Heaf, N.J. The Effect of Marine Growth on the Performance of Fixed Offshore Platforms in the North Sea. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April 1979. [Google Scholar] [CrossRef]
- Degraer, S.; Carey, D.A.; Coolen, J.W.P.; Hutchison, Z.L.; Kerckhof, F.; Rumes, B.; Vanaverbeke, J. Offshore Wind Farm Artificial Reefs Affect Ecosystem Structure and Functioning. Oceanography 2020, 33, 48–57. [Google Scholar] [CrossRef]
- Hutchison, Z.L.; Bartley, M.L.; Degraer, S.; English, P.; Khan, A.; Livermore, J.; Rumes, B.; King, J.W. Offshore Wind Energy and Benthic Habitat Changes. Oceanography 2020, 33, 58–69. [Google Scholar] [CrossRef]
- American Society for Testing and Materials D6990-20; Standard Practice for Evaluating Biofouling Resistance and Physical Performance of Marine Coating Systems. ASTM International: West Conshohocken, PA, USA, 2020.
- Arany, L.; Bhattacharya, S.; Macdonald, J.; Hogan, S.J. Design of monopiles for offshore wind turbines in 10 steps. Soil Dyn. Earthq. Eng. 2017, 92, 126–152. [Google Scholar] [CrossRef]
- Erdogan, C.; Swain, G. The Effects of Biofouling and Corrosion Products on Impressed Current Cathodic Protection System Design for Offshore Monopile Foundations. J. Mar. Sci. Eng. 2022, 10, 1670. [Google Scholar] [CrossRef]





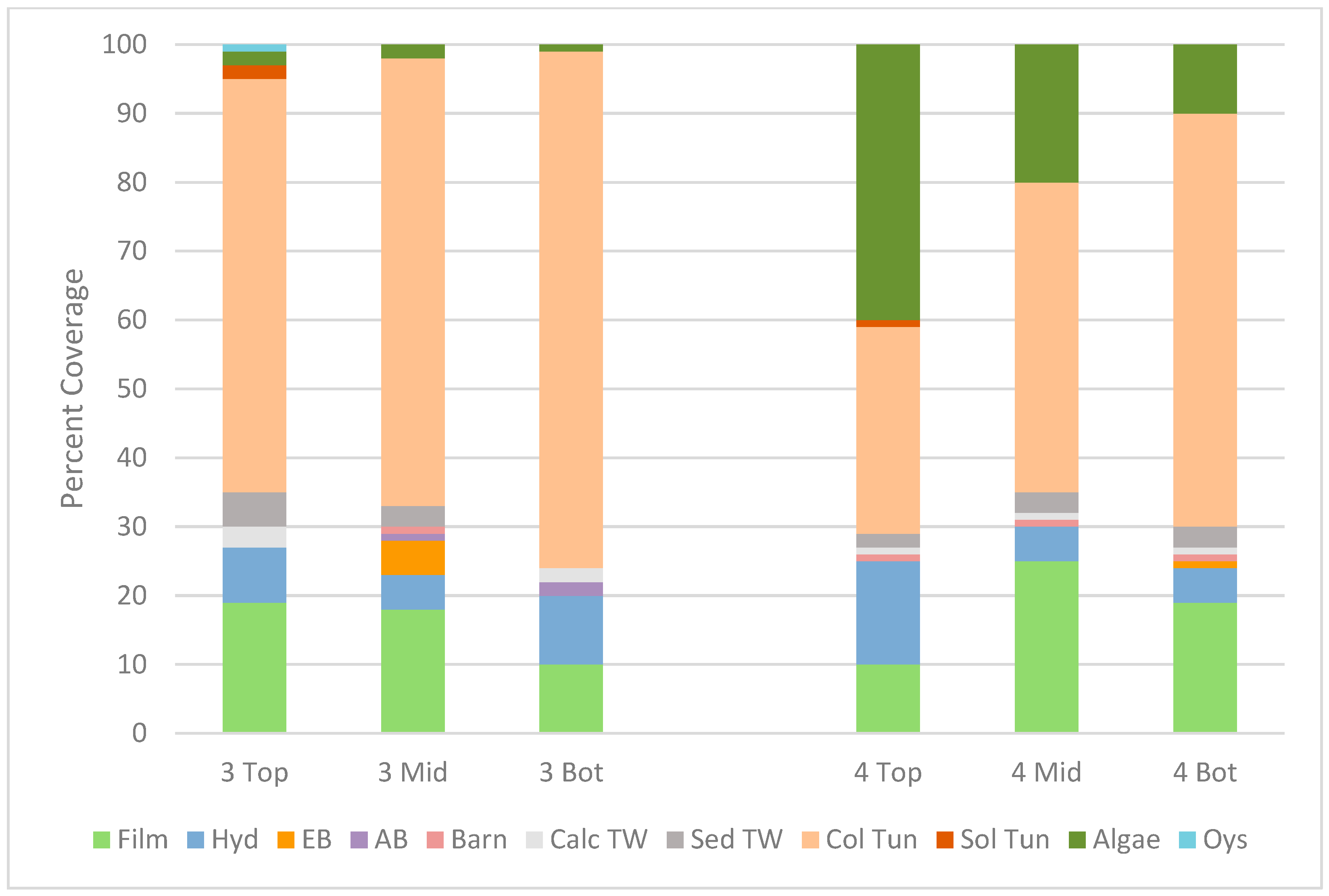
| Internal Coating | External Coating | |||||
|---|---|---|---|---|---|---|
| Pipe | Protection | Splash 1 | Immersed | Splash 1 | Immersed | |
| Sealed | 1 | Al SACP | x | x | ||
| 2 | ICCP | x | x | |||
| Perforated | 3 | Al SACP | x | x | x | |
| 4 | ICCP | x | x | x | ||
| 1B Coupon | 1B Loose Chalking | 1T Coupon | 1T Loose Chalking | 2B Chalking Layer | 2B Non-Chalking Area | 2T | 3B | 3T | 4B | 4T | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | 51.33 | 64.27 | 44.49 | 63.74 | 52.6 | 28.47 | 35.73 | 50.56 | 57.21 | 54.03 | 49.26 |
| Fe | 10.08 | 0.81 | 26.08 | 0.69 | 9.98 | 58.28 | 56.97 | 10.84 | 5.99 | 5.22 | 8.2 |
| Ca | 0 | 0.24 | 0 | 0 | 21.65 | 6.23 | 1.7 | 9.3 | 16.52 | 13.81 | 22 |
| Al | 11.41 | 22.39 | 6.96 | 20.16 | 1.88 | 0.15 | 0 | 5.51 | 5.36 | 4.1 | 1.46 |
| Mg | 8.34 | 3.45 | 12.96 | 3.14 | 2.71 | 0.84 | 0.93 | 6.67 | 5.44 | 4.81 | 1.36 |
| Si | 0.25 | 0 | 0 | 0.45 | 5.81 | 0.53 | 0.83 | 4.95 | 7.02 | 12.43 | 6.12 |
| Mo | 6.88 | 0 | 3.12 | 8.04 | 0 | 0 | 0 | 3.68 | 0 | 0 | 0 |
| Nb | 0 | 1.63 | 0 | 0 | 3.06 | 3.3 | 3.68 | 0 | 0 | 3.3 | 3.05 |
| Zn | 5.87 | 2.5 | 2.51 | 0.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Na | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.32 | 0 | 0.56 | 5.46 |
| Cl | 0 | 0.87 | 0 | 0.9 | 1.05 | 0.51 | 0.16 | 3.7 | 0.04 | 0 | 1.12 |
| Br | 5.84 | 0 | 0.99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S | 0 | 3.84 | 0 | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 0.45 |
| Co | 0 | 0 | 0 | 0 | 0.26 | 1.23 | 0 | 0.28 | 0.09 | 0.19 | 0.3 |
| K | 0 | 0 | 0 | 0 | 0.4 | 0 | 0 | 0.2 | 0.21 | 0.51 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paluzzi, A.; Swain, G.; DeFrancisci, J.; Kuchma, D.; Hansel, C.M. Effects of Perforations on Internal Cathodic Protection and Recruitment of Marine Organisms to Steel Pipes. J. Mar. Sci. Eng. 2024, 12, 1299. https://doi.org/10.3390/jmse12081299
Paluzzi A, Swain G, DeFrancisci J, Kuchma D, Hansel CM. Effects of Perforations on Internal Cathodic Protection and Recruitment of Marine Organisms to Steel Pipes. Journal of Marine Science and Engineering. 2024; 12(8):1299. https://doi.org/10.3390/jmse12081299
Chicago/Turabian StylePaluzzi, Alexander, Geoffrey Swain, John DeFrancisci, Daniel Kuchma, and Colleen M. Hansel. 2024. "Effects of Perforations on Internal Cathodic Protection and Recruitment of Marine Organisms to Steel Pipes" Journal of Marine Science and Engineering 12, no. 8: 1299. https://doi.org/10.3390/jmse12081299






