Feeding Habits of Scomber japonicus Inferred by Stable Isotope and Fatty Acid Analyses
Abstract
:1. Introduction
2. Materials and Methods
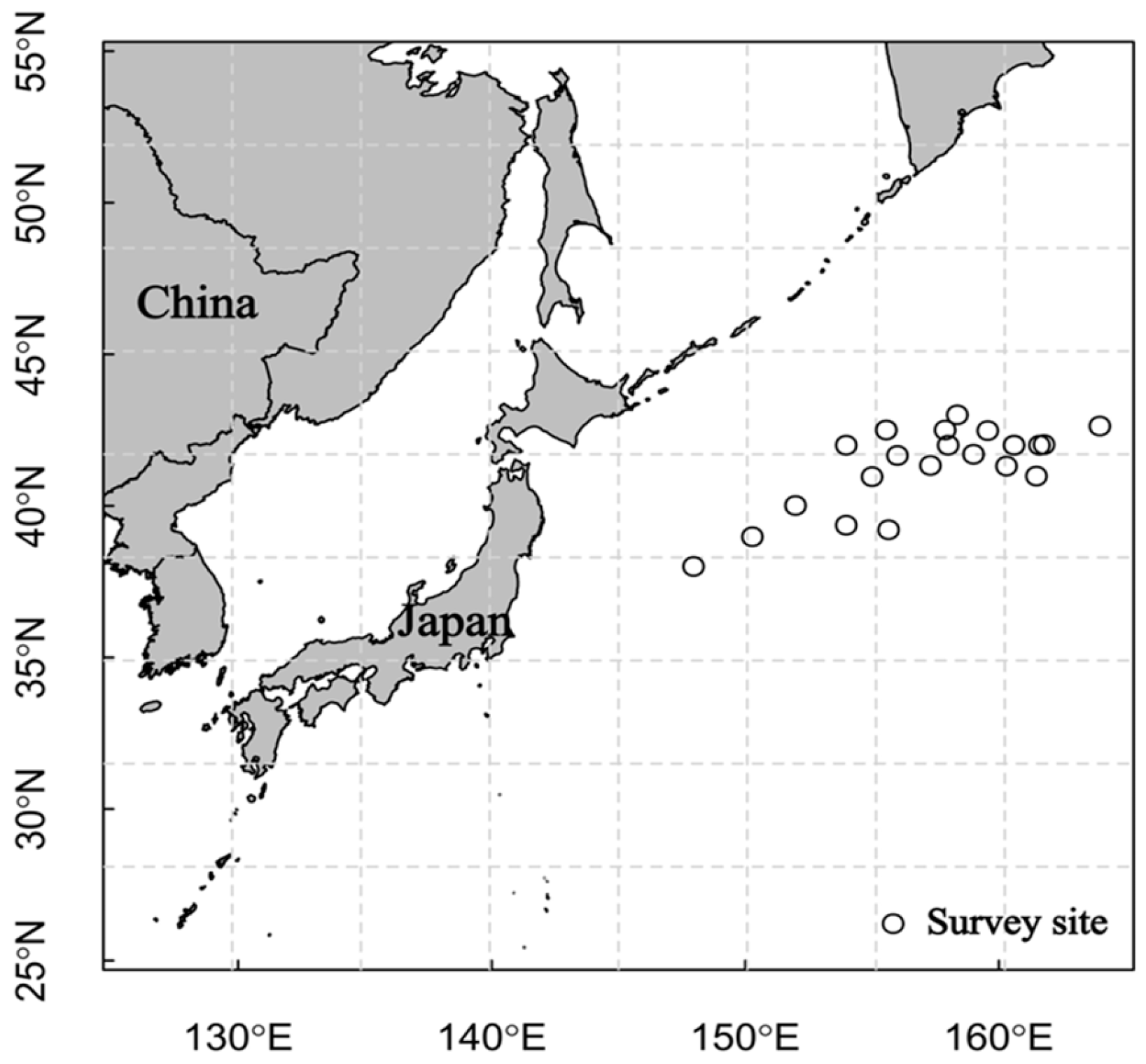
2.1. Sample Collection
2.2. Sample Processing
2.3. Stable Isotope Analysis
2.4. Fatty Acid Analysis
2.5. Trophic Level Calculation
2.6. Data Analysis
3. Results
3.1. Differences between Male and Female Individuals
3.2. Differences among Different Size Groups
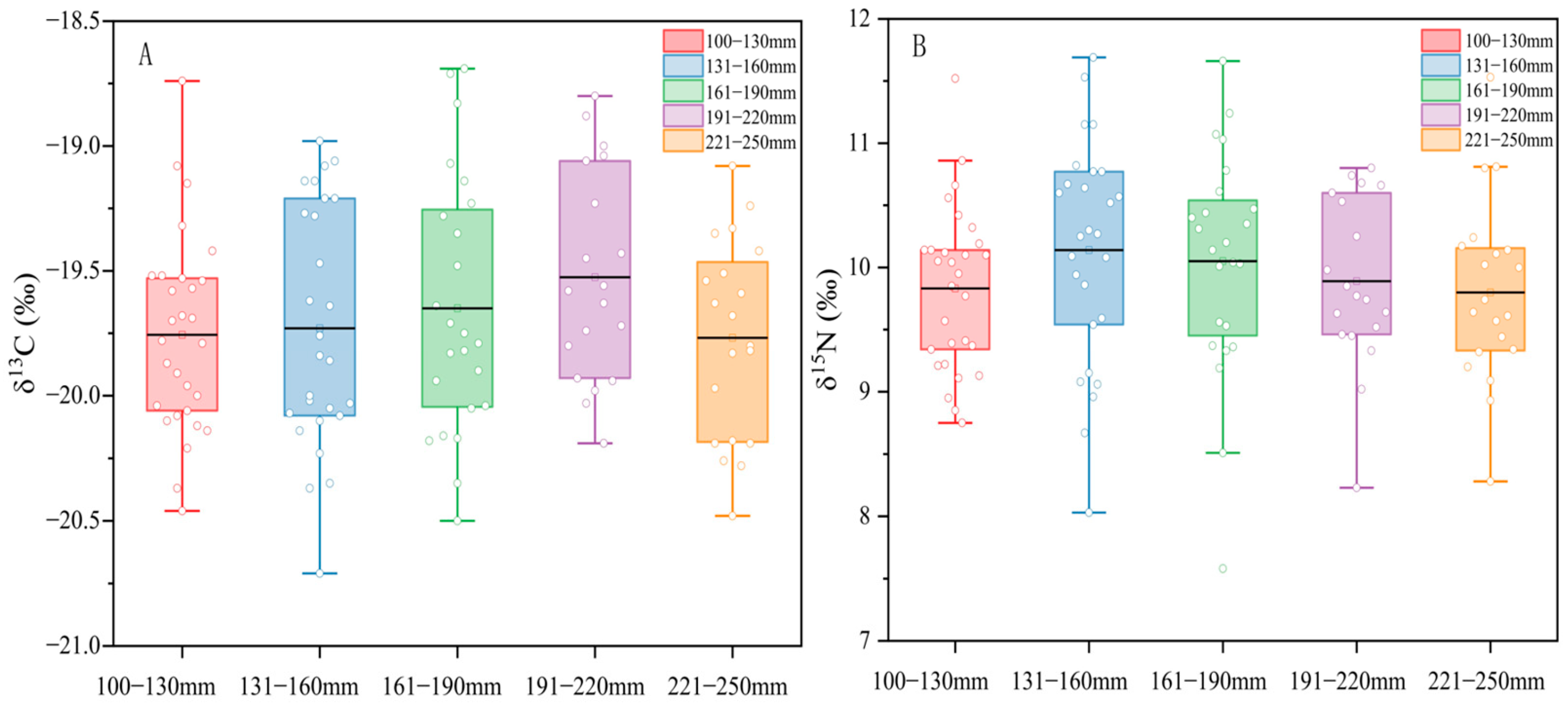
3.2.1. Differences in Stable Isotope Values among Different Size Groups
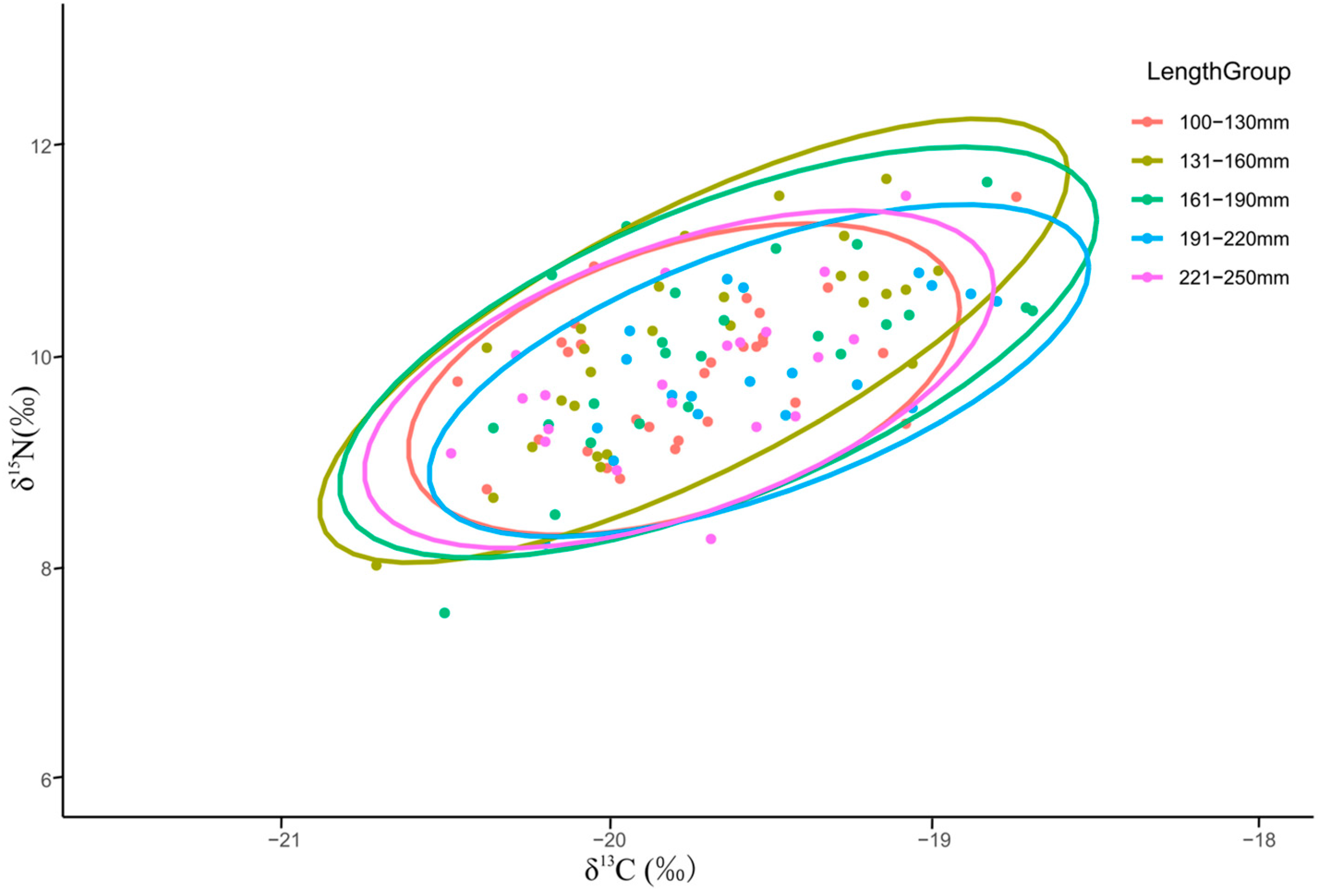
3.2.2. Niche Differences among Different Size Groups
3.3. Differences in Fatty Acids among Different Body Length Groups
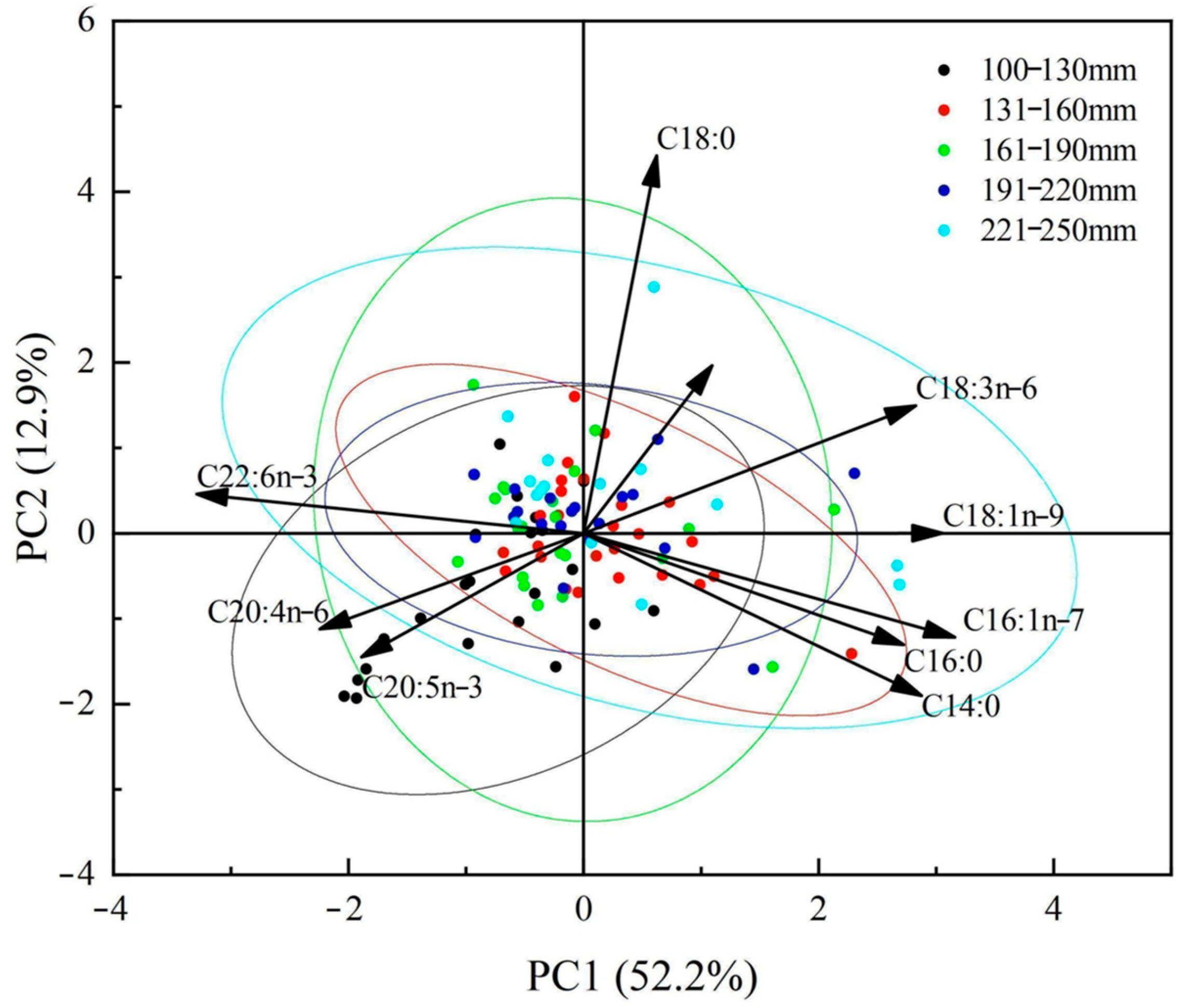
3.3.1. Fatty Acid Composition among Different Body Length Groups
3.3.2. Differences in Fatty Acid Composition among Different Body Length Groups
3.3.3. Characteristic Fatty Acids and Dietary Differences
4. Discussion
4.1. Differences between Males and Females
4.2. Differences among Different Size Groups
4.2.1. Stable Isotope Differences
4.2.2. Trophic Ecology of S. japonicus
4.3. Fatty Acid Analysis
4.3.1. Differences in Fatty Acid Composition among Different Body Length Groups
4.3.2. Dietary Differences
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Zhang, X.; He, Y.; Fan, W.; Tang, F. Stock assessment using length-based Bayesian evaluation method for three small pelagic species in the Northwest Pacific Ocean. Front. Mar. Sci. 2022, 9, 775180. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Xu, B.; Shi, Y.; Zhao, G.; Han, H. Age and Growth of Japanese Mackerel in the High Seas of the Northwest pacific ocean. Open J. Anim. Sci. 2021, 12, 91–102. [Google Scholar] [CrossRef]
- Pan, X.; Ye, Z.; Xu, B.; Jiang, T.; Yang, J.; Tian, Y. Population connectivity in a highly migratory fish, Japanese Spanish mackerel (Scomberomorus niphonius), along the Chinese coast, implications from otolith chemistry. Fish. Res. 2020, 231, 105690. [Google Scholar] [CrossRef]
- Papadimitraki, M.; Maar, K.; Jónasdóttir, S.H. Meso- and bathypelagic fish feeding ecology: A meta-analysis on fatty acids and stable isotope trophic studies. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2023, 198, 104083. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Baird, R.C. Diet of the hatchetfish Sternoptyx diaphana. Mar. Biol. 1973, 21, 34–46. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Taşbozan, O.; Gökçe, M.A. Fatty acids in fish. Fat. Acids 2017, 1, 143–159. [Google Scholar] [CrossRef]
- Su, M.; Liu, H.; Liang, X.; Gui, L.; Zhang, J. Dietary analysis of marine fish species: Enhancing the detection of prey-specific DNA sequences via high-throughput sequencing using blocking primers. Estuaries Coasts 2018, 41, 560–571. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Bode, A.; Alvarez-Ossorio, M.T.; Carrera, P.; Lorenzo, J. Reconstruction of trophic pathways between plankton and the North Iberian sardine (Sardina pilchardus) using stable isotopes. Sci. Mar. 2004, 68, 165–178. [Google Scholar] [CrossRef]
- Bode, A.; Carrera, P.; Lens, S. The pelagic foodweb in the upwelling ecosystem of Galicia (NW Spain) during spring: Natural abundance of stable carbon and nitrogen isotopes. ICES J. Mar. Sci. 2003, 60, 11–22. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, C.; Zhang, H.; Yin, F.; Zhang, S.; Wan, R.; Kitazawa, D. Review of estimating trophic relationships by quantitative fatty acid signature analysis. J. Mar. Sci. Eng. 2020, 8, 1030. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Hu, G.Y.; Chen, X.J.; Liu, B.L.; Liang, J.W. Studies on monthly difference of fatty acid composition and dietary indicator of Dosidicusgigasin offshore waters of Peru. Trans. Oceanol. Limnol. 2022, 44, 98–105. (In Chinese) [Google Scholar]
- Yang, Y.Y. Trophic Interactions in the Northern Slopeof the South China Sea through Fatty Acidand Stable Isotope Analyses; Shanghai Ocean University: Shanghai, China, 2016. (In Chinese) [Google Scholar]
- Huang, H.J. Feeding of Bigeye Tuna (Thunnusobesus) Inthe Tropical Eastern Pacific Ocean Baseon Biomarker Techniques; Shanghai Ocean University: Shanghai, China, 2021. (In Chinese) [Google Scholar]
- Xu, M. Trophic Niche Partitioning of Sympatricpelagic Sharks in the Tropical Eastern PacificInferred by Fatty Acid; Shanghai Ocean University: Shanghai, China, 2022. (In Chinese) [Google Scholar]
- Gao, X.D.; Chen, X.J.; Li, Y.K. A review on the methods used in aquatic food web research: Development and applications. J. Fish. Sci. China 2018, 25, 1347–1360. (In Chinese) [Google Scholar] [CrossRef]
- Rossi, S.; Sabatés, A.; Latasa, M.; Reyes, E. Lipid biomarkers and trophic linkages between phytoplankton, zooplankton and anchovy (Engraulis encrasicolus) larvae in the NW Mediterranean. J. Plankton Res. 2006, 28, 551–562. [Google Scholar] [CrossRef]
- Petursdottir, H.; Gislason, A.; Falk-Petersen, S.; Hop, H.; Svavarsson, J. Trophic interactions of the pelagic ecosystem over the Reykjanes Ridge as evaluated by fatty acid and stable isotope analyses. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 83–93. [Google Scholar] [CrossRef]
- Du, J.; Makatipu, P.C.; Tao, L.S.; Pauly, D.; Cheung, W.W.; Peristiwady, T.; Liao, J.; Chen, B. Comparing trophic levels estimated from a tropical marine food web using an ecosystem model and stable isotopes. Estuarine Coast. Shelf Sci. 2020, 233, 106518. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Hu, S.; Gong, Y. Trophic structure of micronekton in the Northwest Pacific Ocean. Front. Mar. Sci. 2022, 9, 971649. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of δ15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Dalsgaard, J.; John, M.S.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar] [CrossRef] [PubMed]
- Graeve, M.; Kattner, G.; Wiencke, C.; Karsten, U. Fatty acid composition of Arctic and Antarctic macroalgae: Indicator of phylogenetic and trophic relationships. Mar. Ecol. Prog. Ser. 2002, 231, 67–74. [Google Scholar] [CrossRef]
- Parrish, C.C.; Abrajano, T.A.; Budge, S.M.; Helleur, R.J.; Hudson, E.D.; Pulchan, K.; Ramos, C. Lipid and phenolic biomarkers in marine ecosystems: Analysis and applications. Mar. Chem. 2000, 5D, 193–223. [Google Scholar] [CrossRef]
- Martins, E.G.; Minke-Martin, V.; Patterson, D.A.; Power, M. The effects of inter- and intra-population variability on the use of adipose fin-muscle tissue comparisons for stable isotope studies of sockeye salmon. Fish. Res. 2019, 211, 148–157. [Google Scholar] [CrossRef]
- Reimchen, T.E.; Ingram, T.; Hansen, S.C. Assessing niche differences of sex, armour and asymmetry phenotypes using stable isotope analyses in Haida Gwaii sticklebacks. Behaviour 2008, 145, 561–577. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. The effect of season and sex on trophic levels of marine fishes. J. Fish Biol. 2008, 72, 1463–1487. [Google Scholar] [CrossRef]
- Muro-Torres, V.M.; Amezcua, F.; Green, L.; Payan, J.; Balart-Páez, E.F.; Amezcua-Linares, F. Ontogenetic shifts in feeding habits of orangemouth weakfish (Cynoscion xanthulus): From estuarine benthic feeder to marine nektivore top predator. Mar. Ecol. 2023, 44, e12735. [Google Scholar] [CrossRef]
- Baranek, E.; Heraud, C.; Larroquet, L.; Surget, A.; Lanuque, A.; Terrier, F.; Skiba-Cassy, S.; Jérôme, R. Long-term regulation of fat sensing in rainbow trout (Oncorhynchus mykiss) fed a vegetable diet from the first feeding: Focus on free fatty acid receptors and their signalling. Br. J. Nutr. 2024, 131, 1–16. [Google Scholar] [CrossRef]
- Usydus, Z.; Szlifder-Richert, J.; Adamczyk, M. Variations in proximate composition and fatty acid profiles of Baltic sprat (Sprattus sprattus balticus). Food Chem. 2012, 130, 97–103. [Google Scholar] [CrossRef]
- Rumolo, P.; Bonanno, A.; Barra, M.; Fanelli, E.; Calabrò, M.; Genovese, S.; Ferreri, R.; Mazzola, S.; Basilone, G. Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar. Environ. Res. 2016, 115, 65–77. [Google Scholar] [CrossRef]
- Perkins, M.J.; McDonald, R.A.; van Veen, F.J.F.; Kelly, S.D.; Rees, G.; Bearhop, S. Application of nitrogen and carbon stable isotopes (δ15N and δ13C) to quantify food chain length and trophic structure. PLoS ONE 2014, 9, e93281. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aderet, N.; Johnston, E.M.; Cravey, R.; Sandin, S.A. Revisiting the life history of yellowtail jack (Seriola dorsalis) in the Southern California Bight: New evidence for ontogenetic habitat shifts and regional differences in a changing environment. Fish. Bull. 2020, 118, 158–170. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C. Contributions of stable-isotope data to elucidating food webs of Mediterranean rocky littoral fishes. Oecologia 2000, 122, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hussey, N.E.; MacNeil, M.A.; Olin, J.A.; McMeans, B.C.; Kinney, M.J.; Chapman, D.D.; Fisk, A.T. Stable isotopes and elasmobranchs: Tissue types, methods, applications and assumptions. J. Fish Biol. 2012, 80, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wen, J.; Chen, X.; Li, G.; Li, Y.; Zhang, Z. Effects of climate variability on habitat range and distribution of chub mackerel in the east China sea. J. Ocean Univ. China 2021, 20, 1483–1494. [Google Scholar] [CrossRef]
- Kume, G.; Shigemura, T.; Okanishi, M.; Hirai, J.; Shiozaki, K.; Ichinomiya, M.; Komorita, T.; Habano, A.; Makino, F.; Kobari, T. Distribution, feeding habits, and growth of chub mackerel, Scomber japonicus, larvae during a high-stock period in the northern Satsunan area, southern Japan. Front. Mar. Sci. 2021, 8, 725227. [Google Scholar] [CrossRef]
- Ishikawa, K.; Watanabe, C.; Kameda, T.; Tokeshi, T.; Horie, H.; Hashida, D.; Ookawa, T.; Takeda, T.; Kuno, M.; Suzuki, Y.; et al. Spatiotemporal variability in the occurrence of juvenile Japanese jack mackerel Trachurus japonicus along coastal areas of the Kuroshio Current. Fish. Oceanogr. 2021, 30, 569–583. [Google Scholar] [CrossRef]
- Zarzo-Arias, A.; Uhl, B.; Maynard, D.S.; Morales, M.B. Editorial: The ecological niche at different spatial scales. Front. Ecol. Evol. 2023, 11, 1296340. [Google Scholar] [CrossRef]
- Endo, C.; Watanabe, K. Morphological variation associated with trophic niche expansion within a lake population of a benthic fish. PLoS ONE 2020, 15, e0232114. [Google Scholar] [CrossRef] [PubMed]
- Roches, S.D.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Govaert, L.; Hendry, A.P.; Fattahi, F.; Möst, M. Quantifying interspecific and intraspecific diversity effects on ecosystem functioning. Ecology 2024, 105, e4199. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.H.; Dai, S.W.; Fang, W. Study on the stomach contents of Japanese mackerel (Scomber japonicus) in the high seas of the Northwest Pacific and its feeding grade. China Agric. Sci. Technol. Rev. 2020, 22, 138–148. [Google Scholar] [CrossRef]
- Xue, Y.; Jin, X.; Zhang, B.; Liang, Z. Seasonal, diel and ontogenetic variation in feeding patterns of small yellow croaker in the central Yellow Sea. J. Fish Biol. 2005, 67, 33–50. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Svanbäck, R.; I Bolnick, D. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B Biol. Sci. 2007, 274, 839–844. [Google Scholar] [CrossRef]
- Nemova, N.N.; Nefedova, Z.A.; Murzina, S.A.; Veselov, A.E.; Ripatti, P.O.; Pavlov, D.S. The effect of environmental conditions on the dynamics of fatty acids in juveniles of the Atlantic salmon (Salmo salar L.). Russ. J. Ecol. 2015, 46, 267–271. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Killen, S.S.; Glazier, D.S.; Rezende, E.L.; Clark, T.D.; Atkinson, D.; Willener, A.S.T.; Halsey, L.G. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 2016, 187, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Barbeaux, S.J.; Holsman, K.; Zador, S. Marine heatwave stress test of ecosystem-based fisheries management in the gulf of alaska pacific cod fishery. Front. Mar. Sci. 2020, 7, 703. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, Z.; Liu, B.; Lin, D.; Liang, J.; Fang, Z.; Chen, X. Fatty acid profile of jumbo squid (Dosidicus gigas) off the Peruvian exclusive economic zone: Revealing the variability of feeding strategies. Fishes 2022, 7, 221. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Xie, C.; Li, D.; Xu, J.; Zhang, M.; Zhang, M. Lipid contents, fatty acid profiles and nutritional quality of nine wild caught freshwater fish species of the Yangtze Basin, China. J. Food Nutr. Res. 2014, 2, 388–394. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ju, X.R.; Zhou, G.H. Classification and physiological functions of saturated fatty acids. Chin. Fats Oils 2008, 33, 35–39. (In Chinese) [Google Scholar]
- Xie, R.T.; Huang, K.; Xiao, X.; Zhao, X.; Zhang, S.; Lu, H. Analysis of muscle fatty acid composition of juvenile Pelteobagrus fulvidraco Jiang and parent fish of both sexes. Guangxi Agric. Sci. 2009, 40, 911–914. (In Chinese) [Google Scholar]
- Gao, C.X. Trophic Structure of Offshore Fishery Biomes in South-Central Zhejiang Based on Stable Isotope Technique; Shanghai Ocean University: Shanghai, China, 2020. (In Chinese) [Google Scholar]
- Koven, W.; Barr, Y.; Lutzky, S.; Ben-Atia, I.; Weiss, R.; Harel, M.; Behrens, P.; Tandler, A. The effect of dietary arachidonic acid (20:4n−6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae. Aquaculture 2001, 193, 107–122. [Google Scholar] [CrossRef]
- Castell, J.; Blair, T.; Neil, S.; Howes, K.; Mercer, S.; Reid, J.; Young-Lai, W.; Gullison, B.; Dhert, P.; Sorgeloos, P. The effect of different HUFA enrichment emulsions on the nutritional value of rotifers (Brachionus plicatilis) fed to larval haddock (Melanogrammus aeglefinus). Aquac. Int. 2003, 11, 109–117. [Google Scholar] [CrossRef]
- Shi, Y.H.; Xu, J.B.; Liu, Y.S.; Zhang, H.M.; Deng, P.P.; Lu, G.H.; Zhang, Z.F. Changes in fatty acid composition of the chrysanthemum yellow oriental triggerfish during early development. J. Aquat. Sci. 2017, 41, 1203–1212. (In Chinese) [Google Scholar]
- Lu, S.F.; Zhao, N.; Liu, H.B.; Hu, R.G. Changes in fatty acid composition of fertilised eggs and fish during early developmental stages of Pelteobagrus fulvidraco. J. Aquat. Sci. 2008, 5, 711–716. (In Chinese) [Google Scholar]
- Dall, W.; Chandumpai, A.; Smith, D.M. The fate of some 14C-labelled dietary lipids in the tiger prawn Penaeus esculentus. Mar. Biol. 1993, 115, 39–45. [Google Scholar] [CrossRef]
- Dickey-Collas, M.; Geffen, A.J. Importance of the fatty acids 20: 5ω 3 and 22: 6ω 3 in the diet of plaice (Pleuronectes platessa) larvae. Mar. Biol. 1992, 113, 463–468. [Google Scholar] [CrossRef]
- Hulbert, A.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Theor. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.; Frost, K.; Lowry, L. Fatty acid signatures reveal fine scale structure of foraging distribution of harbor seals and their prey in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 1997, 151, 255–271. [Google Scholar] [CrossRef]
- Gonçalves, A.; Azeiteiro, U.; Pardal, M.; De Troch, M. Fatty acid profiling reveals seasonal and spatial shifts in zooplankton diet in a temperate estuary. Estuarine Coast. Shelf Sci. 2012, 109, 70–80. [Google Scholar] [CrossRef]
- Liu, M.T. Indicative Role of Fatty Acid Markers in the Study of Trophic Relationships in the Yellow Sea Ecosystem; Graduate School of Chinese Academy of Sciences (Institute of Oceanography): Qingdao, China, 2010. (In Chinese) [Google Scholar]
- Iverson, S.J. Tracing aquatic food webs using fatty acids: From qualitative indicators to quantitative determination. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; pp. 281–308. [Google Scholar] [CrossRef]
- Mejri, S.C.; Tremblay, R.; Audet, C.; Wills, P.S.; Riche, M. Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles. Front. Mar. Sci. 2021, 8, 680003. [Google Scholar] [CrossRef]

| Species | Sex/BL | Number | δ15N (‰) | δ15C (‰) | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | p | Range | Mean ± SD | p | |||
| S. japonicus | Male | 70 | 7.58~11.69 | 10 ± 0.79 | 0.684 | −20.50~−18.69 | −19.65 ± 0.45 | 0.331 |
| Female | 49 | 8.03~11.98 | 10.02 ± 0.76 | −20.71~−18.43 | −19.62 ± 0.42 | |||
| 100–130 | 29 | 8.75~11.52 | 9.83 ± 0.65 | >0.05 | −20.46~−18.74 | −19.76 ± 0.39 | >0.05 | |
| 131–160 | 27 | 8.03~11.69 | 10.14 ± 0.9 | −20.71~−18.98 | −19.73 ± 0.49 | |||
| 161–190 | 24 | 7.58~11.66 | 10.05 ± 0.9 | −20.50~−18.69 | −19.65 ± 0.51 | |||
| 191–220 | 19 | 8.23~10.8 | 9.89 ± 0.68 | −20.19~−18.80 | −19.53 ± 0.42 | |||
| 221–250 | 20 | 8.28~11.53 | 9.8 ± 0.74 | −20.48~−19.08 | −19.77 ± 0.40 | |||
| Fatty Acid | Female/% | Male/% | p |
|---|---|---|---|
| C14:0 | 1.92 ± 1.52 | 1.71 ± 1.41 | 0.39 |
| C15:0 | 0.46 ± 0.21 | 0.42 ± 0.17 | 0.27 |
| C16:0 | 25.30 ± 8.36 | 22.45 ± 7.84 | 0.13 |
| C17:0 | 0.76 ± 0.27 | 0.74 ± 0.25 | 0.61 |
| C18:0 | 4.53 ± 0.94 | 4.63 ± 1.44 | 0.63 |
| C20:0 | 0.08 ± 0.05 | 0.08 ± 0.08 | 0.87 |
| C21:0 | 0.05 ± 0.13 | 0.03 ± 0.02 | 0.14 |
| C22:0 | 0.03 ± 0.05 | 0.03 ± 0.07 | 0.97 |
| C23:0 | 0.04 ± 0.10 | 0.02 ± 0.03 | 0.20 |
| C24:0 | 0.03 ± 0.04 | 0.03 ± 0.06 | 0.66 |
| ΣSFA | 33.20 ± 11.67 | 30.14 ± 11.37 | 0.35 |
| C14:1n-5 | 0.05 ± 0.10 | 0.03 ± 0.04 | 0.10 |
| C15:1n-5 | 0.29 ± 0.27 | 0.30 ± 0.26 | 0.07 |
| C16:1n-7 | 3.39 ± 2.10 | 2.78 ± 1.97 | 0.09 |
| C17:1n-7 | 0.45 ± 0.60 | 0.33 ± 0.20 | 0.07 |
| C18:1n-9 | 6.13 ± 4.12 | 4.72 ± 2.85 | 0.20 |
| C22:1n-9 | 0.27 ± 0.34 | 0.20 ± 0.26 | 0.13 |
| C24:1n-9 | 0.39 ± 0.38 | 0.33 ± 0.31 | 0.30 |
| C20:1 | 0.85 ± 0.60 | 0.72 ± 0.60 | 0.12 |
| ΣMUFA | 11.82 ± 8.51 | 9.41 ± 6.49 | 0.41 |
| C18:2n-6 | 1.00 ± 0.35 | 1.05 ± 0.31 | 0.33 |
| C18:3n-3 | 0.26 ± 0.14 | 0.31 ± 0.24 | 0.22 |
| C18:3n-6 | 6.11 ± 1.86 | 5.38 ± 2.00 | 0.17 |
| C20:2 | 0.34 ± 0.29 | 0.28 ± 0.13 | 0.18 |
| C20:3n-3 | 0.56 ± 0.62 | 0.54 ± 0.33 | 0.66 |
| C20:3n-6 | 0.46 ± 0.33 | 0.45 ± 0.18 | 0.71 |
| ARAC20:4n-6 | 7.61 ± 2.95 | 8.17 ± 3.04 | 0.26 |
| EPAC20:5n-3 | 2.42 ± 2.47 | 2.80 ± 3.03 | 0.41 |
| C22:2n-6 | 0.26 ± 0.37 | 0.20 ± 0.26 | 0.24 |
| DHAC22:6n-3 | 35.33 ± 12.90 | 40.68 ± 12.79 | 0.10 |
| ΣPUFA | 54.35 ± 22.28 | 59.86 ± 22.31 | 0.08 |
| Species | Isotope | Variable | Degree of Freedom | F | p | DE% |
|---|---|---|---|---|---|---|
| S. japonicus | δ13C | BL | 4 | 10.09 | 0.33 | 37.6% |
| δ15N | BL | 4 | 1.902 | 0.844 | 58.4% |
| Body Length (mm) | 100–130 | 131–160 | 161–190 | 191–220 | 221–250 |
|---|---|---|---|---|---|
| 100–130 | 0.653‰2 | ||||
| 131–160 | 0.63 | 0.895‰2 | |||
| 161–190 | 0.63 | 0.79 | 0.901‰2 | ||
| 191–220 | 0.72 | 0.55 | 0.69 | 0.735‰2 | |
| 221–250 | 0.89 | 0.71 | 0.70 | 0.66 | 0.680‰2 |
| Fatty Acid | 100–130 mm | 131–160 mm | 161–190 mm | 191–220 mm | 221–250 mm | DF | F | p |
|---|---|---|---|---|---|---|---|---|
| C14:0 | 1.26 ± 0.78 | 2.34 ± 1.97 | 1.82 ± 1.25 | 1.34 ± 1.37 | 1.26 ± 1.03 | 4 | 2.95 | <0.05 * |
| C15:0 | 0.39 ± 0.17 | 0.51 ± 0.16 | 0.40 ± 0.13 | 0.35 ± 0.17 | 0.47 ± 0.23 | 4 | 3.30 | <0.05 * |
| C16:0 | 19.02 ± 4.89 | 25.00 ± 4.26 | 18.92 ± 7.14 | 22.55 ± 5.90 | 24.32 ± 9.28 | 4 | 5.23 | <0.01 ** |
| C17:0 | 0.78 ± 0.19 | 0.93 ± 0.27 | 0.77 ± 0.19 | 0.62 ± 0.16 | 0.68 ± 0.24 | 4 | 6.62 | <0.01 ** |
| C18:0 | 4.36 ± 1.02 | 4.93 ± 0.84 | 4.69 ± 1.83 | 4.35 ± 0.69 | 4.75 ± 1.05 | 4 | 1.17 | 0.33 |
| C20:0 | 0.10 ± 0.05 | 0.08 ± 0.03 | 0.09 ± 0.14 | 0.06 ± 0.05 | 0.08 ± 0.05 | 4 | 0.80 | 0.53 |
| C21:0 | 0.06 ± 0.17 | 0.02 ± 0.01 | 0.03 ± 0.04 | 0.04 ± 0.06 | 0.06 ± 0.08 | 4 | 0.68 | 0.61 |
| C22:0 | 0.03 ± 0.06 | 0.02 ± 0.01 | 0.04 ± 0.13 | 0.02 ± 0.01 | 0.04 ± 0.04 | 4 | 0.87 | 0.49 |
| C23:0 | 0.04 ± 0.14 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.05 ± 0.05 | 4 | 0.85 | 0.50 |
| C24:0 | 0.03 ± 0.05 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.03 | 0.11 ± 0.14 | 4 | 0.82 | 0.51 |
| ΣSFA | 26.07 ± 7.52 | 33.36 ± 6.85 | 26.27 ± 10.65 | 29.37 ± 8.45 | 32.90 ± 13.13 | 4 | 5.34 | <0.05 * |
| C14:1n-5 | 0.04 ± 0.11 | 0.02 ± 0.01 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0.11 ± 0.14 | 4 | 3.44 | <0.05 * |
| C15:1n-5 | 0.16 ± 0.23 | 0.28 ± 0.19 | 0.49 ± 0.33 | 0.36 ± 0.19 | 0.44 ± 0.31 | 4 | 6.51 | <0.01 ** |
| C16:1n-7 | 1.66 ± 1.37 | 3.22 ± 1.96 | 2.30 ± 1.36 | 2.62 ± 0.99 | 3.58 ± 2.12 | 4 | 5.39 | <0.01 ** |
| C17:1n-7 | 0.26 ± 0.29 | 0.34 ± 0.17 | 0.34 ± 0.22 | 0.30 ± 0.16 | 0.69 ± 1.05 | 4 | 2.80 | <0.05 * |
| C18:1n-9 | 2.56 ± 1.62 | 4.71 ± 2.78 | 4.37 ± 2.19 | 4.98 ± 2.18 | 6.12 ± 3.03 | 4 | 7.43 | <0.01 ** |
| C22:1n-9 | 0.23 ± 0.40 | 0.24 ± 0.31 | 0.31 ± 0.43 | 0.15 ± 0.08 | 0.32 ± 0.28 | 4 | 6.89 | 0.49 |
| C24:1n-9 | 0.30 ± 0.33 | 0.28 ± 0.19 | 0.32 ± 0.39 | 0.27 ± 0.23 | 0.64 ± 0.61 | 4 | 3.84 | <0.01 ** |
| C20:1 | 0.39 ± 0.32 | 0.77 ± 0.50 | 0.79 ± 0.54 | 0.74 ± 0.50 | 1.13 ± 0.59 | 4 | 6.89 | <0.01 ** |
| ΣMUFA | 5.60 ± 4.67 | 9.86 ± 6.11 | 8.95 ± 5.49 | 9.45 ± 4.36 | 13.03 ± 8.13 | 4 | 2.34 | <0.01 ** |
| C18:2n-6 | 0.94 ± 0.26 | 1.06 ± 0.44 | 1.12 ± 0.47 | 1.02 ± 0.23 | 1.11 ± 0.20 | 4 | 1.12 | 0.35 |
| C18:3n-3 | 0.33 ± 0.33 | 0.25 ± 0.18 | 0.22 ± 0.14 | 0.27 ± 0.15 | 0.29 ± 0.15 | 4 | 0.91 | 0.46 |
| C18:3n-6 | 3.45 ± 1.57 | 6.66 ± 1.20 | 6.26 ± 2.10 | 5.81 ± 1.96 | 5.60 ± 1.34 | 4 | 15.26 | <0.01 ** |
| C20:2 | 0.28 ± 0.20 | 0.30 ± 0.13 | 0.36 ± 0.28 | 0.30 ± 0.14 | 0.39 ± 0.38 | 4 | 0.89 | 0.47 |
| C20:3n-3 | 0.84 ± 0.76 | 0.48 ± 0.21 | 0.65 ± 0.48 | 0.52 ± 0.48 | 0.50 ± 0.33 | 4 | 2.34 | 0.06 |
| C20:3n-6 | 0.57 ± 0.48 | 0.38 ± 0.07 | 0.45 ± 0.19 | 0.48 ± 0.26 | 0.45 ± 0.15 | 4 | 1.68 | 0.16 |
| ARAC20:4n-6 | 7.65 ± 3.60 | 8.52 ± 2.15 | 8.92 ± 3.45 | 9.14 ± 2.06 | 7.37 ± 2.77 | 4 | 1.53 | 0.2 |
| EPAC20:5n-3 | 5.10 ± 4.73 | 1.90 ± 0.60 | 3.40 ± 2.88 | 1.96 ± 0.52 | 1.58 ± 0.57 | 4 | 7.50 | <0.01 ** |
| C22:2n-6 | 0.27 ± 0.45 | 0.17 ± 0.18 | 0.22 ± 0.27 | 0.22 ± 0.41 | 0.31 ± 0.33 | 4 | 0.59 | 0.67 |
| DHAC22:6n-3 | 34.96 ± 16.14 | 36.98 ± 8.92 | 42.97 ± 10.95 | 41.18 ± 9.97 | 48.04 ± 8.44 | 4 | 5.63 | <0.01 ** |
| ΣPUFA | 54.39 ± 28.53 | 56.70 ± 14.08 | 64.12 ± 21.07 | 60.69 ± 15.56 | 65.64 ± 14.66 | 4 | 4.59 | <0.05 * |
| Body Length | Similarity Coefficients | R | p |
|---|---|---|---|
| GroupA/GroupB | Bray–Curtis | −0.04 | 0.03 |
| GroupA/GroupC | Bray–Curtis | 0.18 | 0.04 |
| GroupA/GroupD | Bray–Curtis | 0.21 | 0.32 |
| GroupA/GroupE | Bray–Curtis | 0.33 | 0.23 |
| GroupB/GroupC | Bray–Curtis | 0.17 | 0.22 |
| GroupB/GroupD | Bray–Curtis | 0.16 | 0.76 |
| GroupB/GroupE | Bray–Curtis | 0.28 | 0.48 |
| GroupC/GroupD | Bray–Curtis | 0.44 | 0.35 |
| GroupC/GroupE | Bray–Curtis | 0.49 | 0.01 |
| GroupD/GroupE | Bray–Curtis | 0.06 | 0.22 |
| Fatty Acid | PC1 | PC2 |
|---|---|---|
| C14:0 | 0.35 | −0.31 |
| C16:0 | 0.34 | −0.21 |
| C18:0 | 0.07 | 0.74 |
| C16:1n-7 | 0.39 | −0.20 |
| C18:1n-9 | 0.38 | 0.13 |
| C18:3n-6 | 0.35 | 0.25 |
| EPAC20:5n-3 | −0.24 | −0.24 |
| ARAC20:4n-6 | −0.28 | −0.19 |
| DHAC22:6n-3 | −0.41 | 0.08 |
| Signature Fatty Acid | Corresponding Sources | 100–130 mm | 131–160 mm | 161–190 mm | 191–220 mm | 221–250 mm |
|---|---|---|---|---|---|---|
| C18:1n-9 | Zooplankton [19] | 2.56 ± 1.62 | 4.71 ± 2.78 | 4.37 ± 2.19 | 4.98 ± 2.18 | 6.12 ± 3.03 |
| C16:1n-7 | Diatoms [20] | 1.66 ± 1.37 | 3.22 ± 1.96 | 2.30 ± 1.36 | 2.62 ± 0.99 | 3.58 ± 2.12 |
| DHAC22:6n-3 | Fish [20] | 34.96 ± 16.14 | 36.98 ± 8.92 | 42.97 ± 10.95 | 41.18 ± 9.97 | 48.04 ± 8.44 |
| ARAC20:4n-6 | Crustacea [7] | 7.65 ± 3.60 | 8.52 ± 2.15 | 8.92 ± 3.45 | 9.14 ± 2.06 | 7.37 ± 2.77 |
| EPAC20:5n-3 | Krill [26] | 5.10 ± 4.73 | 1.90 ± 0.60 | 3.40 ± 2.88 | 1.96 ± 0.52 | 1.58 ± 0.57 |
| C18:3n-3 C18:2n-6 | Green Algae [27] | 0.94 ± 0.26/ 0.94 ± 0.26 | 1.06 ± 0.44/ 0.25 ± 0.18 | 1.12 ± 0.47/ 0.22 ± 0.14 | 1.02 ± 0.23/ 0.27 ± 0.15 | 1.11 ± 0.20/ 0.29 ± 0.15 |
| C15:0 C17:0 | Planktonic Bacteria [28] | 0.39 ± 0.17/ 0.78 ± 0.19 | 0.51 ± 0.16/ 0.93 ± 0.27 | 0.40 ± 0.13/ 0.77 ± 0.19 | 0.35 ± 0.17/ 0.62 ± 0.16 | 0.47 ± 0.23/ 0.62 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Hu, G.; Zhao, Z.; Chen, X.; Liu, B. Feeding Habits of Scomber japonicus Inferred by Stable Isotope and Fatty Acid Analyses. J. Mar. Sci. Eng. 2024, 12, 1335. https://doi.org/10.3390/jmse12081335
Chen Y, Hu G, Zhao Z, Chen X, Liu B. Feeding Habits of Scomber japonicus Inferred by Stable Isotope and Fatty Acid Analyses. Journal of Marine Science and Engineering. 2024; 12(8):1335. https://doi.org/10.3390/jmse12081335
Chicago/Turabian StyleChen, Yingcong, Guanyu Hu, Zhenfang Zhao, Xinjun Chen, and Bilin Liu. 2024. "Feeding Habits of Scomber japonicus Inferred by Stable Isotope and Fatty Acid Analyses" Journal of Marine Science and Engineering 12, no. 8: 1335. https://doi.org/10.3390/jmse12081335





