Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions
Abstract
:1. Introduction
2. Soft Rock Softening Test
2.1. Softening Test of Soft Rock under Seawater Conditions
2.1.1. Purpose of the Test
2.1.2. Test Content and Process
- (1)
- The rock samples were immersed in two kinds of soaking solutions for 30 days. The pH value and main cationic concentration of the solution were measured in the first 7 days, and the pH value was measured every 12 h, while the ion concentration was measured every 24 h.
- (2)
- During the initial 24 h of soaking, macroscopic changes in the soft rock were observed at intervals of 1 h, and the fracture development and disintegration were recorded.
- (3)
- A uniaxial compressive strength test was carried out on the natural rock samples after 30 days of immersion.
2.2. Inhibition Testing of the Ions on Soft Rock Softening
2.2.1. Testing Programme
- (1)
- After preparation of the solution was completed and maintaining the solution for 30 min, the initial concentration of the solution remained constant, and it was assumed that there was no solute precipitation.
- (2)
- After each sampling, it is assumed that the concentration of the solution remains consistent before and after sampling and does not change with the change in volume.
2.2.2. Test Process
3. Softening Law of Soft Rock under Seawater Conditions
3.1. Effect of Seawater on Soft Rock Softening
3.1.1. Macroscopic Variation Law of the Rock
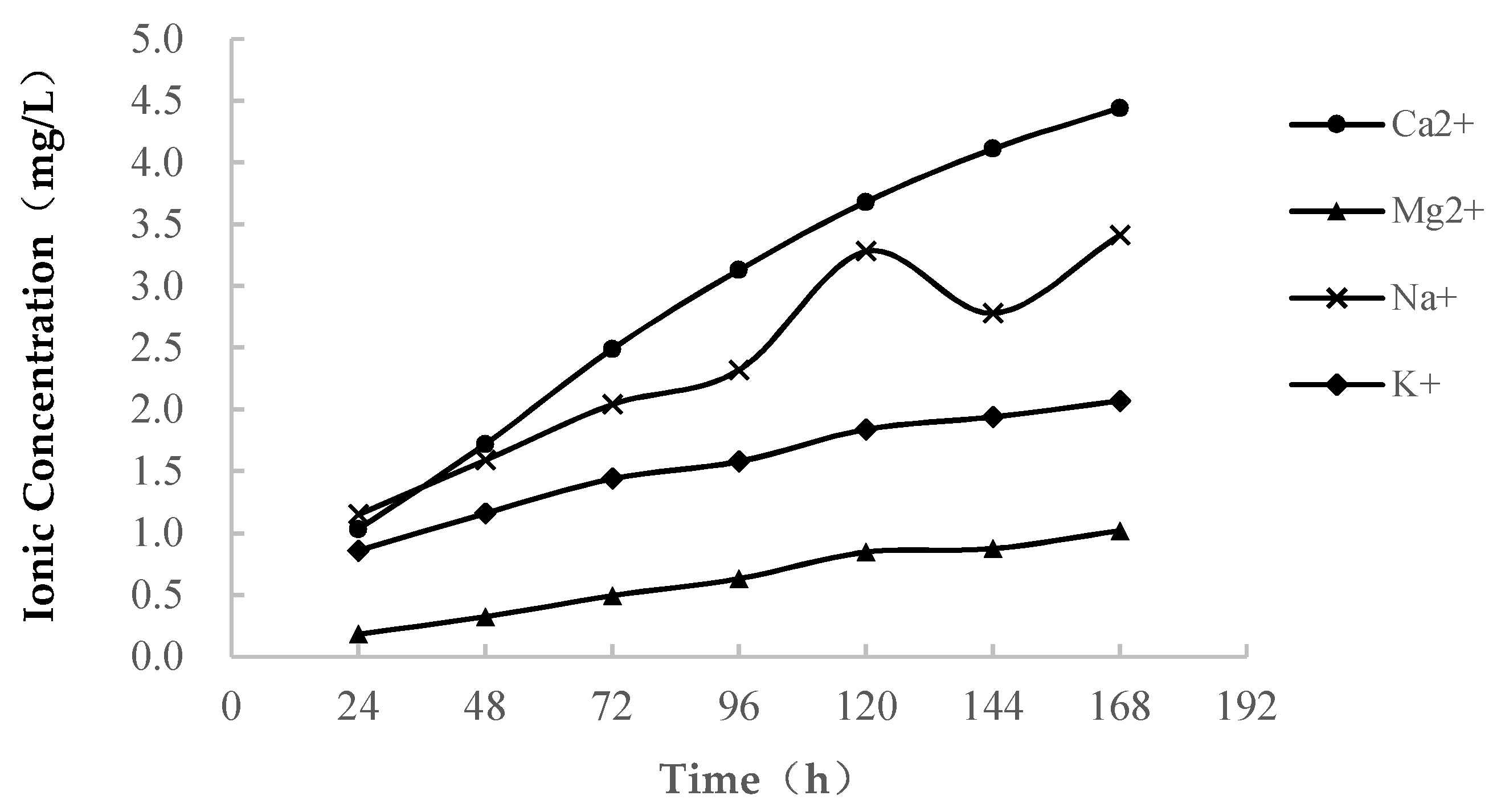
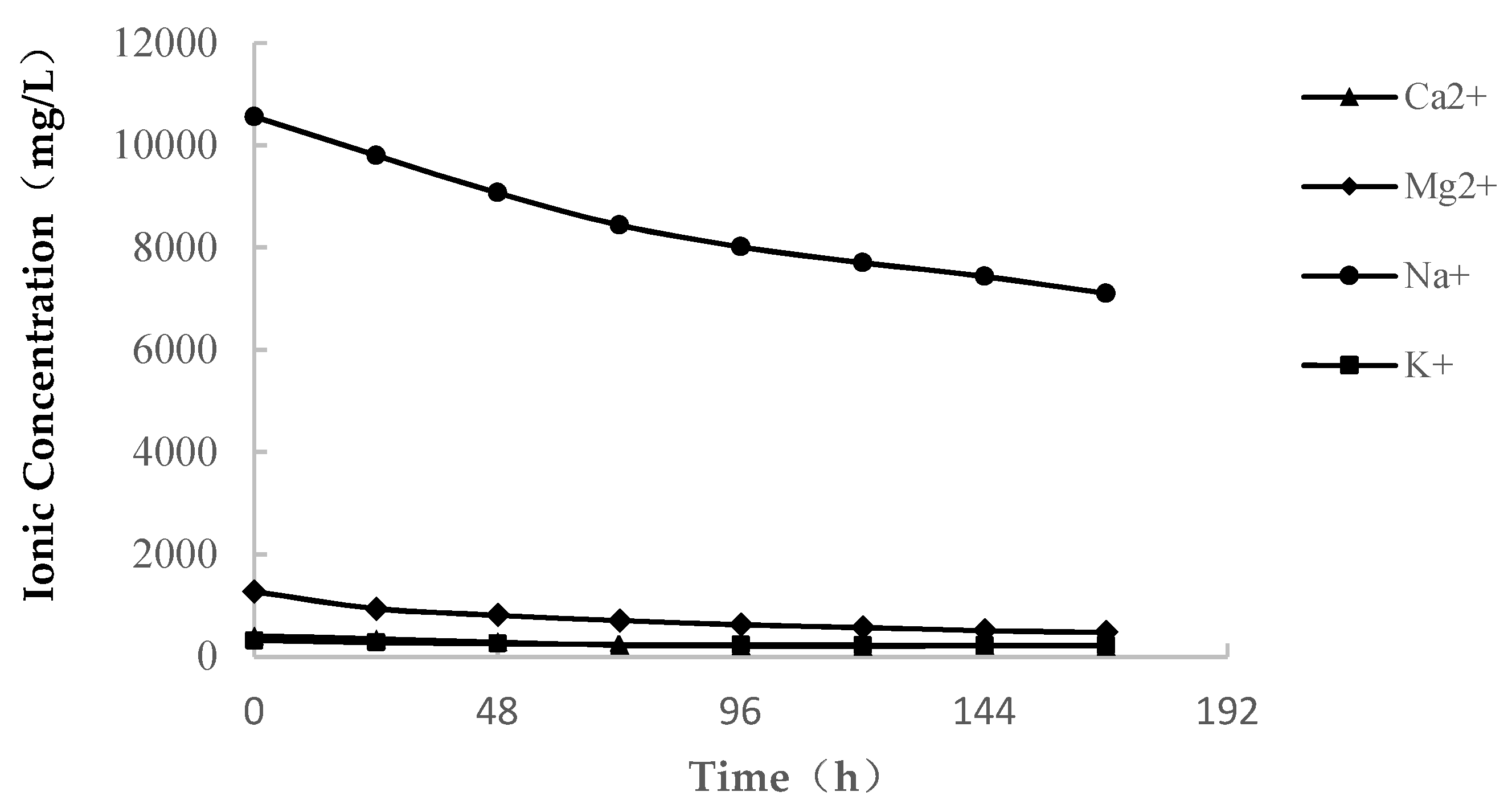
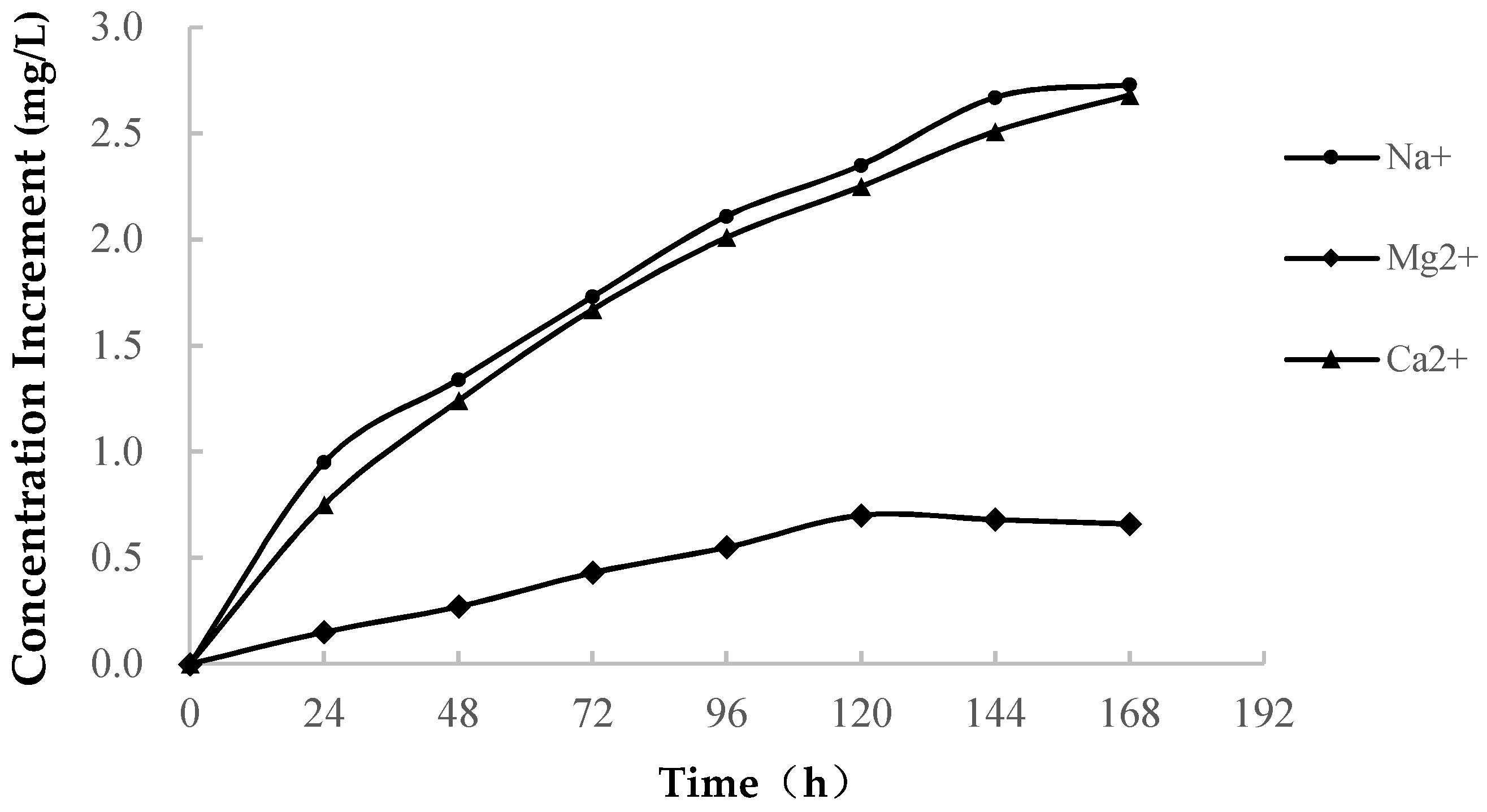
3.1.2. Change in the Ion Concentration
- (1)
- In pure water immersion, the dissolution of soft rock is very strong, large amounts of Ca2+, Mg2+, Na+ and K+ are ionized, and the concentration of each ion increases. These ions are the main components of soluble minerals, which are often present in the ionic state in solutions. When sample immersion lasts approximately 120 h, the dissolution of minerals gradually weakens, the upward trend of the ion concentration slows down, and ion exchange increases.
- (2)
- In seawater immersion, there are high concentrations of Ca2+, Mg2+, Na+ and K+ in the initial solution. With the process of softening, the cations are continuously consumed under the actions of water and rock, and the ion concentration decreases gradually. When the initial solution reaches a certain saturated state, the softened soft rock tends to be stable, and the concentration of metal cations in the solution remained stable.
- (3)
- Through the variation curve of the cationic concentration of the solution during the soft rock saturated with water, it is found that the concentrations of Ca2+, Mg2+, Na+ and K+ change greatly, which indicates that the softening and disintegration of soft rock in seawater may be related to the change in the main ion content in seawater.
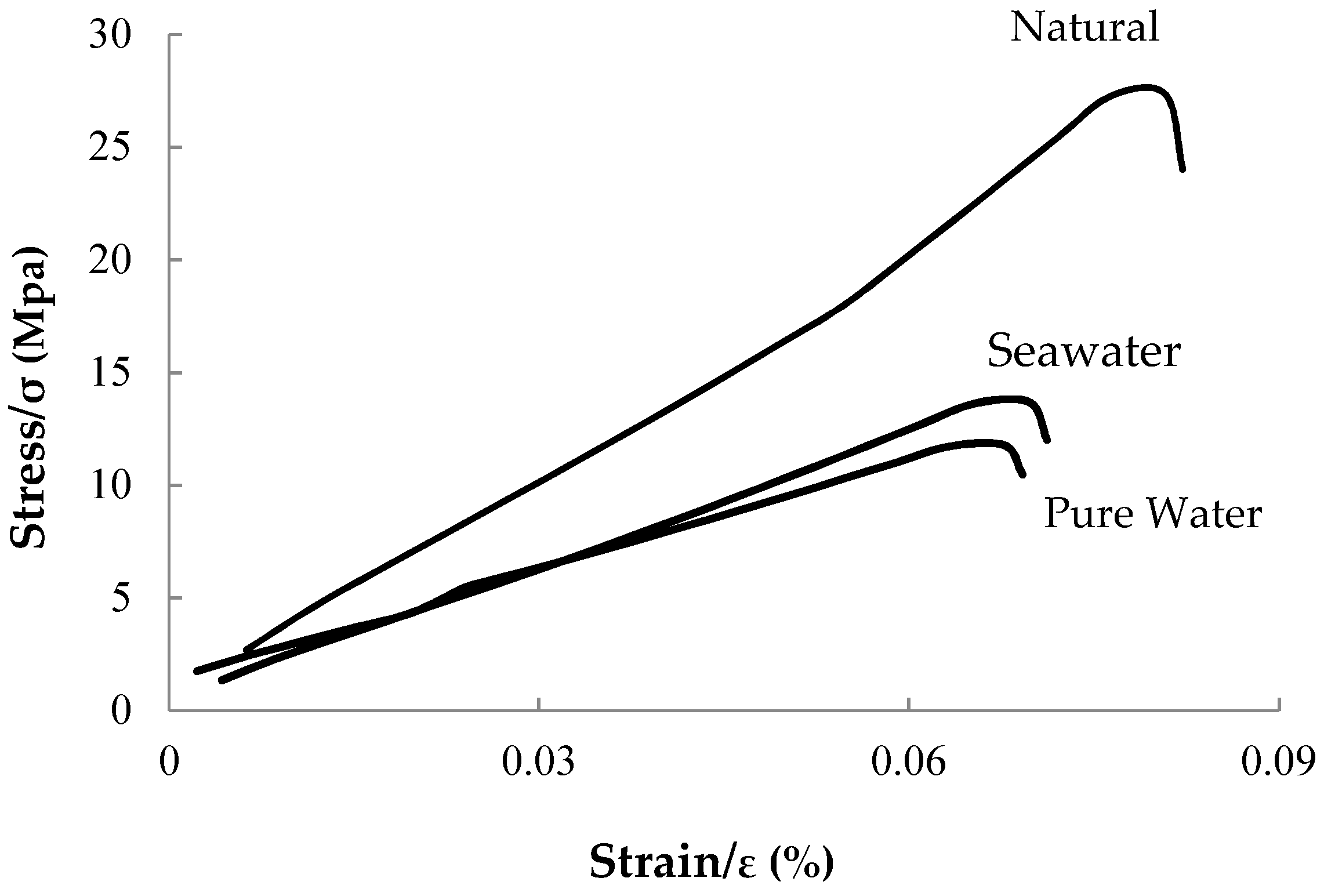
3.1.3. Uniaxial Compressive Strength Test
3.2. Inhibitory Influences of the Ions on the Softening of Soft Rock
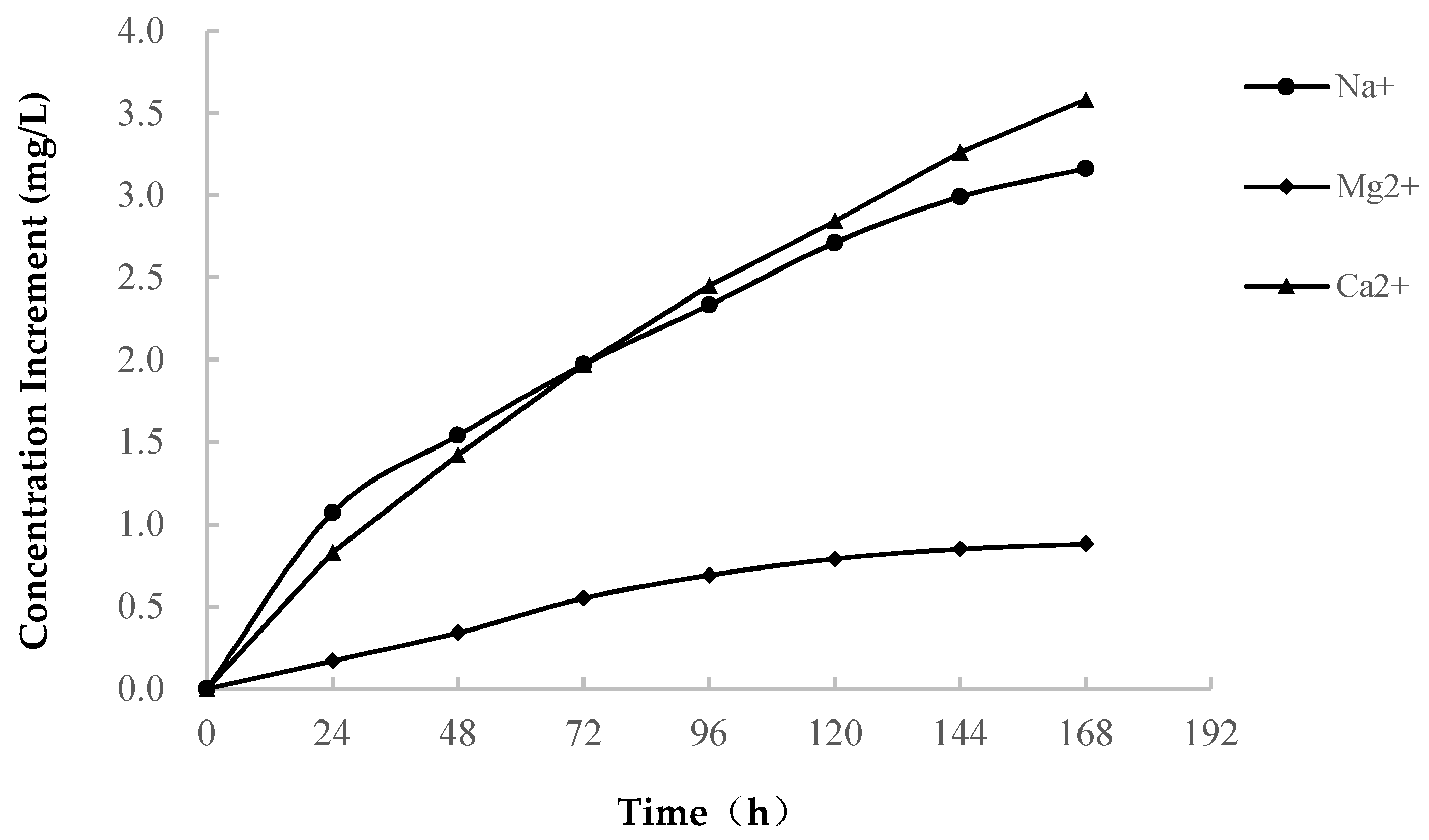
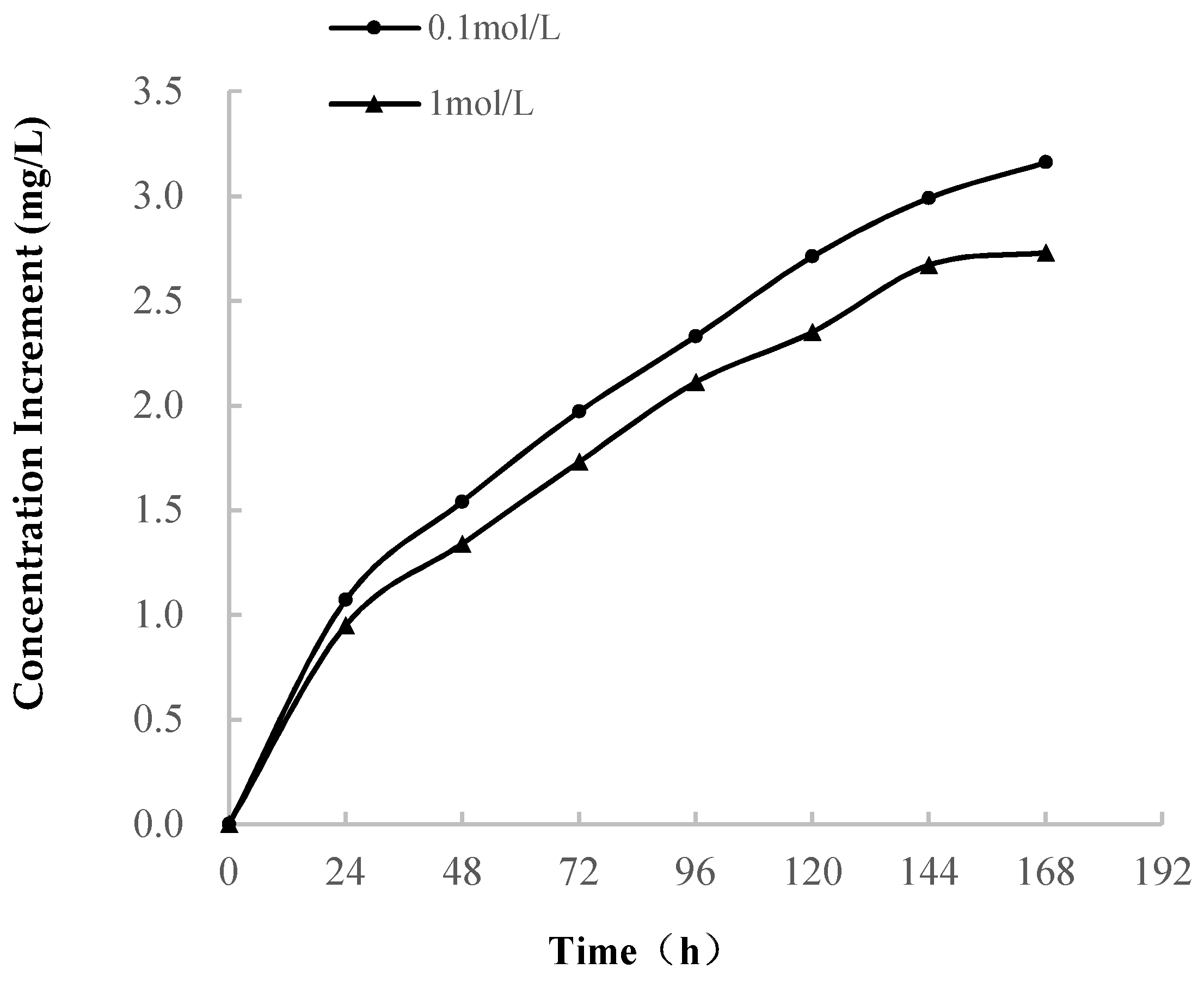
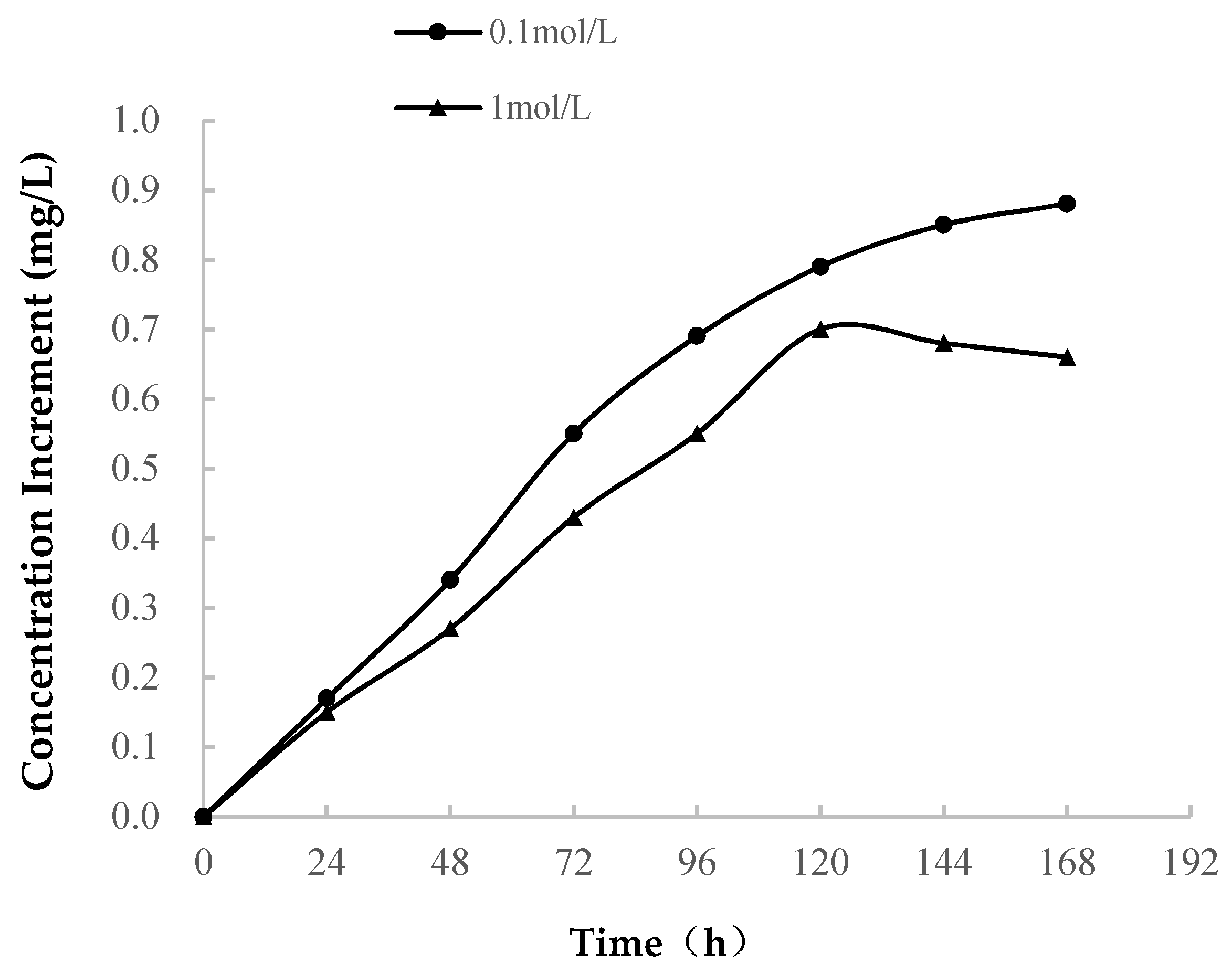
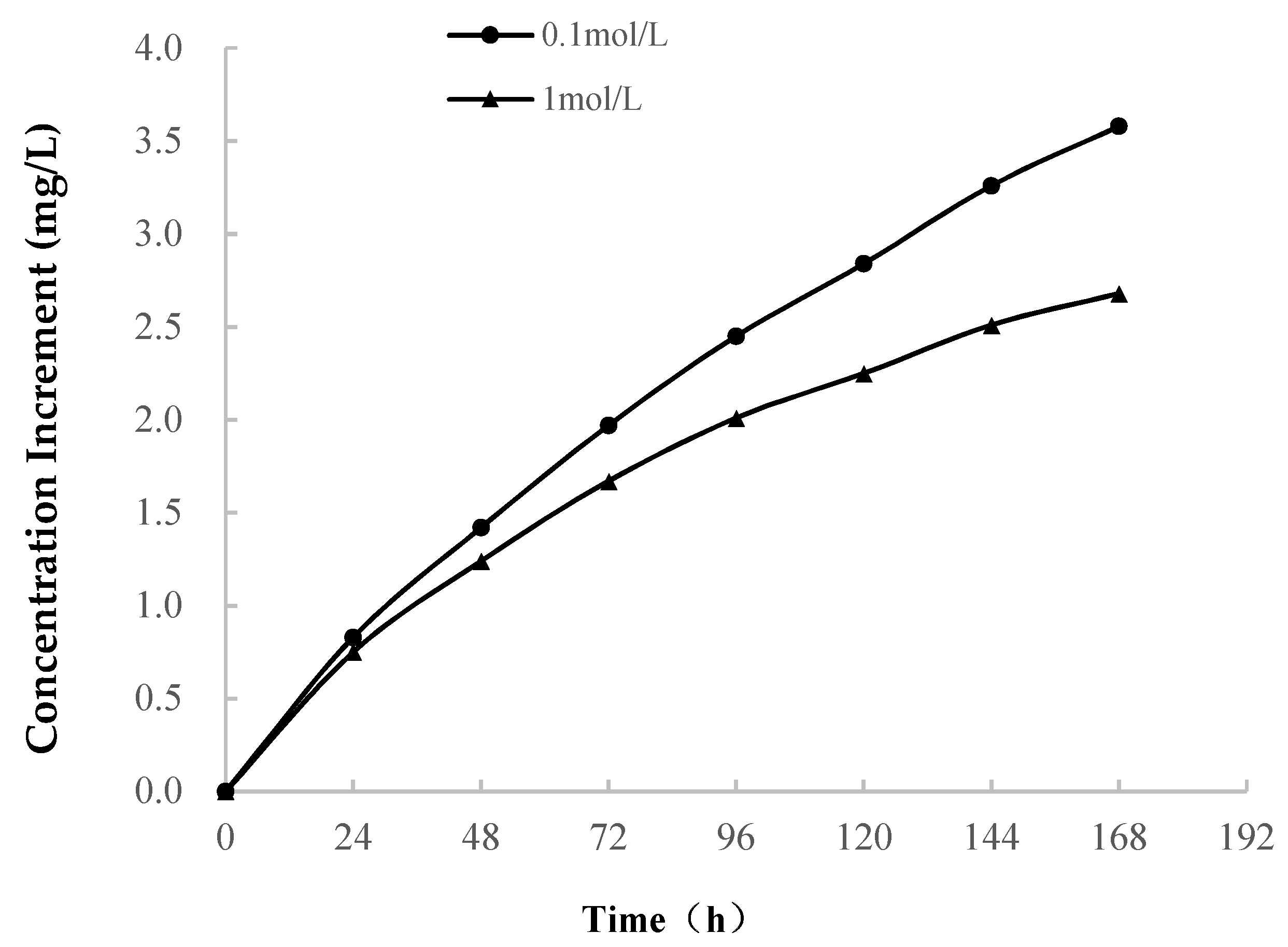
3.2.1. Variations in Ion Concentration
- (1)
- As shown in Table 1, Ca is the main component of soft rock, which occurs in the calcareous cement that connects the clay particles. When the soft rock encounters water, the soluble calcareous cement dissolves. The concentration of Ca2+ increases at a high rate in the early stage of softening. The rapid increase in the Na+ concentration is related to the dissolution of soluble minerals, and it is possible that albite dissolves and Na+ precipitates.(Dissolution of albite)
- (2)
- In the MgCl2 solution, the lattice substitution of Mg2+ is limited to the montmorillonite structure, and the negative charge formed after substitution is compensated by Na+ and Ca2+. In the NaCl and CaCl2 solutions, the amounts of Na+ and Ca2+ consumed to compensate the negative charges are smaller than that of Mg2+ consumed by substitution. Moreover, the Mg content in soft rock is low, and the amount of Mg2+ formed by the dissolution reaction is also low.
3.2.2. Analysis of the Softening Coefficient
- (1)
- Ca2+ and Mg2+ are bivalent cations. In the lattice substitution of montmorillonite and kaolinite, the exchange capacity of divalent ions is greater than that of the monovalent Na+.
- (2)
- According to the influence of the ion radius on the exchange capacity, because the order of the ion radius is Ca2+ > Mg2+ > Na+, the order of the ion exchange capacity is Ca2+ > Mg2+ > Na+.
- (3)
- In the three solutions, the higher the concentration of the ionic solution is, the more soft rock softening is inhibited.
4. Softening Mechanism of Soft Rock under Seawater Conditions
4.1. Mechanism of Water Absorption, Expansion and Disintegration of the Clay Minerals
- (1)
- Because kaolinite, Illite and other clay minerals have small particles and are strongly hydrophilic, when interacting with seawater, the water molecules enter the layered clay mineral particles and form polarized water molecular layers between the particles. These water molecular layers can continuously absorb water and expand, resulting in an increasing gap between the particles. At the same time, water molecules enter the unit cell interlayers of the clay minerals, forming an interlayer water layer in the minerals. Relatively speaking, it is easier for water molecules to enter the interparticle gap than to enter the interlayer of each particle. The former causes the external expansion of clay minerals, called intragranular expansion, and the latter causes internal expansion, called intra-particle expansion. Existing studies [40] show that the physical and chemical reactions between Illite and water can cause the expansion of soft rock, which can increase the original volume by 50% to 60%. The chemical reaction process is as follows:
- (2)
- According to the two kinds of expansion of clay minerals, the clay particles of soft rock can absorb much water during water immersion, which increases the unit cell distance or thickens the diffusion layer, and the minerals expand. When the expansion stress exceeds the bonding effect, the clay cements are destroyed and disintegrated, while the clastic particles gravitationally disintegrate due to the lost connections. In addition, due to the uneven water absorption and expansion of clay minerals, an uneven stress distribution gradually develops in the soft rock, resulting in a large number of micropores, which destroy the original internal structure of the rock sample. Finally, the phenomenon leads to the fragmentation and disintegration of rock particles.
4.2. Ion Exchange-Adsorption
- (1)
- Ion valence number: the larger the ion valence number is, the more prone to ion exchange;
- (2)
- Ion radius: the larger the ion radius is, the smaller the ion hydration radius is, the stronger the adsorption and exchange capacities of ions are. Usually, the order of the ion exchange capacity from weak to strong is as follows:Li+ < Na+ < K+ (NH4+) < Mg2+ < Ca2+ < Ba2+ < Al3+ < Fe3+ < H+
- (3)
- Ion concentration: the higher the ion concentration is, the stronger the exchange capacity.
4.3. Dissolution and Corrosion of the Soluble Minerals
5. Soft Rock Softening Control under Seawater Conditions
5.1. Control Principle Based on the Solubility Product Rule and Same Ion Effect
- (1)
- Qi = Ksp: the solution is a saturated solution with no precipitation occurring and has obtained dynamic equilibrium;
- (2)
- Qi < Ksp: the solution is not saturated and there is no precipitation. If there are solids in the system, the solids will dissolve until the solution becomes saturated.
- (3)
- Qi > Ksp: the solution is oversaturated, and the equilibrium moves to the left with increasing precipitation, while the ion concentration in the solution is reduced depending on the saturation of the solution.
5.2. Soft Rock Softening Control Method under Seawater Conditions
- (1)
- When a project is executed in a coastal area, a certain proportion or volume of seawater can be considered a soft rock softening inhibitor, which improves the mechanical properties of the soft rock packing structure to a certain extent. For marine engineering or coastal engineering, when the engineering strength requirement is not high, soft rock can be considered as foundation filling for the project, which will greatly reduce the construction cost.
- (2)
- In addition, when the project is executed in an inland area, a certain amount of calcium salt can be added to the soft rock packing structure, and the overall stability and mechanical properties of the soft rock can be improved by relying on the inhibitory effect of calcium ions on the softening of soft rock.
6. Conclusions
- (1)
- In the same external environment, seawater has a certain inhibitory effect on the softening of soft rock compared with pure water, and the inhibitory effect is related to the main cations. The order of the inhibitory effect was Ca2+ > Mg2+ > Na+.
- (2)
- According to the inhibitory effect of the main cations on soft rock, calcium salt can be added to soft rock, supplemented by a cement slurry, silicone and other traditional chemical modifiers to modify the soft rock to reduce its softness and the disintegration of soft rock.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Kang, T.H.; Han, W.M.; Liu, Z.P.; Chai, Z.Y. Electrochemical modification of the porosity and zeta potential of montmorillonitic soft rock. Geomech. Eng. 2010, 2, 191–202. [Google Scholar] [CrossRef]
- Miščević, P.; Vlastelica, G. Shear strength of weathered soft rock–proposal of test method additions. In Proceedings of the Regional Symposium of the International Society for Rock Mechanics, Leiden, The Netherlands, 10 January 2009; pp. 303–308. [Google Scholar]
- Chai, Z.Y. Feasibility analysis of electrochemical modification of materialized soft rock. In Proceedings of the Ninth National Congress on Rock Mechanics and Engineering, Beijing, China, 2015. [Google Scholar]
- Miščević, P.; Vlastelica, G. Durability Characterization of Marls from the Region of Dalmatia, Croatia. Geotech. Geol. Eng. 2011, 29, 771–781. [Google Scholar] [CrossRef]
- Liu, X.M.; Xiong, L.; Zhang, L.L.; Zhao, M.H. Experimental study on collapse inhibition measures of Class I Red Sandstone. Highw. Traffic Sci. Technol. 2011, 28, 25–29. [Google Scholar]
- Zhang, P. Study on Physical Properties and Modification Mechanism of Mudstone; Taiyuan University of Technology: Taiyuan, China, 2014. [Google Scholar]
- Wang, L.G.; Zhang, P.; Yang, J.L.; Li, X.L. Study on modification of soft rock and its microscopic mechanism. Silic. Circ. 2015, 34, 99–105. [Google Scholar]
- Vlastelica, G.; Miščević, P.; Štambuk Cvitanović, N. Durability of soft rocks in Eocene flysch formation (Dalmatia, Croatia). Eng. Geol. 2018, 245, 207–217. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, D.; Kang, G.X.; Kang, T.H. Mechanism and Effect of Electrochemical Modification on Physicochemical Soft Rock. Adv. Mater. Res. 2011, 415–417, 2275–2280. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, D.; Kang, T.H. Electrochemical Modification of Physicochemical Soft Rock. Energy Procedia 2012, 16, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.O. Expansion and deformation of mudstone in different hydrochemical environments. Min. Technol. 2015, 15, 44–45. [Google Scholar]
- Yang, J.L.; Wang, L.G.; Li, X.L.; Zhao, G.C.; Li, C. Study on water damage law and chemical modification of silty mudstone. Silic. Notif. 2016, 35, 1883–1890. [Google Scholar]
- Liu, B.; Wang, M. Experimental study on the mechanical modification of soft rock grouting. Coal Technol. 2014, 33, 293–306. [Google Scholar]
- Wang, Q.; Pan, R.; Jiang, B.; Li, S.C.; He, M.C.; Sun, H.B.; Wang, L.; Qin, Q.; Yu, H.C.; Luan, Y.C. Study on failure mechanism of roadway with soft rock in deep coal mine and confined concrete support system. Eng. Fail. Anal. 2017, 81, 155–177. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, Y.J.; Wang, W.J.; Yu, W.J. Failure mechanism of Mesozoic soft rock roadway in Shajihai coal mine and its surrounding rock control. Int. J. Min. Sci. Technol. 2014, 24, 853–858. [Google Scholar] [CrossRef]
- Yu, W.J.; Wang, W.J.; Chen, X.Y.; Du, S.H. Field investigations of high stress soft surrounding rocks and deformation control. J. Rock Mech. Geotech. Eng. 2015, 7, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Vlastelica, G.; Miščević, P.; Pavić, N. Testing the shear strength of soft rock at different stages of laboratory simulated weathering. Građevinar 2016, 68, 955–966. [Google Scholar]
- Chen, S.M.; Wu, A.X.; Wang, Y.M.; Chen, X.; Yan, Y.F.; Ma, H.J. Study on repair control technology of soft surrounding rock roadway and its application. Eng. Fail. Anal. 2018, 92, 443–455. [Google Scholar] [CrossRef]
- Yang, S.Q.; Chen, M.; Jing, H.W.; Chen, K.F.; Meng, B. A case study on large deformation failure mechanism of deep soft rock roadway in Xin’An coal mine, China. Eng. Geol. 2017, 217, 89–101. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, C.K.; Chong, D.Y.; Liu, Y.; Li, S.C. Catastrophe mechanism and disaster countermeasure for soft rock roadway surrounding rock in Meihe mine. Int. J. Min. Sci. Technol. 2015, 25, 407–413. [Google Scholar] [CrossRef]
- Li, G.C.; Jiang, Z.H.; Lv, C.X.; Huang, C.; Chen, G.; Li, M.Y. Instability mechanism and control technology of soft rock roadway affected by mining and high confined water. Int. J. Min. Sci. Technol. 2015, 25, 573–580. [Google Scholar] [CrossRef]
- Liu, J.X. Simulating quasi-brittle failures including damage-induced softening based on the mechanism of stress redistribution. Appl. Math. Model. 2018, 55, 685–697. [Google Scholar] [CrossRef]
- Meng, B.; Jing, H.W.; Chen, K.F.; Su, H.J. Failure mechanism and stability control of a large section of very soft roadway surrounding rock shear slip. Int. J. Min. Sci. Technol. 2013, 23, 127–134. [Google Scholar] [CrossRef]
- Yang, X.J.; Wang, E.Y.; Wang, Y.J.; Gao, Y.B.; Wang, P. A study of the large deformation mechanism and control techniques for deep soft rock roadways. Sustainability 2018, 10, 1100. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhao, M.H.; Su, Y.H. Study on the control method of the disintegrable road of the red layer soft rock. Rock Soil Mech. 2005, 19, 111–114. [Google Scholar]
- Hu, P.; Wang, Y.H.; Qing, Q.X. Laboratory experimental study on the characteristics of soft rock improved soil. J. Hunan Univ. Technol. 2007, 2, 96–99. [Google Scholar]
- Li, X.H.; Yao, Q.L.; Man, J.K.; Chen, C.Q.; He, L.H. Development of fractures in soft rock surrounding a roadway and their control. Min. Sci. Technol. 2011, 21, 573–579. [Google Scholar] [CrossRef]
- Vakhnenko, O.O.; Vakhnenko, V.O.; Shankland, T.J. Soft-ratchet modeling of end-point memory in the nonlinear resonant response of sedimentary rocks. Phys. Rev. B 2005, 71, 174103. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.L.; Wang, L.G.; Li, X.L.; Zhang, P. Study on double Modification of mudstone subgrade in Open Pit Mine. J. Geotech. Eng. 2015, 37, 1469–1477. [Google Scholar]
- Ermano, D.A.; Marcel, R.A.G.; Bas, H. Damage Characterization of Rock Slopes. J. Mar. Sci. Eng. 2019, 7, 10. [Google Scholar] [Green Version]
- Marcel, R.A.G.; Ermano, D.A.; Bas, H. Statistical Analysis of the Stability of Rock Slopes. J. Mar. Sci. Eng. 2019, 7, 60. [Google Scholar] [Green Version]
- Liu, Z.; He, X.F.; Zhou, C.Y. Influence Mechanism of Different Flow Patterns on the Softening of Red-Bed Soft Rock. J. Mar. Sci. Eng. 2019, 7, 155. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Lu, Y.Q.; Liu, Z.; Zhang, L.H. An Innovative Acousto-optic-Sensing-Based Triaxial Testing System for Rocks. Rock Mech. Rock Eng. 2019. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Zhu, F.X. An elasto-plastic damage constitutive model with double yield surfaces for saturated soft rock. Int. J. Rock Mech. Min. Sci. 2010, 47, 385–395. [Google Scholar] [CrossRef]
- Miščević, P.; Vlastelica, G. Estimation of embankment settlement caused by deterioration of soft rock grains. Bull. Eng. Geol. Environ. 2019, 78, 1843–1853. [Google Scholar] [CrossRef]
- ASTM D4644-16; Standard Test Method for Slake Durability of Shales and Other Similar Weak Rocks; ASTM International: West Conshohocken, PA, USA, 2016.
- Franklin, J.A.; Chandra, R. Slake-durability test. Int. J. Rock Mech. Min. Sci. 1972, 9, 325–341. [Google Scholar] [CrossRef]
- Czerewko, M.A.; Cripps, J.C. Assessing the durability of mudrocks using the modified jar slake index test. Q. J. Eng. Geol. Hydrogeol. 2001, 34, 153–163. [Google Scholar] [CrossRef]
- ASTM D7012-14e1; Standard Test Methods for Compressive Strength and Elastic Moduli of Intact Rock Core Specimens under Varying States of Stress and Temperatures; ASTM International: West Conshohocken, PA, USA, 2014.
- Wang, L.; Deng, H.; Deng, T.H.; Zhu, J.J. Experimental study on the correlation between collapse resistance and particle size of mudstone. J. Yangtze Acad. Sci. 2017, 34, 120–124. [Google Scholar]
- Zhan, X.Y. Mineralogy and sintered brick and tile production. Brick Tile World. 2007, 01, 49–56. [Google Scholar]
- Dixon, J.B.; Schulze, D.G. Soil Mineralogy with Environmental Applications; Soil Science Society of America, Inc.: Madison, AM, USA, 2002. [Google Scholar]
- Cao, F.Q.; Mao, J.Y. Basic Chemistry; Nanjing Southeast University Press: Nanjing, China, 2006; pp. 71–72. [Google Scholar]
- Xiong, L. Study on Collapse Mechanism of Red Bed Soft Rock and its Engineering Application; Hunan University: Changsha, China, 2011. [Google Scholar]

| Sample Label | Relative Content Percentage of Each Element (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | K | Ca | Fe | |
| 01 | 11.79 | 48.46 | 0.52 | 5.01 | 21.21 | 2.51 | 7.85 | 2.66 |
| 02 | 13.73 | 49.33 | 0.37 | 3.43 | 16.24 | 1.49 | 14.44 | 0.96 |
| 03 | 12.89 | 49.54 | — | 1.81 | 13.91 | 1.06 | 20.15 | 0.64 |
| Content | Compressive Strength (MPa) | |||
|---|---|---|---|---|
| Group | I | II | Average Value | |
| Natural | 25.8 | 26.7 | 26.25 | |
| Pure Water | 10.02 | 10.21 | 10.12 | |
| Seawater | 13.36 | 13.97 | 13.67 | |
| Concentration | Compressive Strength (MPa) | ||
|---|---|---|---|
| Solution | 0.1 mol/L | 1 mol/L | |
| NaCl | 11.68 | 13.47 | |
| CaCl2 | 13.67 | 17.38 | |
| MgCl2 | 12.86 | 15.47 | |
| Concentration | Softening Coefficient | ||
|---|---|---|---|
| Solution | 0.1 mol/L | 1 mol/L | |
| NaCl | 0.44 | 0.46 | |
| CaCl2 | 0.52 | 0.58 | |
| MgCl2 | 0.49 | 0.51 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; He, X.; Fan, J.; Zhou, C. Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions. J. Mar. Sci. Eng. 2019, 7, 235. https://doi.org/10.3390/jmse7070235
Liu Z, He X, Fan J, Zhou C. Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions. Journal of Marine Science and Engineering. 2019; 7(7):235. https://doi.org/10.3390/jmse7070235
Chicago/Turabian StyleLiu, Zhen, Xinfu He, Jin Fan, and Cuiying Zhou. 2019. "Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions" Journal of Marine Science and Engineering 7, no. 7: 235. https://doi.org/10.3390/jmse7070235
APA StyleLiu, Z., He, X., Fan, J., & Zhou, C. (2019). Study on the Softening Mechanism and Control of Red-Bed Soft Rock under Seawater Conditions. Journal of Marine Science and Engineering, 7(7), 235. https://doi.org/10.3390/jmse7070235





