Multi-Stage Screening Purification: A New and Effective Method for Cleaning Diatom Samples from Marine Sediments
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
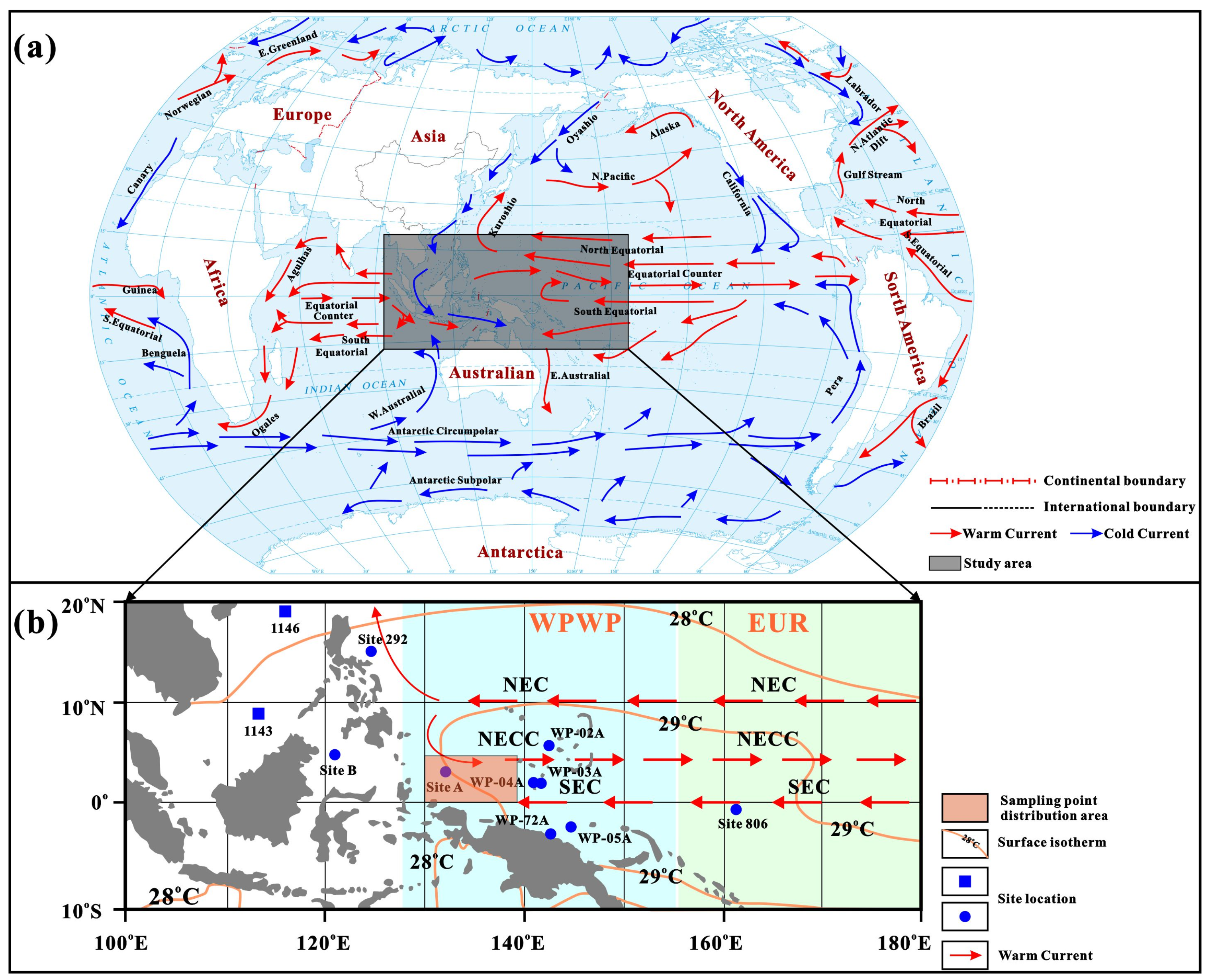
3.1. Sampling
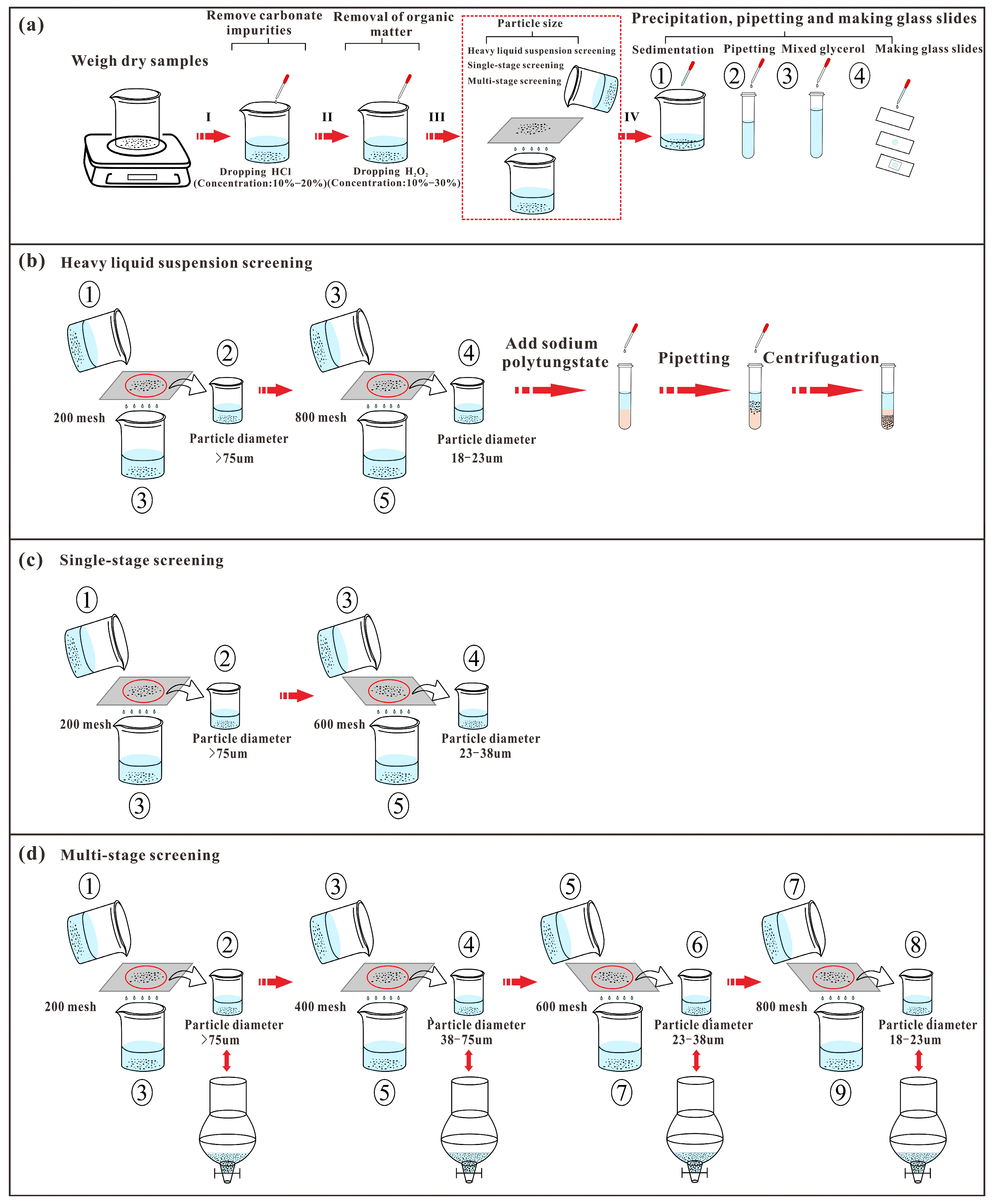
3.2. Experimental Method
3.3. Analytical Method
4. Results
4.1. Morphology and Size of Diatom Remains
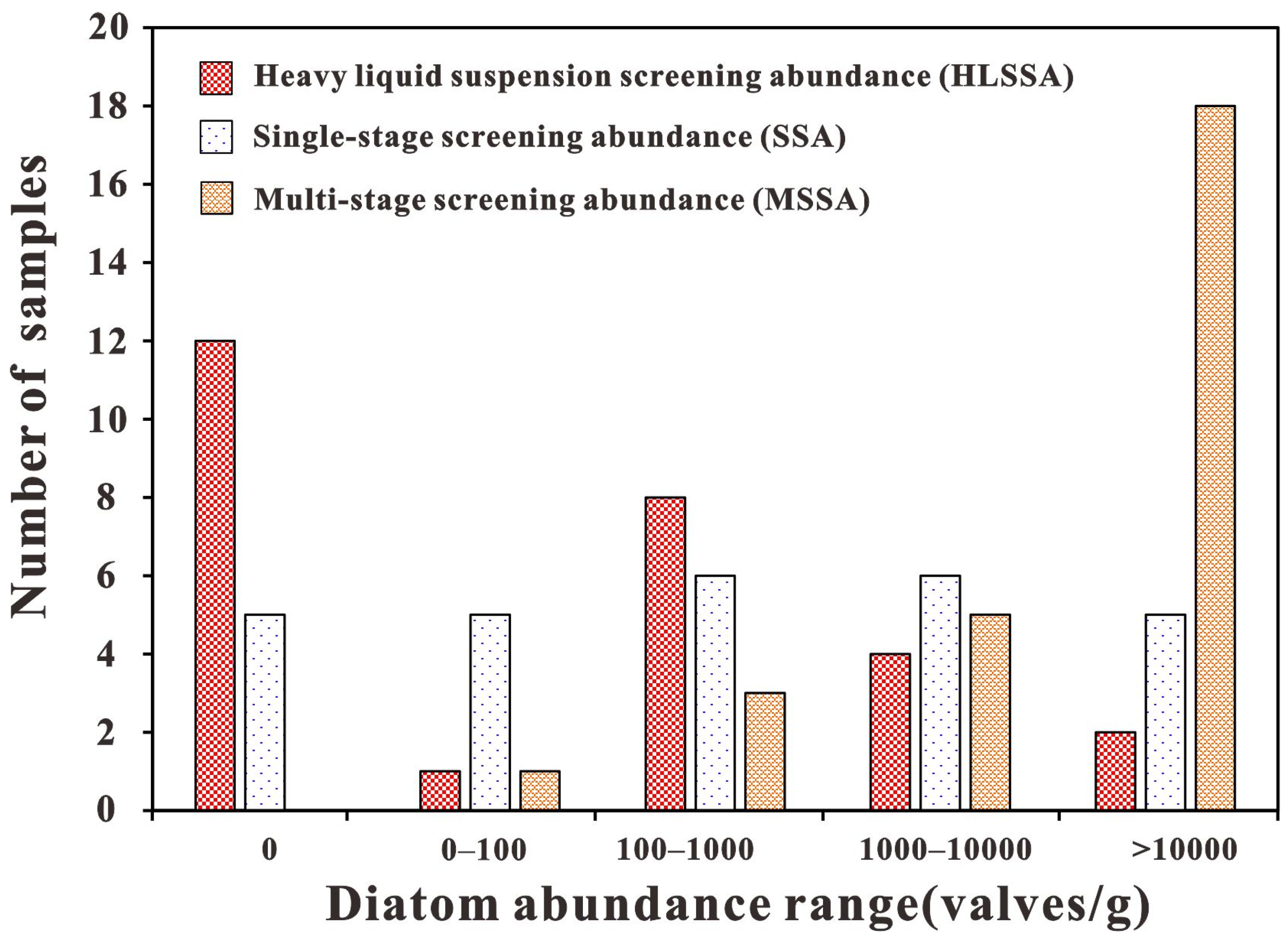
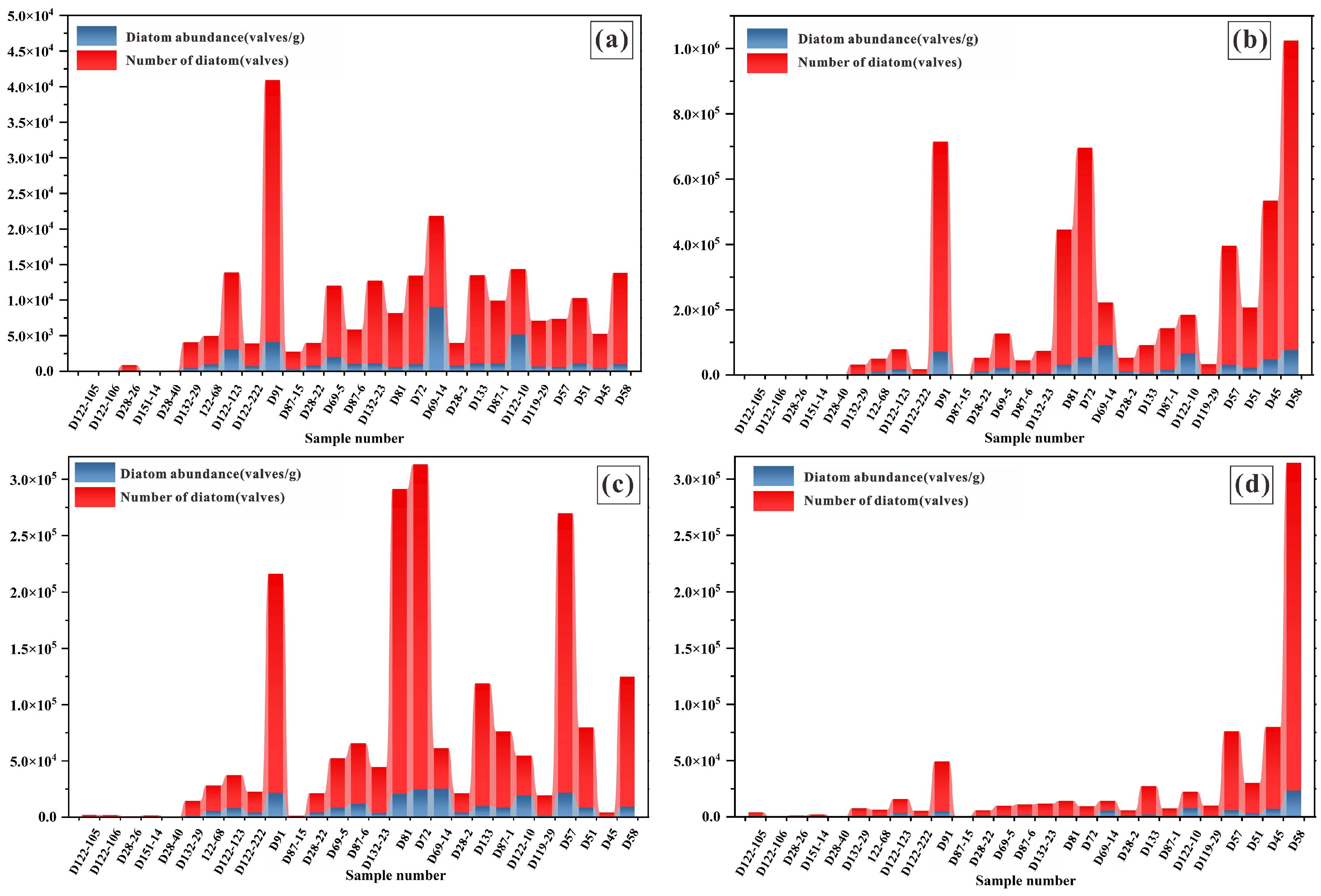
4.2. Distribution Characteristics of Diatom Abundance
4.2.1. Heavy Liquid Suspension Purification
4.2.2. Single-Stage Screening Purification
4.2.3. Multi-Stage Screening Purification
5. Discussion
5.1. The Influencing Factors of Diatom Abundance in Different Purification Methods
5.2. Application and Significance of the Multi-Stage Screening Method for the Purification of Diatom Valves
6. Conclusions
- (1)
- The multi-stage screening purification method greatly enhanced diatom abundance, yielding 21.9 times higher abundance than the heavy liquid suspension method and 6.53 times higher than the single-stage method. This method is also cost-effective, easy to operate, and results in low impurity residuals, significantly improving microscopic observation and the accuracy of diatom taxonomic identification.
- (2)
- The abundance of diatoms was primarily influenced by sieve pore size, heavy liquid concentration, and the size and density of diatom valves of different sizes. The type of oxidant had minimal impact on diatom abundance, highlighting the importance of sieve size and particle characteristics in effective diatom purification.
- (3)
- The multi-stage screening method allowed for the classification of diatom sizes, revealing a comprehensive distribution, including nano-sized diatoms (diameter < 20 μm).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Liu, Y. Marine sediments as archives of environmental change. Earth-Sci. Rev. 2020, 204, 103178. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Battarbee, R.W.; Donald, F.; Charles, C.B.; Cumming, B.F.; Renberg, I. Diatoms as indicators of surface water acidity. In The Diatoms: Applications for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 2001; pp. 85–127. [Google Scholar]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Science; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Horton, B.P. Diatom analysis in coastal environments. Quat. Int. 1999, 56, 155–168. [Google Scholar]
- Barker, P.; Williamson, D. Diatom preservation in lake sediments: An experimental study. Limnol. Oceanogr. 1988, 33, 785–793. [Google Scholar]
- Shemesh, A.; Mortlock, R.A.; Smith, R.J.; Froelich, P.N. Determination of Ge/Si in marine siliceous microfossils: Separation, cleaning and dissolution of diatoms and radiolaria. Mar. Chem. 1988, 25, 305–323. [Google Scholar] [CrossRef]
- Shemesh, A.; Burckle, L.H.; Hays, J.D. Late Pleistocene oxygen isotope records of biogenic silica from the Atlantic sector of the Southern Ocean. Paleoceanography 1995, 10, 179–196. [Google Scholar] [CrossRef]
- Smart, S.M.; Fawcett, S.E.; Ren, H.; Schiebel, R.; Tompkins, E.M.; Martínez-García, A.; Stirnimann, L.; Roychoudhury, A.; Haug, G.H.; Sigman, D.M. The nitrogen isotopic composition of tissue and shell-bound organic matter of planktic foraminifera in Southern Ocean surface waters. Geochem. Geophys. Geosystems 2020, 21, e2019GC008440. [Google Scholar] [CrossRef]
- Vilhena, J.C.; Amorim, A.; Ribeiro, L.; Ferreira, I.M.; Pombo, M. Assessing the efficiency of low-cost cleaning methods for diatoms from intertidal sediment samples. J. Microbiol. Methods 2021, 187, 106255. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Sullivan, L.A. Distribution of diatoms in peat and lake sediments. J. Paleolimnol. 2005, 33, 465–482. [Google Scholar]
- Robinson, R.S.; Brunelle, B.G.; Sigman, D.M. Revisiting nutrient utilization in the glacial Antarctic: Evidence from a new method for diatom-bound N isotopic analysis. Paleoceanography 2004, 19, 2003PA000996. [Google Scholar] [CrossRef]
- Hatté, C.; Hodgins, G.; Jull, A.T.; Bishop, B.; Tesson, B. Marine chronology based on 14C dating on diatoms proteins. Mar. Chem. 2008, 109, 143–151. [Google Scholar] [CrossRef]
- Strugnell, J.M.; McGregor, H.V.; Wilson, N.G.; Meredith, K.T.; Chown, S.L.; Lau, S.C.; Robinson, S.A.; Saunders, K.M. Emerging biological archives can reveal ecological and climatic change in Antarctica. Glob. Change Biol. 2022, 28, 6483–6508. [Google Scholar] [CrossRef] [PubMed]
- Stoermer, E.F.; Smol, J.P. The diatoms: Indications for modern and fossil records. Phycologia 1999, 38, 388–408. [Google Scholar]
- Flower, R.J.; Battarbee, R.W. Diatoms and climate change. Quat. Sci. Rev. 2001, 20, 1829–1848. [Google Scholar]
- Westacott, S.; Planavsky, N.J.; Zhao, M.Y.; Hull, P.M. Revisiting the sedimentary record of the rise of diatoms. Proc. Natl. Acad. Sci. USA 2021, 118, e2103517118. [Google Scholar] [CrossRef]
- Saros, J.E.; Anderson, N. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol. Rev. 2015, 90, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Watts, A.E.; Kirika, A. Integrating molecular and imaging technologies in diatom analysis. Prog. Oceanogr. 2001, 51, 275–303. [Google Scholar]
- Lelchat, F.; Dussauze, M.; Lemaire, P.; Theron, M.; Toffin, L.; Le Floch, S. Measuring the biological impact of drilling waste on the deep seafloor: An experimental challenge. J. Hazard. Mater. 2020, 389, 122132. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, T.; Yokoyama, Y.; Ikehara, M.; Kuwae, M. Shoaling of the western equatorial Pacific thermocline during the last glacial maximum inferred from multispecies temperature reconstruction of planktonic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 346, 120–129. [Google Scholar] [CrossRef]
- Hu, D.; Wu, L.; Cai, W.; Gupta, A.S.; Ganachaud, A.; Qiu, B.; Gordon, A.L.; Lin, X.; Chen, Z.; Hu, S.; et al. Pacific western boundary currents and their roles in climate. Nature 2015, 522, 299–308. [Google Scholar] [CrossRef]
- Shi, J.; Jia, Q.; Nürnberg, D.; Li, T.; Xiong, Z.; Qin, B. Coupled nutricline and productivity variations during the Pliocene in the western Pacific warm pool and their paleoceanographic implications. Glob. Planet. Change 2022, 212, 103810. [Google Scholar] [CrossRef]
- Max, L.; Rippert, N.; Lembke-Jene, L.; Mackensen, A.; Nürnberg, D.; Tiedemann, R. Evidence for enhanced convection of North Pacific Intermediate Water to the low-latitude Pacific under glacial conditions. Paleoceanography 2017, 32, 41–55. [Google Scholar] [CrossRef]
- Ramos, R.D.; Goodkin, N.F.; Fan, T.Y. Coral records at the northern edge of the Western Pacific Warm Pool reveal multiple drivers of sea surface temperature, salinity, and rainfall variability since the end of the Little Ice Age. Paleoceanogr. Paleoclimatol. 2020, 35, e2019PA003826. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Murty, S.A.; Sprintall, J.; Lee, T.; Abram, N.J. Heat and freshwater changes in the Indian Ocean region. Nat. Rev. Earth Environ. 2021, 2, 525–541. [Google Scholar] [CrossRef]
- Kobayashi, F.; Takahashi, K. Distribution of diatoms along the equatorial transect in the western and central Pacific during the 1999 La Niña conditions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 2801–2821. [Google Scholar] [CrossRef]
- Morley, D.W.; Leng, M.J.; Mackay, A.W.; Sloane, H.J.; Rioual, P.; Battarbee, R.W. Cleaning of lake sediment samples for diatom oxygen isotope analysis. J. Paleolimnol. 2004, 31, 391–401. [Google Scholar] [CrossRef]
- Morley, D.W.; Leng, M.J.; Mackay, A.W.; Sloane, H.J. Late glacial and Holocene environmental change in the Lake Baikal region documented by oxygen isotopes from diatom silica. Glob. Planet. Change 2005, 46, 221–233. [Google Scholar] [CrossRef]
- Crosta, X.; Koç, N. Chapter eight diatoms: From micropaleontology to isotope geochemistry. Dev. Mar. Geol. 2007, 1, 327–369. [Google Scholar]
- Cristóbal, G.; Blanco, S.; Bueno, G. Modern Trends in Diatom Identification; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Tseng, C.K. The past, present and future of phycology in China. In Asian Pacific Phycology in the 21st Century: Prospects and Challenges, Proceeding of the Second Asian Pacific Phycological Forum, Hong Kong, China, 21–25 June 1999; Springer Netherlands: Dordrecht, The Netherlands, 21–25 June 2004; pp. 11–20. [Google Scholar]
- Guillard, R.; Kilham, P.E.T.E.R. The ecology of marine planktonic diatoms. Biol. Diatoms 1977, 13, 372–469. [Google Scholar]
- Leblanc, K.; Queguiner, B.; Diaz, F.; Cornet, V.; Michel-Rodriguez, M.; Durrieu de Madron, X.; Bowler, C.; Malviya, S.; Thyssen, M.; Grégori, G.; et al. Nanoplanktonic diatoms are globally overlooked but play a role in spring blooms and carbon export. Nat. Commun. 2018, 9, 953. [Google Scholar] [CrossRef]
- Tang, H.; Ye, H.; Yang, W.; Huang, Y.; Sun, T.; Ge, Y.; Xie, Z. East Asian Winter Monsoon and enhanced stratification in the western Pacific promoted giant marine diatom (Ethmodiscus rex) blooms during the last deglaciation. Quat. Sci. Rev. 2024, 336, 108798. [Google Scholar] [CrossRef]
- Hollstein, M.; Mohtadi, M.; Rosenthal, Y.; Moffa Sanchez, P.; Oppo, D.; Martínez Méndez, G.; Hebbeln, D. Stable oxygen isotopes and Mg/Ca in planktic foraminifera from modern surface sediments of the Western Pacific Warm Pool: Implications for thermocline reconstructions. Paleoceanography 2017, 32, 1174–1194. [Google Scholar] [CrossRef]
- Steinke, S.; Mohtadi, M.; Groeneveld, J.; Lin, L.C.; Löwemark, L.; Chen, M.T.; Rendle-Bühring, R. Reconstructing the southern South China Sea upper water column structure since the Last Glacial Maximum: Implications for the East Asian winter monsoon development. Paleoceanography 2010, 25. [Google Scholar] [CrossRef]
- Rutgers van der Loeff, M.; Kipp, L.; Charette, M.A.; Moore, W.S.; Black, E.; Stimac, I.; Charkin, A.; Bauch, D.; Valk, O.; Karcher, M.; et al. Radium isotopes across the Arctic Ocean show time scales of water mass ventilation and increasing shelf inputs. J. Geophys. Res. Ocean. 2018, 123, 4853–4873. [Google Scholar] [CrossRef]
- Gdaniec, S.; Roy-Barman, M.; Levier, M.; Valk, O.; van Der Loeff, M.R.; Foliot, L.; Andersson, P.S. 231Pa and 230Th in the Arctic Ocean: Implications for boundary scavenging and 231Pa230Th fractionation in the Eurasian Basin. Chem. Geol. 2020, 532, 119380. [Google Scholar] [CrossRef]
- Daniels, C.J.; Poulton, A.J.; Esposito, M.; Paulsen, M.L.; Bellerby, R.; St John, M.; Martin, A.P. Phytoplankton dynamics in contrasting early stage North Atlantic spring blooms: Composition, succession, and potential drivers. Biogeosciences 2015, 12, 2395–2409. [Google Scholar] [CrossRef]

| Diatom Abundance Range (valves/g) | Heavy Liquid Suspension Screening Abundance (HLSSA) | Percentage of Each Abundance (%) | Single-Stage Screening Abundance (SSA) | Percentage of Each Abundance (%) | Multi-Stage Screening Abundance (MSSA) | Percentage of Each Abundance (%) |
|---|---|---|---|---|---|---|
| 0 | 12 | 44.40% | 5 | 18.50% | 0 | 0.00% |
| 0–100 | 1 | 3.70% | 5 | 18.50% | 1 | 3.70% |
| 100–1000 | 8 | 29.60% | 6 | 22.20% | 3 | 11.10% |
| 1000–10,000 | 4 | 14.80% | 6 | 22.20% | 5 | 18.50% |
| >10,000 | 2 | 7.40% | 5 | 18.50% | 18 | 66.70% |
| Sample Number | Heavy Liquid Suspension Screening Abundance (HLSSA) | Single-Stage Screening Abundance (SSA) | Multi-Stage Screening Abundance (MSSA) | Ratio of Abundance (MSSA/HLSSA) | Ratio of Abundance (MSSA/SSA) |
|---|---|---|---|---|---|
| D 122-105 | 0.00 | 0.00 | 2056.91 | / | / |
| D 122-106 | 0.00 | 0.00 | 558.19 | / | / |
| D 28-26 | 0.00 | 0.00 | 558.78 | / | / |
| D 151-14 | 0.00 | 0.00 | 685.49 | / | / |
| D 28-40 | 0.00 | 0.00 | 57.28 | / | / |
| D 132-29 | 0.00 | 40.91 | 7117.24 | / | 173.97 |
| 122-68 | 0.00 | 42.86 | 18,023.40 | / | 420.55 |
| D 122-123 | 0.00 | 44.98 | 32,815.20 | / | 729.49 |
| D 122-222 | 103.56 | 56.57 | 9606.24 | / | 169.82 |
| D 91 | 0.00 | 90.91 | 103,282.37 | / | 1136.09 |
| D 87-15 | 69.17 | 259.74 | 7266.08 | / | 27.97 |
| D 28-22 | 0.00 | 327.27 | 17,150.06 | / | 52.40 |
| D 69-5 | 627.45 | 467.53 | 1002.35 | 1.60 | 2.14 |
| D 87-6 | 0.00 | 556.82 | 23,737.26 | / | 42.63 |
| D 132-23 | 341.25 | 681.81 | 12,797.50 | 37.50 | 18.77 |
| D 81 | 123.14 | 686.87 | 54,703.51 | 444.24 | 79.64 |
| D 72 | 0.00 | 1036.36 | 82,093.81 | / | 79.21 |
| D 69-14 | 128.62 | 1818.18 | 132,579.51 | 1030.78 | 72.92 |
| D 28-2 | 1621.21 | 2102.27 | 181,244.06 | 111.80 | 86.21 |
| D 133 | 721.83 | 2636.36 | 21,042.08 | 29.15 | 7.98 |
| D 87-1 | 952.44 | 3636.36 | 27,214.05 | 28.57 | 7.48 |
| D 122-10 | 644.13 | 4886.36 | 34,395.42 | 53.40 | 7.04 |
| D 119-29 | 6985.76 | 15,378.79 | 99,550.56 | 14.25 | 6.47 |
| D 57 | 7296.32 | 19,103.03 | 61,074.66 | 8.37 | 3.20 |
| D 51 | 10,132.02 | 31,846.10 | 35,702.08 | 3.52 | 1.12 |
| D 45 | 9675.26 | 36,007.13 | 56,930.79 | 5.88 | 1.58 |
| D 58 | 12,456.23 | 52,106.95 | 111,633.99 | 8.96 | 2.14 |
| Total abundance of diatoms | 1921.4 | 6437.6 | 42,032.6 | 21.9 | 6.53 |
| Experimental Method | Average Abundance (valves/g) | Lowest Abundance (valves/g) | Highest Abundance (valves/g) |
|---|---|---|---|
| Heavy liquid suspension screening abundance (HLSSA) | 1921.42 | 0.00 | 12,456.23 |
| Single-stage screening abundance (SSA) | 6437.56 | 0.00 | 52,106.95 |
| Multi-stage screening abundance (MSSA) | 42,032.55 | 57.28 | 181,244.06 |
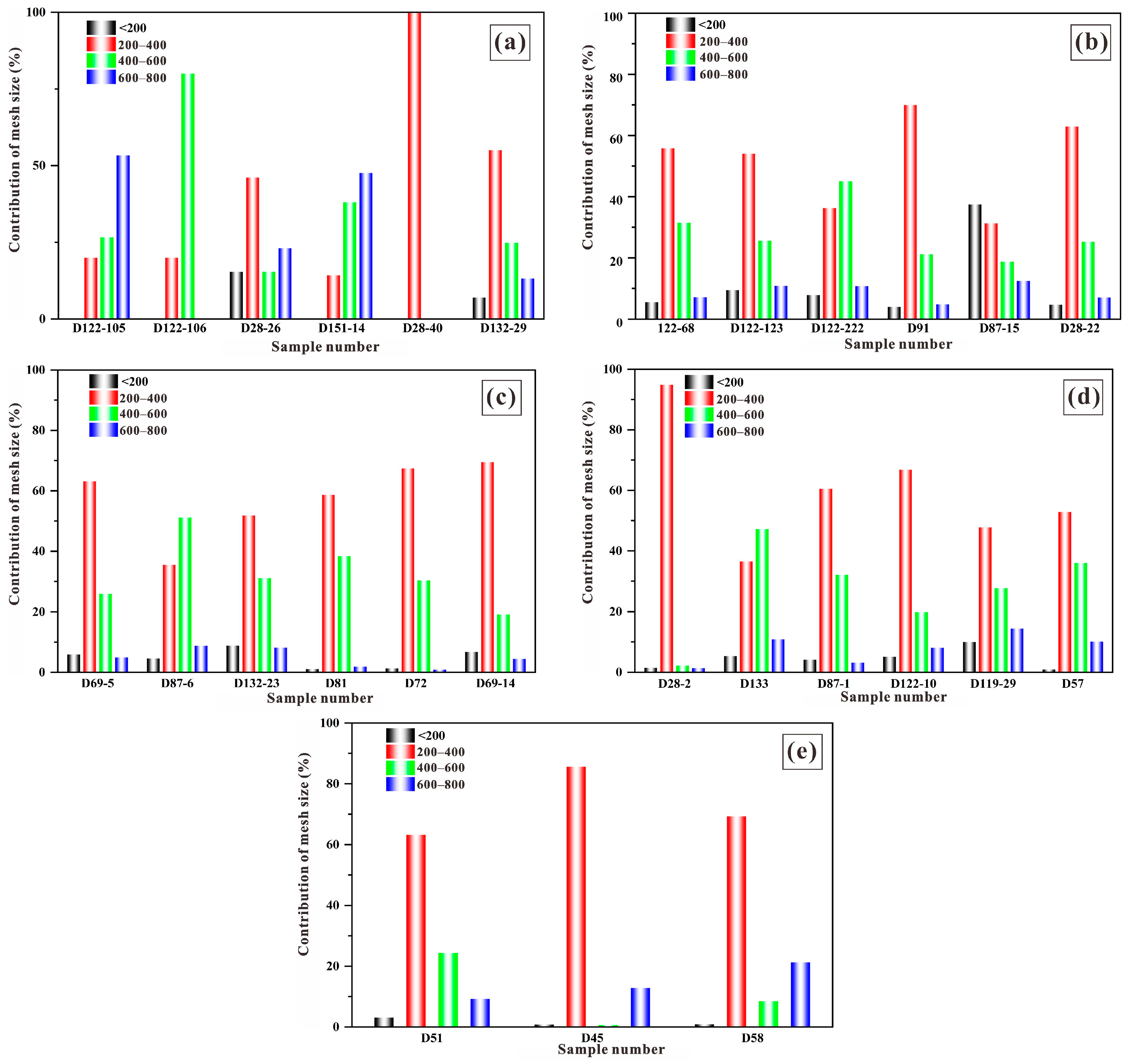
| Mesh Size (L) | Average Contribution Rate of Diatoms |
|---|---|
| >75 μm | 5.64% |
| 38~75 μm | 55.20% |
| 23~38 μm | 27.70% |
| 18~23 μm | 11.50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, K.; Meng, M.; Lu, Y.; Ji, W.; Tang, G.; Cai, G.; Cui, Q.; Lu, Y.; Yang, F.; Zhang, D.; et al. Multi-Stage Screening Purification: A New and Effective Method for Cleaning Diatom Samples from Marine Sediments. J. Mar. Sci. Eng. 2025, 13, 187. https://doi.org/10.3390/jmse13020187
Deng K, Meng M, Lu Y, Ji W, Tang G, Cai G, Cui Q, Lu Y, Yang F, Zhang D, et al. Multi-Stage Screening Purification: A New and Effective Method for Cleaning Diatom Samples from Marine Sediments. Journal of Marine Science and Engineering. 2025; 13(2):187. https://doi.org/10.3390/jmse13020187
Chicago/Turabian StyleDeng, Kong, Mianmo Meng, Yangbo Lu, Wenming Ji, Guanxiong Tang, Guanqiang Cai, Qinyu Cui, Yongchao Lu, Feng Yang, Dingjie Zhang, and et al. 2025. "Multi-Stage Screening Purification: A New and Effective Method for Cleaning Diatom Samples from Marine Sediments" Journal of Marine Science and Engineering 13, no. 2: 187. https://doi.org/10.3390/jmse13020187
APA StyleDeng, K., Meng, M., Lu, Y., Ji, W., Tang, G., Cai, G., Cui, Q., Lu, Y., Yang, F., Zhang, D., & Liu, Z. (2025). Multi-Stage Screening Purification: A New and Effective Method for Cleaning Diatom Samples from Marine Sediments. Journal of Marine Science and Engineering, 13(2), 187. https://doi.org/10.3390/jmse13020187









