Genetic Differentiation in Red and Green Noctiluca scintillans in Jakarta Bay, Indonesia
Abstract
1. Introduction
2. Materials and Methods
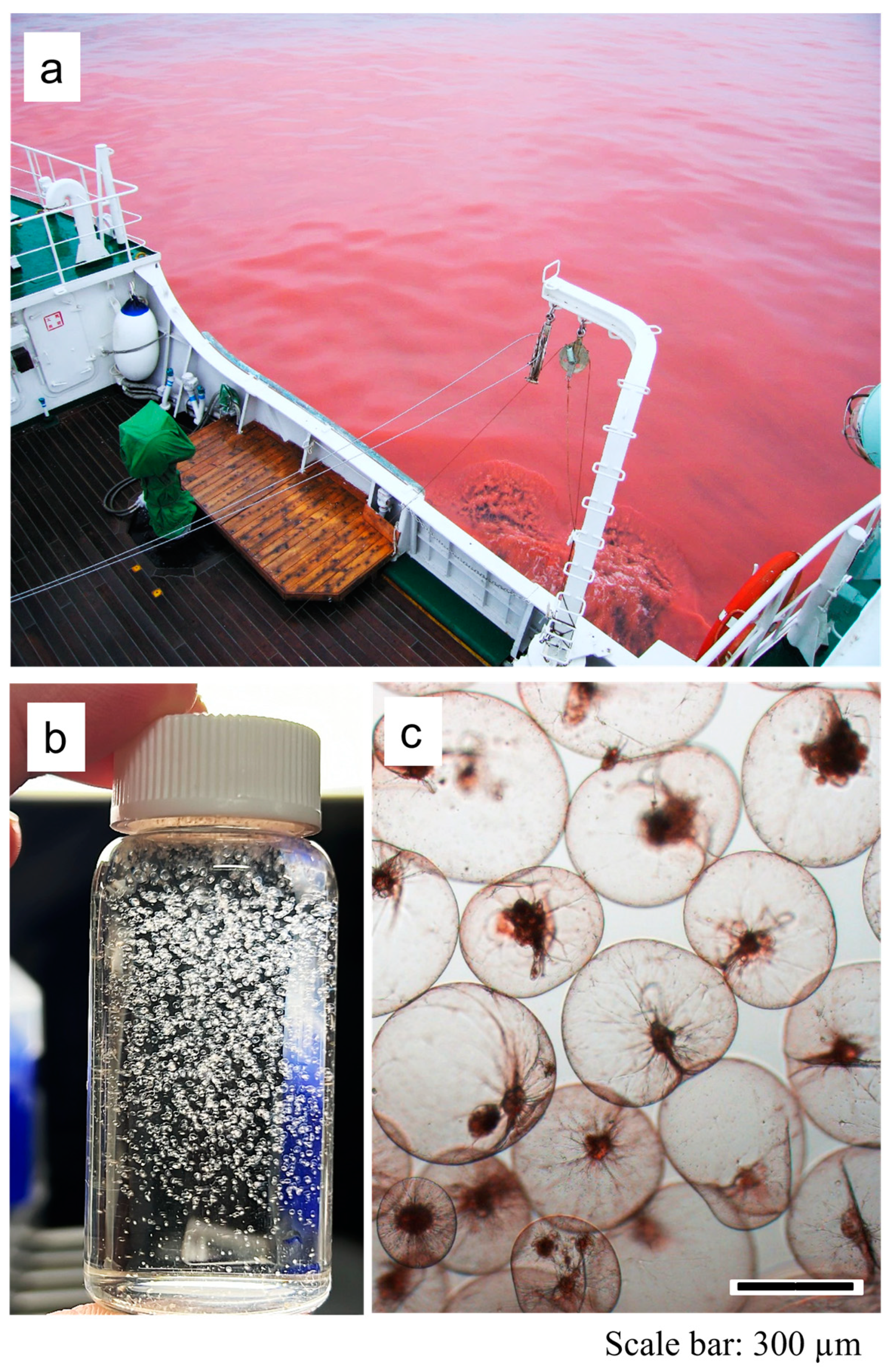
2.1. Sample Collection and Morphological Study
2.2. Genetic Analysis
3. Results
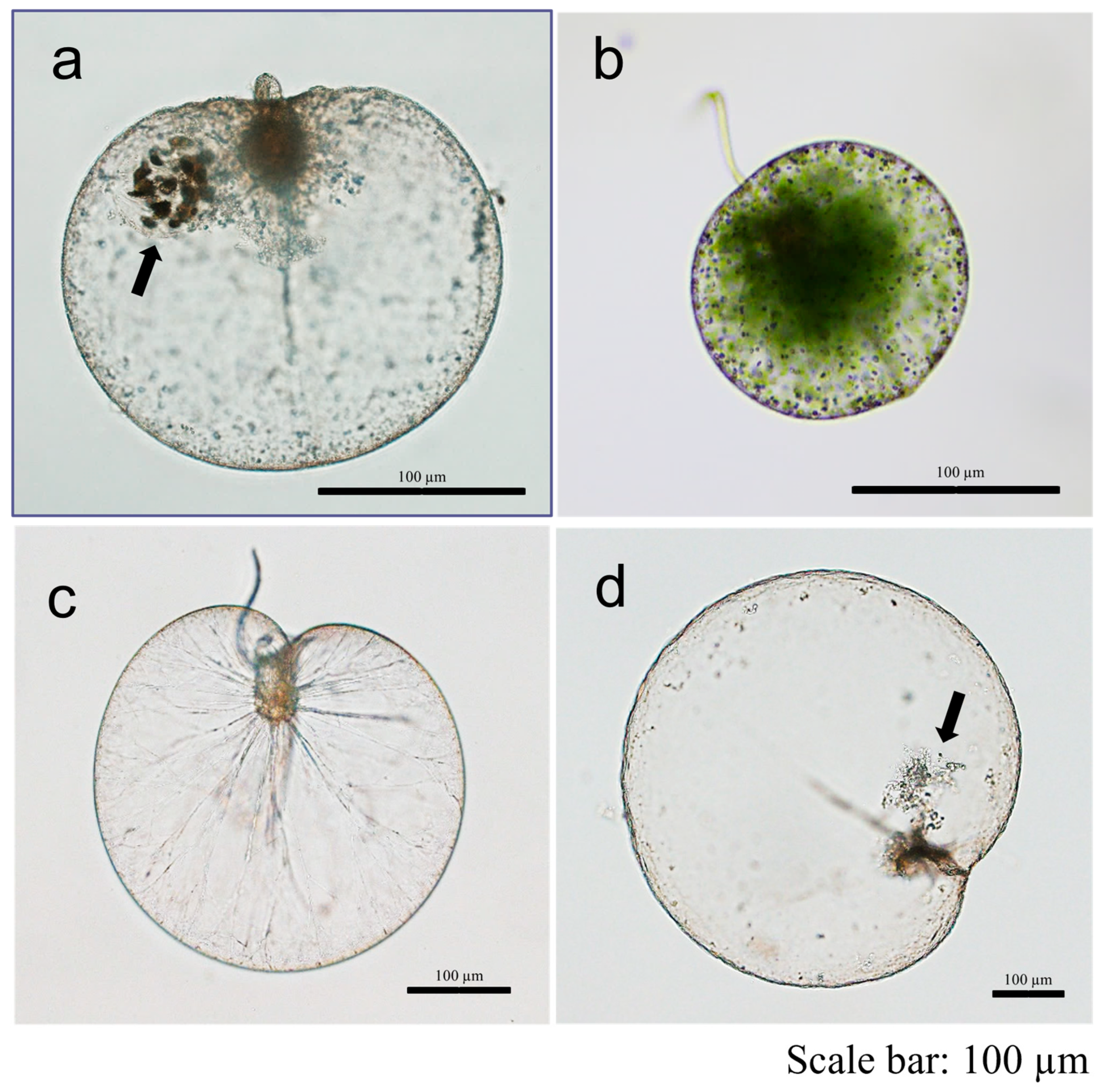
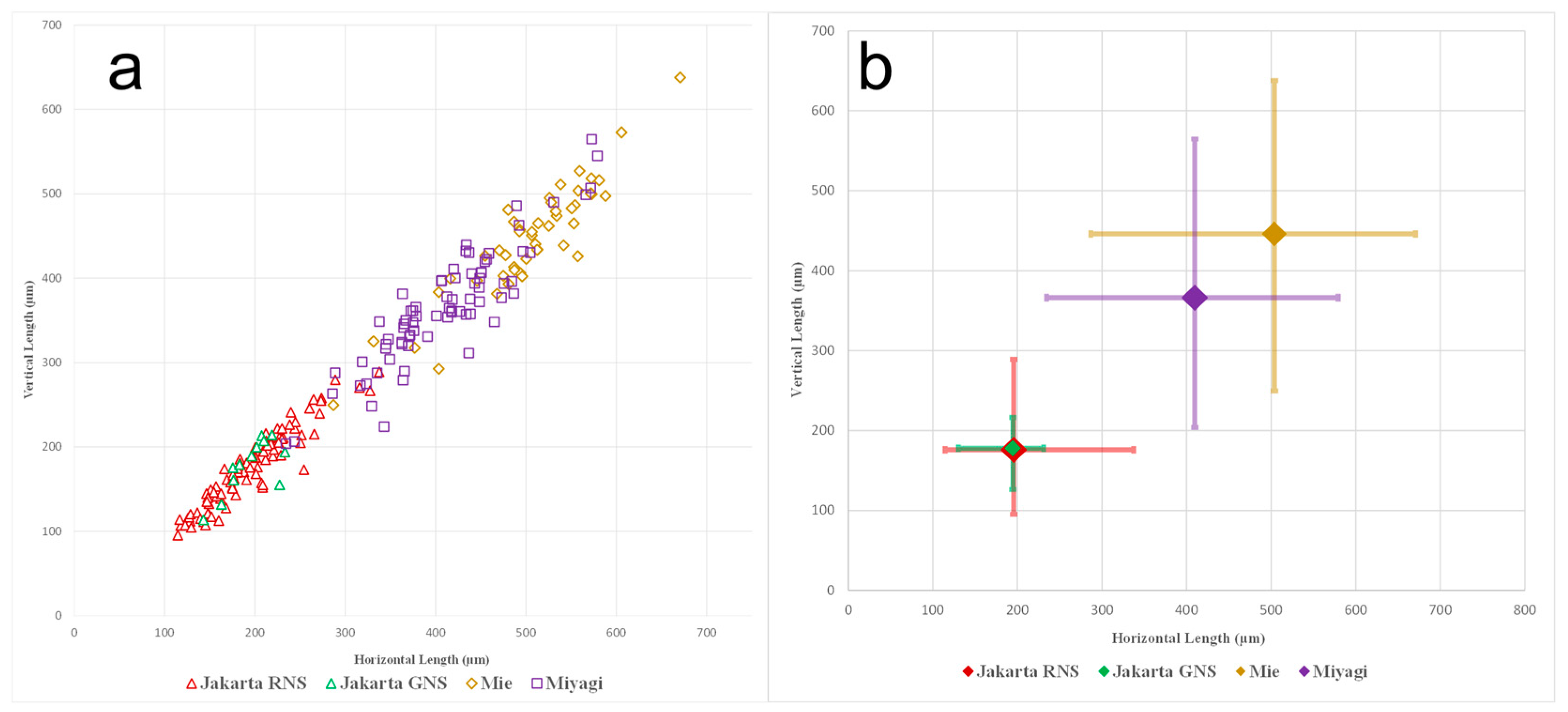
3.1. Morphological Observation and Cell Size Measurement
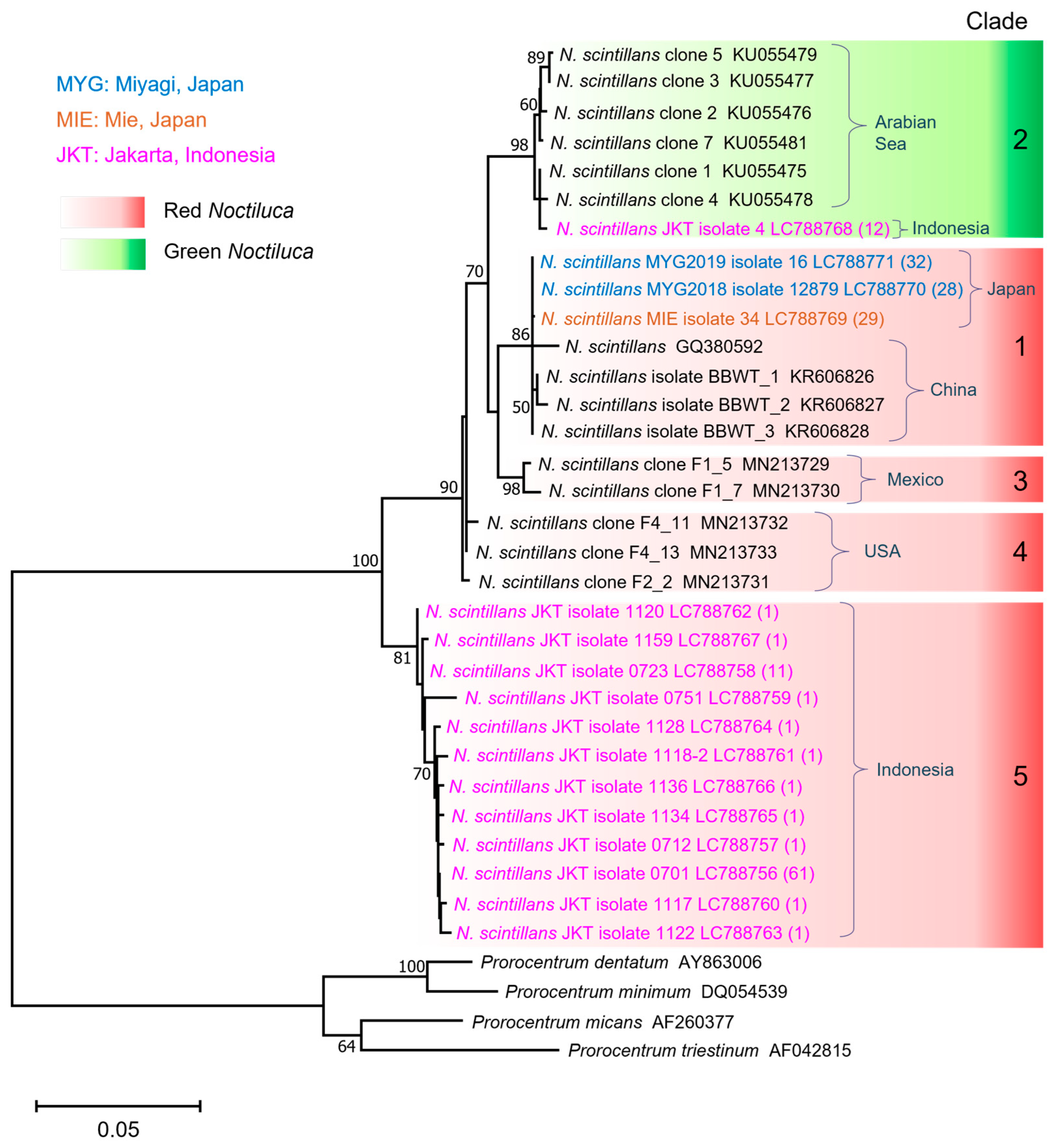
3.2. Phylogenetic Relationship and Genetic Divergence
4. Discussion
4.1. Morphology and Cell Size
4.2. Insights into Phylogenetic Patterns and Genetic Divergence
4.3. Red and Green Noctiluca scintillans
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAB | Harmful algal bloom |
| RNS | Red Noctiluca scintillans |
| GNS | Green Noctiluca scintillans |
| PCR | Polymerase chain reaction |
References
- Huang, C.; Qi, Y. The abundance cycle and influence factors on red tide phenomena of Noctiluca scintillans (Dinophyceae) in Dapeng Bay, the South China Sea. J. Plankton Res. 1997, 19, 303–318. [Google Scholar] [CrossRef]
- Escalera, L.; Pazos, Y.; Moroño, Á.; Reguera, B. Noctiluca scintillans may act as a vector of toxigenic microalgae. Harmful Algae 2007, 6, 317–320. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef]
- Kofoid, C.A.; Swezy, O. The Free-Living Unarmored Dinoflagellata; Memoirs of the University of California: Berkeley, CA, USA, 1921. [Google Scholar]
- Saldarriaga, J.F.; Taylor, F.J.R.; Cavalier-Smith, T.; Menden-Deuer, S.; Keeling, P.J. Molecular data and the evolutionary history of dinoflagellates. Eur. J. Protistol. 2004, 40, 85–111. [Google Scholar] [CrossRef]
- Cooney, E.C.; Leander, B.S.; Keeling, P.J. Phylogenomics shows unique traits in Noctilucales are derived rather than ancestral. PNAS Nexus 2022, 1, pgac202. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Shimode, S.; Kim, H.C.; Han, M.S.; Kikuchi, T. Strong bottom-up effects on phytoplankton community caused by a rainfall during spring and summer in Sagami Bay, Japan. J. Mar. Syst. 2009, 75, 253–264. [Google Scholar] [CrossRef]
- do Rosário Gomes, H.; Goes, J.I.; Matondkar, S.P.; Buskey, E.J.; Basu, S.; Parab, S.; Thoppil, P. Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia. Nat. Commun. 2014, 5, 4862. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Malej, A.; Shiganova, T.A.; Turk, V.; Sivkovitch, A.E.; Musaeva, E.I.; Kogovšek, T.; Lukasheva, T.A. Populations of the red tide forming dinoflagellate Noctiluca scintillans (Macartney): A comparison between the Black Sea and the northern Adriatic Sea. Harmful Algae 2014, 33, 29–40. [Google Scholar]
- Quevedo, M.; Gonzalez-Quiros, R.; Anadon, R. Evidence of heavy predation by Noctiluca scintillans on Acartia clausi (Copepoda) eggs off the central Cantabrian coast (NW Spain). Oceanol. Acta 1999, 22, 127–131. [Google Scholar] [CrossRef]
- Nishitani, G.; Shiromoto, M.; Sato-Okoshi, W.; Ishikawa, A. Molecular approach for analysis of in situ feeding by the dinoflagellate Noctiluca scintillans. Harmful Algae 2020, 99, 101928. [Google Scholar] [CrossRef]
- Okaichi, T. Identification of ammonia as the toxic principle of red tide of Noctiluca miliaris. Bull Plank Soc Jpn. 1976, 23, 75–80. [Google Scholar]
- Drits, A.V.; Nikishina, A.B.; Sergeeva, V.M.; Solov’ev, K.A. Feeding, respiration, and excretion of the Black Sea Noctiluca scintillans MacCartney in summer. Oceanology 2013, 53, 442–450. [Google Scholar] [CrossRef]
- Almeda, R.; Connelly, T.L.; Buskey, E.J. Novel insight into the role of heterotrophic dinoflagellates in the fate of crude oil in the sea. Sci. Rep. 2014, 4, 7560. [Google Scholar] [CrossRef]
- San Diego-McGlone, M.L.; Yñiguez, A.T.; Benico, G.; Lum, W.M.; Hii, K.S.; Leong, S.C.Y.; Leaw, C.P.; Iwataki, M.; Lim, P.T. Fish Kills Related to Harmful Algal Bloom Events in Southeast Asia. Sustainability 2024, 16, 10521. [Google Scholar] [CrossRef]
- Raj, K.D.; Mathews, G.; Obura, D.O.; Laju, R.L.; Bharath, M.S.; Kumar, P.D.; Arasamuthu, A.; Kumar, T.A.; Edward, J.P. Low oxygen levels caused by Noctiluca scintillans bloom kills corals in Gulf of Mannar, India. Sci. Rep. 2020, 10, 22133. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J.; Miranda, L.; Azanza, R. Green Noctiluca scintillans: A dinoflagellate with its own greenhouse. Mar. Ecol. Prog. Ser. 2004, 275, 78–87. [Google Scholar] [CrossRef]
- Harrison, P.J.; Furuya, K.; Glibert, P.M.; Xu, J.; Liu, H.B.; Yin, K.; Lee, J.H.; Anderson, D.M.; Gowen, R.; Al-Azri, A.R.; et al. Geographical distribution of red and green Noctiluca scintillans. Chin. J. Oceanol. Limnol. 2011, 29, 807–831. [Google Scholar]
- Wang, L.; Lin, X.; Goes, J.I.; Lin, S. Phylogenetic Analyses of Three Genes of Pedinomonas noctilucae, the Green Endosymbiont of the Marine Dinoflagellate Noctiluca scintillans, Reveal its Affiliation to the Order Marsupiomonadales (Chlorophyta, Pedinophyceae) under the Reinstated Name Protoeuglena noctilucae. Protist 2016, 167, 205–216. [Google Scholar]
- Manigandan, V.; Muthukumar, C.; Shah, C.; Logesh, N.; Sivadas, S.K.; Ramu, K.; Murthy, M.R. Phylogenetic affiliation of Pedinomonas noctilucae and green Noctiluca scintillans nutritional dynamics in the Gulf of Mannar, Southeastern Arabian Sea. Protist 2024, 175, 126019. [Google Scholar] [CrossRef]
- Elbrachter, M.; Qi, Y.Z. Aspects of Noctiluca (Dinophyceae) population dynamics. Physiol. Ecol. Harmful Algal Blooms 1998, 41, 315–335. [Google Scholar]
- Tibbs, J.F. On Some Planktonic Protozoa Taken from the Track of Drift Station ARLIS I, 1960–1961. Arct. Inst. N. Am. 1967, 20, 247–254. [Google Scholar] [CrossRef][Green Version]
- Sulochanan, B.; Dineshbabu, A.P.; Saravanan, R.; Subramanya Bhat, G.; Lavanya, S. Occurrence of Noctiluca scintillans bloom off Mangalore in the Arabian Sea. Indian J. Fish. Sci. 2014, 61, 42–48. [Google Scholar]
- Valiadi, M.; de Rond, T.; Amorim, A.; Gittins, J.R.; Gubili, C.; Moore, B.S.; Iglesias-Rodriguez, M.D.; Latz, M.I. Molecular and biochemical basis for the loss of bioluminescence in the dinoflagellate Noctiluca scintillans along the west coast of the U.S.A. Limnol. Oceanogr. 2019, 64, 2709–2724. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, L.; Zhang, W.; Liu, G.; Lin, S. Genetic analysis of Noctiluca scintillans populations indicates low latitudinal differentiation in China but high China-America differences. J. Exp. Mar. Biol. Ecol. 2016, 477, 31–39. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rodríguez, R.A.; Ochoa, J.L.; Uribe Alcocer, M. Grazing of heterotrophic dinoflagellate Noctiluca scintillans (Mcartney) Kofoid on Gymnodinium catenatum Graham. Rev. Latinoam. Microbiol. 2005, 47, 6–10. [Google Scholar]
- Ollevier, A.; Mortelmans, J.; Aubert, A.; Deneudt, K.; Vandegehuchte, M.B. Noctiluca scintillans: Dynamics, Size Measurements and Relationships With Small Soft-Bodied Plankton in the Belgian Part of the North Sea. Front. Mar. Sci. 2021, 8, 777999. [Google Scholar] [CrossRef]
- Ara, K.; Nakamura, S.; Takahashi, R.; Shiomoto, A.; Hiromi, J. Seasonal variability of the red tide-forming heterotrophic dinoflagellate Noctiluca scintillans in the neritic area of Sagami Bay, Japan: Its role in the nutrient-environment and aquatic ecosystem. Plankton Benthos Res. 2013, 8, 9–30. [Google Scholar] [CrossRef]
- Padmakumar, K.B.; SreeRenjima, G.; Fanimol, C.L.; Menon, N.R.; Sanjeevan, V.N. Preponderance of heterotrophic Noctiluca scintillans during a multi-species diatom bloom along the southwest coast of India. Int. J. Ocean. Oceanogr. 2010, 4, 55–63. [Google Scholar]
- Cardoso, L.d.S. Bloom of Noctiluca scintillans (Macartney) Kofoid & Swezy (Dinophyceae) in Southern Brazil. Braz. J. Oceanogr. 2012, 60, 265–268. [Google Scholar]
- Asefi, M.A.; Attaran-Fariman, G. Harmful blooming of Noctiluca scintillans in the southeast coastal waters of Iran, Oman Sea. Iran. J. Fish. Sci. 2023, 22, 261–277. [Google Scholar]
- Gopakumar, G.; Sulochanan, B.; Venkatesan, V. Bloom of Noctiluca scintillans (Macartney) in Gulf of Mannar, southeast coast of India. J. Mar. Biol. Assoc. India 2009, 51, 75–80. [Google Scholar]
- Fukuda, Y.; Endoh, H. New details from the complete life cycle of the red-tide dinoflagellate Noctiluca scintillans (Ehrenberg) McCartney. Eur. J. Protistol. 2006, 42, 209–219. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X.; Ding, X.; Chen, N. The high molecular diversity in Noctiluca scintillans is dominated by intra-genomic variations revealed by single cell high-throughput sequencing of 18S rDNA V4. Harmful Algae 2024, 132, 102568. [Google Scholar] [CrossRef]
- Miranda, L.N.; Zhuang, Y.; Zhang, H.; Lin, S. Phylogenetic analysis guided by intragenomic SSU rDNA polymorphism refines classification of “Alexandrium tamarense” species complex. Harmful Algae 2012, 16, 35–48. [Google Scholar] [CrossRef]
- Tea, Y.K.; Xu, X.; Dibattista, J.D.; Lo, N.; Cowman, P.F.; Ho, S.Y.W. Phylogenomic Analysis of Concatenated Ultraconserved Elements Reveals the Recent Evolutionary Radiation of the Fairy Wrasses (Teleostei: Labridae: Cirrhilabrus). Syst. Biol. 2022, 71, 1–12. [Google Scholar] [CrossRef]
- Funaki, H.; Nishimura, T.; Yoshioka, T.; Ataka, T.; Tanii, Y.; Hashimoto, K.; Yamaguchi, H.; Adachi, M. Toxicity and growth characteristics of epiphytic dinoflagellate Gambierdiscus silvae in Japan. Harmful Algae 2022, 115, 102230. [Google Scholar]
- Leung, P.T.; Yan, M.; Lam, V.T.; Yiu, S.K.; Chen, C.Y.; Murray, J.S.; Harwood, D.T.; Rhodes, L.L.; Lam, P.K.; Wai, T.C. Phylogeny, morphology and toxicity of benthic dinoflagellates of the genus Fukuyoa (Goniodomataceae, Dinophyceae) from a subtropical reef ecosystem in the South China Sea. Harmful Algae 2018, 74, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, K.; Horiguchi, T. Paragymnodinium verecundum sp. nov. (Gymnodiniales, Dinophyceae), a new species of mixotrophic dinoflagellate from Japan. Phycol. Res. 2021, 69, 124–136. [Google Scholar] [CrossRef]
- Cen, J.; Wang, J.; Huang, L.; Lin, Y.; Ding, G.; Qi, Y.; Lü, S. Karlodinium elegans sp. nov. (Gymnodiniales, Dinophyceae), a novel species isolated from the East China Sea in a dinoflagellate bloom. J. Oceanol. Limnol. 2021, 39, 242–258. [Google Scholar] [CrossRef]
- Shaju, S.S.; Akula, R.R.; Jabir, T. Characterization of light absorption coefficient of red Noctiluca scintillans bloom in the South Eastern Arabian Sea. Oceanologia 2018, 60, 419–425. [Google Scholar] [CrossRef]

| Sampling Location | Collection Time | Number of Isolated Cells | ||
|---|---|---|---|---|
| Japan | Ise Bay, Mie Pref. | June 2018 | 29 (RNS) | |
| Matsushima Bay, Miyagi Pref. | May 2018 | 28 (RNS) | ||
| August 2019 | 32 (RNS) | |||
| Indonesia | Jakarta Bay | September 2019 | 51 (RNS) | |
| September 2020 | 31 (RNS) | |||
| September 2020 | 12 (GNS) | |||
| Total | 183 | |||
| Location | Size (µm) | RNS/GNS | Reference |
|---|---|---|---|
| Jakarta, Indonesia | 95–338 | RNS | This study |
| Miyagi, Japan | 203–580 | RNS | This study |
| Mie, Japan | 250–671 | RNS | This study |
| Mazatlan Bay, Mexico | 220–500 | RNS | Rodriguez et al. (2005) [30] |
| North Sea, Belgium | 350–570 | RNS | Ollevier et al. (2021) [31] |
| Sagami Bay, Japan | 300–800 | RNS | Ara et al. (2013) [32] |
| Southwest coast of India | 500–1000 | RNS | Padmakumar et al. (2010) [33] |
| Rio Grande do Sul, Brazil | 600–1000 | RNS | Cardoso (2012) [34] |
| Jakarta, Indonesia | 114–233 | GNS | This study |
| Chabahar Bay, Iran | 150–500 | GNS | Asefi & Attaran-Fariman (2023) [35] |
| Gulf of Mannar, India | 400–1200 | GNS | Gopakumar et al. (2009) [36] |
| Genus | No. of Species | p-Distance * | Reference |
|---|---|---|---|
| Dinophysis | 11 | 0.026 | GenBank, calculated in this study |
| Gambierdiscus | 4 | 0.033 | Funaki et al. (2022) [41] |
| Noctiluca | 1 | 0.039 | This study |
| Fukuyoa | 6 | 0.042 | Leung et al. (2018) [42] |
| Paragymnodinium | 4 | 0.067 | Yokouchi et al. (2020) [43] |
| Karlodinium | 16 | 0.071 | Cen et al. (2021) [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, M.I.; Nishitani, G.; Ishikawa, A.; Hadi, S.; Sato-Okoshi, W.; Subhan, B. Genetic Differentiation in Red and Green Noctiluca scintillans in Jakarta Bay, Indonesia. J. Mar. Sci. Eng. 2025, 13, 866. https://doi.org/10.3390/jmse13050866
Nugraha MI, Nishitani G, Ishikawa A, Hadi S, Sato-Okoshi W, Subhan B. Genetic Differentiation in Red and Green Noctiluca scintillans in Jakarta Bay, Indonesia. Journal of Marine Science and Engineering. 2025; 13(5):866. https://doi.org/10.3390/jmse13050866
Chicago/Turabian StyleNugraha, Muhammad Izzat, Goh Nishitani, Akira Ishikawa, Sutanto Hadi, Waka Sato-Okoshi, and Beginer Subhan. 2025. "Genetic Differentiation in Red and Green Noctiluca scintillans in Jakarta Bay, Indonesia" Journal of Marine Science and Engineering 13, no. 5: 866. https://doi.org/10.3390/jmse13050866
APA StyleNugraha, M. I., Nishitani, G., Ishikawa, A., Hadi, S., Sato-Okoshi, W., & Subhan, B. (2025). Genetic Differentiation in Red and Green Noctiluca scintillans in Jakarta Bay, Indonesia. Journal of Marine Science and Engineering, 13(5), 866. https://doi.org/10.3390/jmse13050866









