Depth Selection and In Situ Validation for Offshore Mussel Aquaculture in Northeast United States Federal Waters
Abstract
1. Introduction
2. Materials & Methods
2.1. Depth Suitability for Offshore Mussel Farming in New England
2.1.1. Study Area
2.1.2. Temperature Data
2.1.3. Chlorophyll a Data
2.1.4. Statistical Analysis
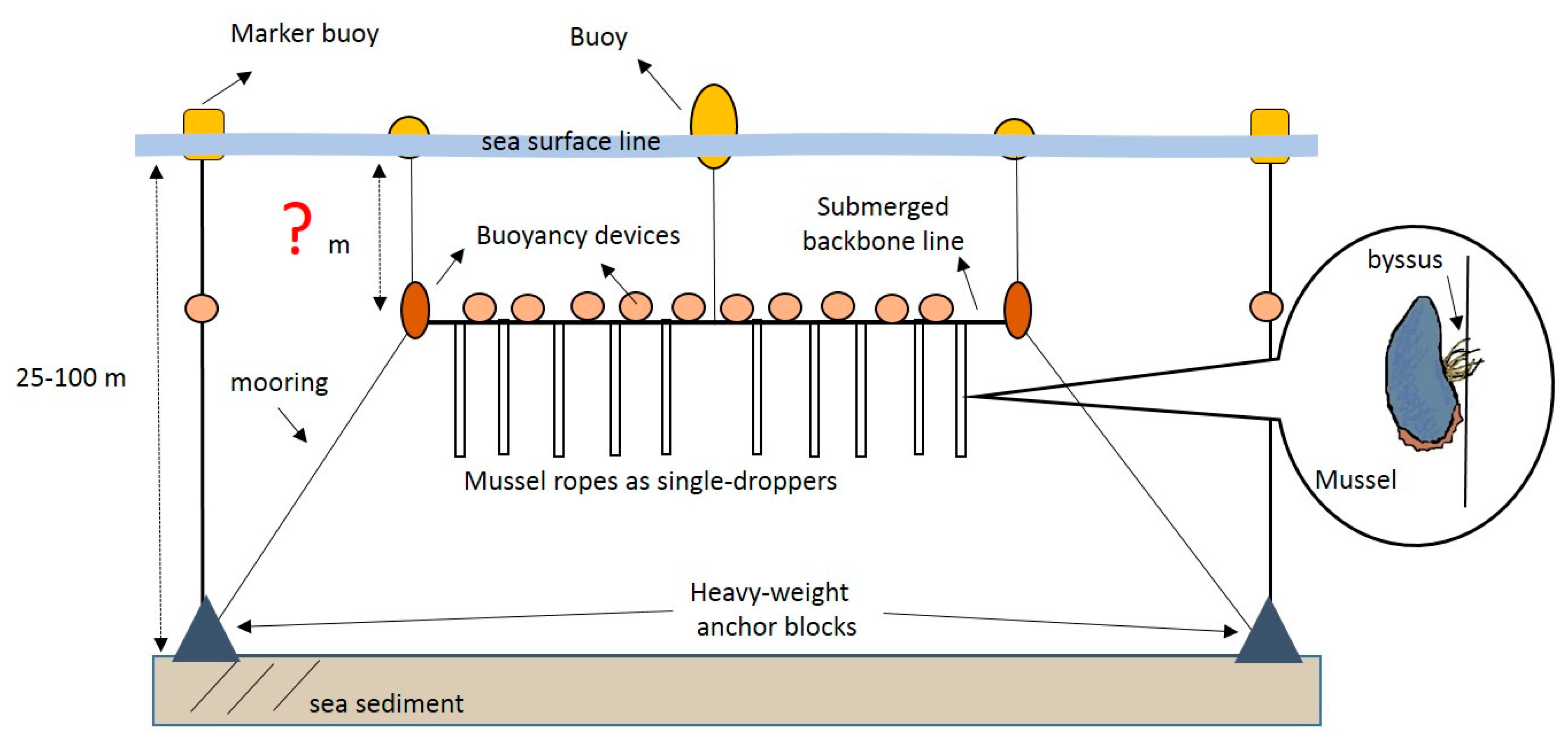
2.2. Mussel Biodeposition Measurements at Experimental Farm in Cape Ann
2.2.1. Study Site
2.2.2. Experimental Design
2.2.3. Vertical Profiles of Water Column
2.2.4. Chlorophyll a Data
2.2.5. Phytoplankton Identification
2.2.6. Particle Differentiation and Quantification by Flow Cytometer
2.2.7. Statistical Analysis
3. Results
3.1. Depth Suitability Analysis
3.1.1. Bathymetry
3.1.2. Temperature Trends
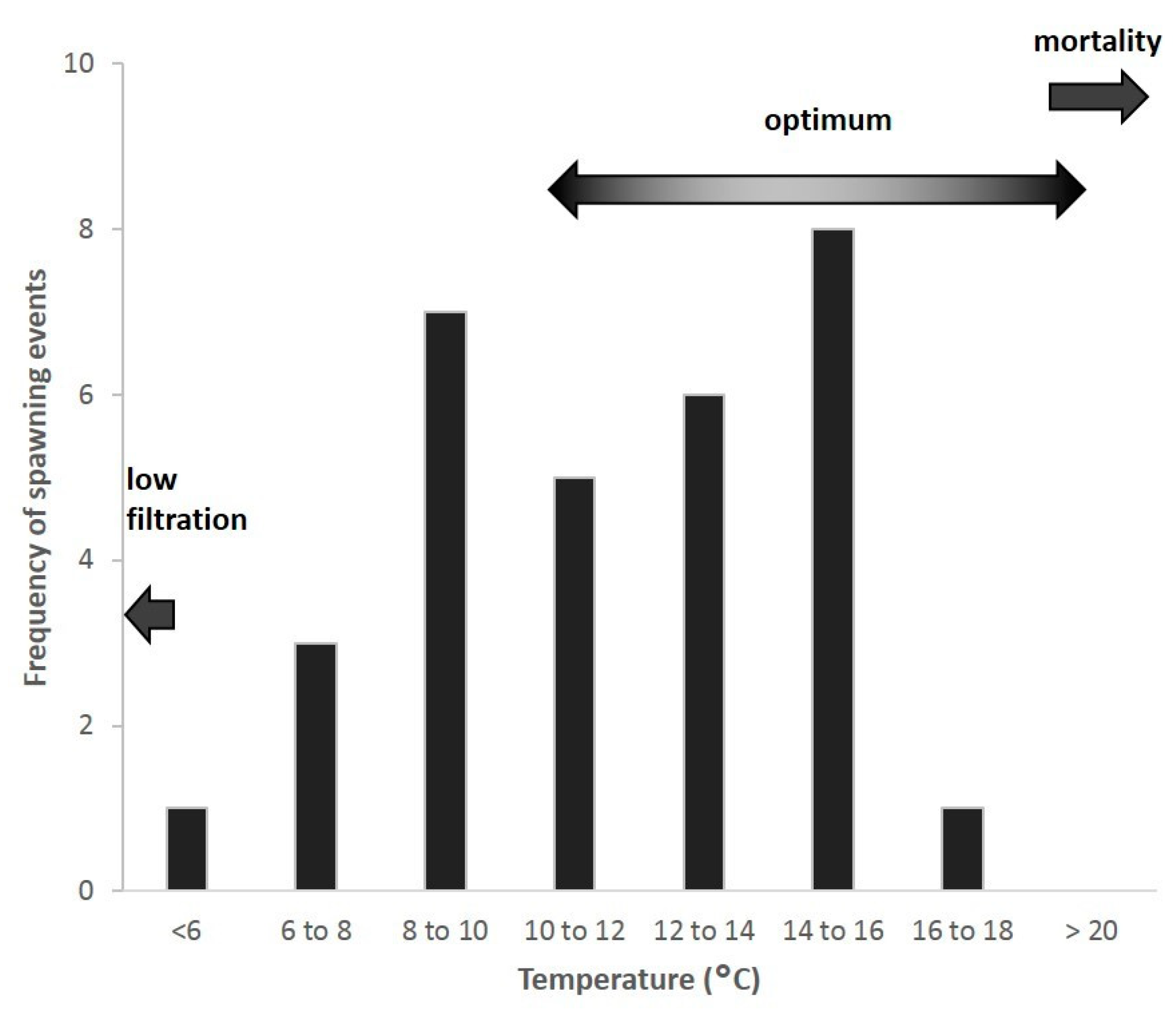
3.1.3. Temperature Thresholds
3.1.4. Thermal Stratification
3.1.5. Temperature Spatial Distribution
3.1.6. Thermal Characteristics of Prospective Farming Sites
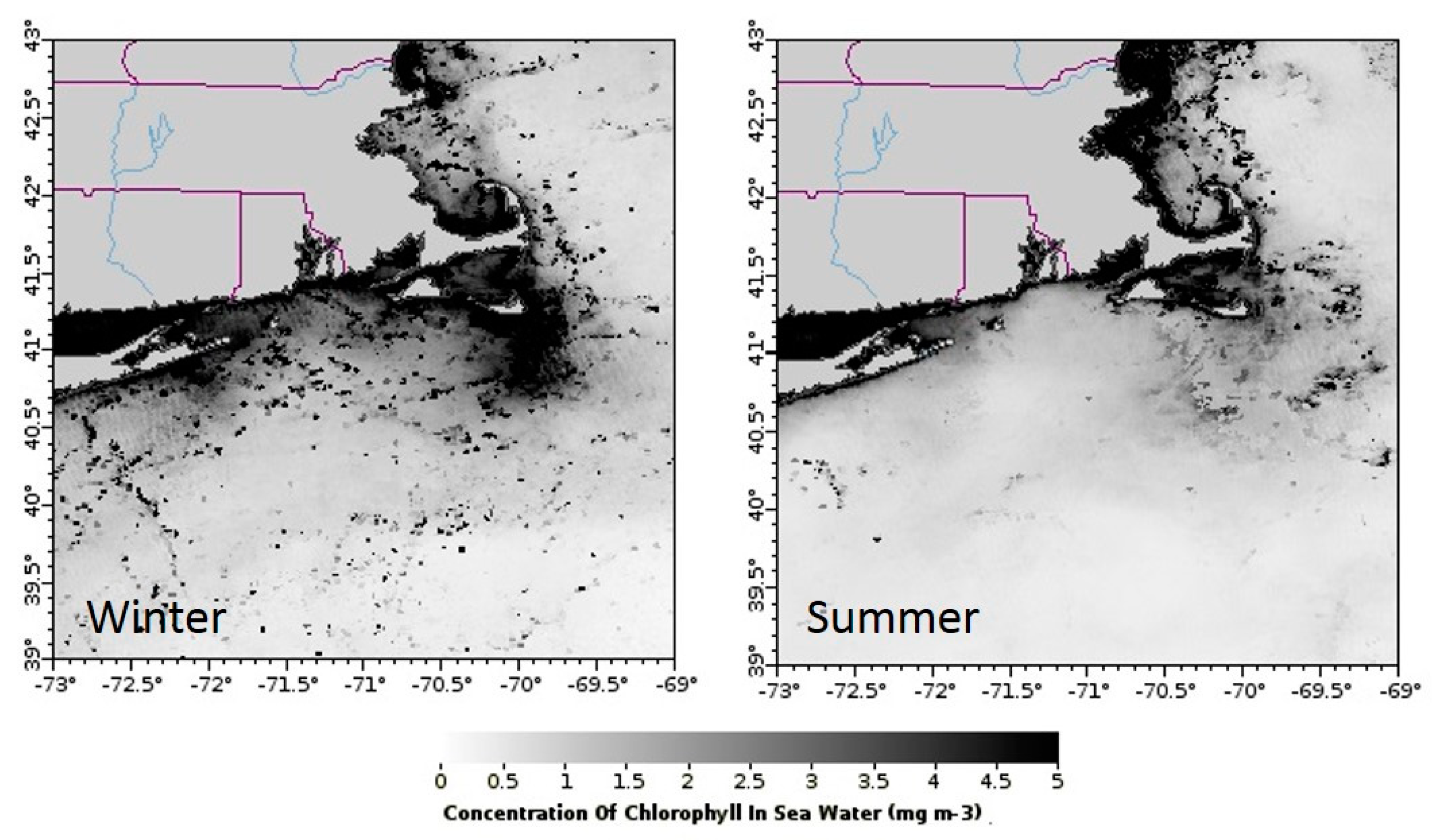
3.1.7. Chlorophyll a
3.2. In Situ Biodeposition
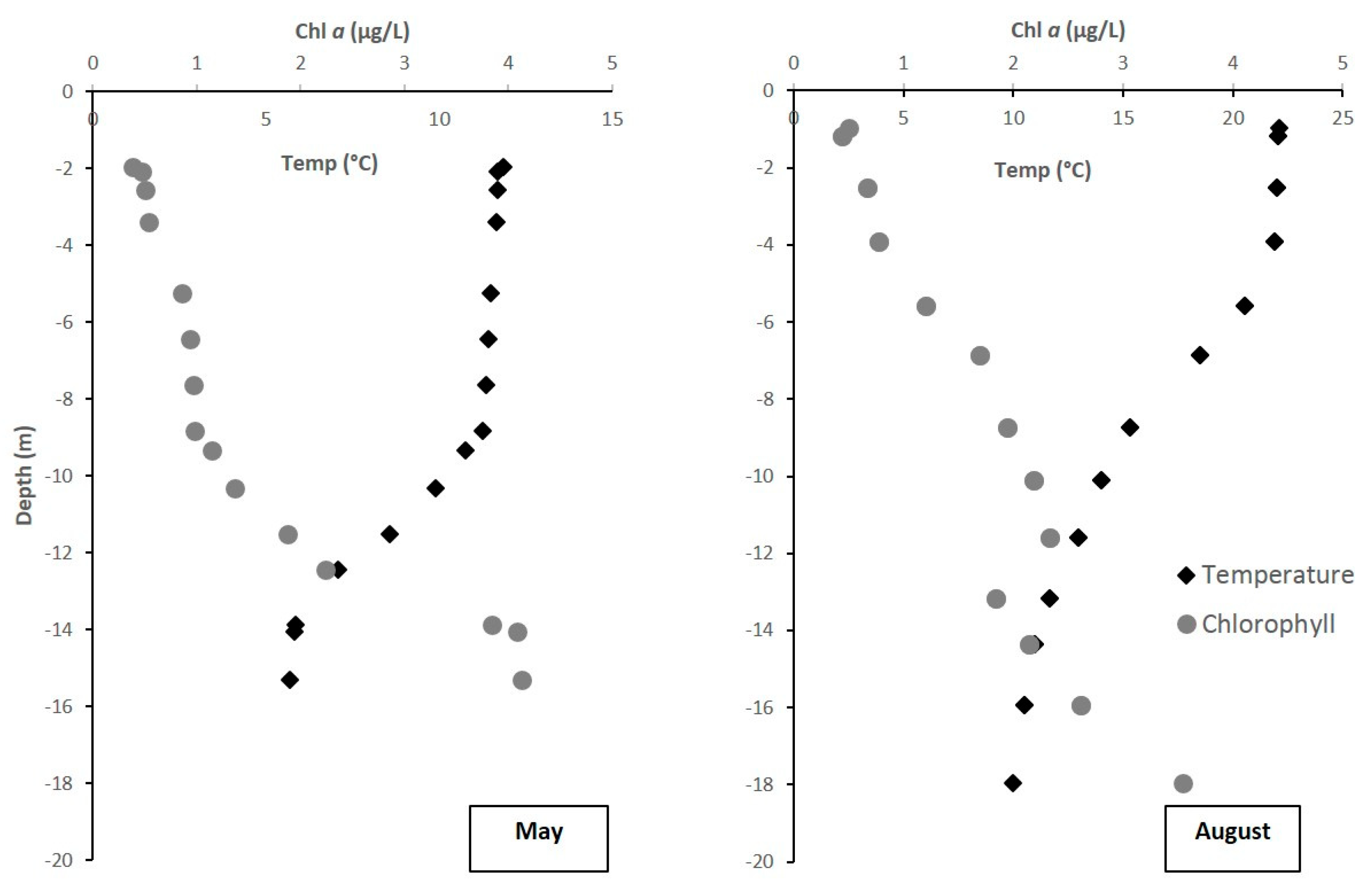
3.2.1. Environmental Characteristics
3.2.2. Mussel Performance
4. Discussion
4.1. General New England Area
4.2. Experimental Farm at Cape Ann
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA). Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; p. 200. [Google Scholar]
- Rubino, M. (Ed.) Offshore Aquaculture in the United States: Economic Considerations, Implications & Opportunities; NOAA Technical Memorandum NMFS F/SPO, 103; U.S. Department of Commerce: Silver Spring, MD, USA, 2008; p. 263.
- Knapp, G.; Rubino, M.C. The political economics of marine aquaculture in the United States. Rev. Fish. Sci. Aquac. 2016, 24, 213–229. [Google Scholar] [CrossRef]
- Environmental Law Institute. U.S. Army Corps of Engineers Regulation of Offshore Aquaculture. 2015. Available online: https://www.eli.org/tags/us-army-corps-engineers (accessed on 12 December 2017).
- Gimpel, A.; Stelzenmuller, V.; Grote, B.; Buck, B.H.; Floeter, J.; Núñez-Riboni, I.; Pogoda, B.; Temming, A. A GIS modeling framework to evaluate marine spatial planning scenarios: Co-location of offshore wind farms and aquaculture in the German EEZ. Mar. Pol. 2015, 55, 102–115. [Google Scholar] [CrossRef]
- Wever, L.; Krause, G.; Buck, B.H. Lessons from stakeholder dialogues on marine aquaculture in offshore wind farms: Perceived potentials, constraints and research gaps. Mar. Pol. 2015, 51, 251–259. [Google Scholar] [CrossRef]
- Island Institute. The Maine Guide to Mussel Raft Culture; Island Institute: Rockland, ME, USA, 1999. [Google Scholar]
- Goseberg, N.; Chambers, M.D.; Heasman, K.; Fredriksson, D.; Fredheim, A.; Schlurmann, T. Technological approaches to longlined and cage-based aquaculture in open ocean environments. In Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene; Buck, B., Langan, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–95. [Google Scholar]
- Crawford, C.M.; Macleod, C.K.A.; Mitchell, I.M. Effects of shellfish farming on the benthic environment. Aquaculture 2003, 224, 117–140. [Google Scholar] [CrossRef]
- Lester, S.E.; Gentry, R.R.; Kappel, C.V.; White, C.; Gaines, S.D. Offshore aquaculture in the United States: Untapped potential in need of smart policy. PNAS 2018, 115, 7126–7165. [Google Scholar] [CrossRef] [PubMed]
- Price, C.S.; Keane, E.; Morin, D.; Vaccaro, C.; Bean, D.; Morris, J.A., Jr. Protected Species & Longline Mussel Aquaculture Interactions; NOAA Technical Memorandum NOS NCCOS; NOAA: Silver Spring, MD, USA, 2016; p. 85.
- Benetti, D.D.; Benetti, G.I.; Rivera, J.A.; Sardenberg, B.; O’Hanlon, B. Site selection criteria for open ocean aquaculture. Mar. Technol. Soc. J. 2010, 44, 22–35. [Google Scholar] [CrossRef]
- Tlusty, M.F.; Wikgren, B.; Laguex, K.; Kite-Powell, H.; Jin, D.; Hoagland, P.; Kenney, R.D.; Kraus, S.D. Co-ocurrence mapping of disparate data sets to assess potential aquaculture sites in the Gulf of Maine. Rev. Fish. Sci. Aquac. 2018, 26, 70–85. [Google Scholar] [CrossRef]
- Lester, S.E.; Costello, C.; Halpern, B.S.; Gaines, S.D.; White, C.; Barth, J.A. Evaluating tradeoffs among ecosystem services to inform marine spatial planning. Mar. Pol. 2013, 38, 80–89. [Google Scholar] [CrossRef]
- Gentry, R.R.; Lester, S.E.; Kappel, C.V.; White, C.; Bell, T.W.; Stevens, J.; Gaines, S.D. Offshore aquaculture: Spatial planning principles for sustainable development. Ecol. Evol. 2017, 7, 733–743. [Google Scholar] [CrossRef]
- Kite-Powell, H.L. Business Planning for Mussel Longline Operations. In Proceedings of the Offshore Mussel Aquaculture Farming Workshop, Shelton, CT, USA, 7 February 2011; Available online: http://www.mbl.edu/bell/scientific-aquaculture/2011-mussel-farming-workshop/ (accessed on 15 October 2017).
- Rosland, R.; Bacher, C.; Strand, O.; Aure, J.; Strohmeier, T. Modelling growth variability in longline mussel farms as a function of stocking density and farm design. J. Sea Res. 2011, 66, 318–330. [Google Scholar] [CrossRef]
- Thomas, Y.; Mazurie, J.; Alunno-Bruscia, M.; Bacher, C.; Bouget, J.F.; Gohin, F.; Pouvreau, S.; Struski, C. Modelling spatio-temporal variability of Mytilus edulis (L.) growth by forcing a dynamic energy budget model with satellite-derived environmental data. J. Sea Res. 2011, 66, 308–317. [Google Scholar] [CrossRef]
- Sara, G.; Reid, G.K.; Rinaldi, A.; Palmeri, V.; Troell, M.; Kooijman, S.A.L.M. Growth and reproductive simulation of candidate shellfish species at fish cages in the Southern Mediterranean: Dynamic Energy Budget (DEB) modelling for integrated multi-trophic aquaculture. Aquaculture 2012, 324, 259–266. [Google Scholar] [CrossRef]
- Kapetsky, J.M.; Aguilar-Manjarrez, J.; Jenness, J. A Global Assessment of Offshore Mariculture Potential from a Spatial Perspective; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2013. [Google Scholar]
- NOAA. Trade Specific US Custom. Commercial Fisheries Statistics. 2019. Available online: https://www.st.nmfs.noaa.gov/commercial-fisheries/foreign-trade/ (accessed on 14 February 2019).
- Buck, B.H.; Nevejan, N.; Wille, M.; Chambers, M.D.; Chopin, T. Offshore and multi-use aquaculture with extractive species: Seaweeds and bivalves. In Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene; Buck, B., Langan, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–95. [Google Scholar]
- Buck, B.H. Experimental trial on the feasibility of offshore seed production of the mussel Mytilus edulis in the German Bight: Installation, technical requirements and environmental conditions. Helgol. Mar. Res. 2007, 61, 87–101. [Google Scholar] [CrossRef]
- Atalah, J.; Fletcher, L.M.; Hopkins, G.A.; Heasman, K.; Woods, C.M.C.; Forrest, B.M. Preliminary assessment of biofouling on offshore mussel farms. J. World Aquacult. Soc. 2016, 47, 376–386. [Google Scholar] [CrossRef]
- Holmyard, J. Offshore Mussel farm Theory and Reality. In Proceedings of the Oceanology International 2016, London, UK, 15–17 March 2016; Available online: www.oceanologyinternational.com/_novadocuments/230188?v=635950419875030000 (accessed on 13 February 2018).
- Van den Burg, S.W.K.; Kamermans, P.; Blanch, M.; Pletsas, D.; Poelman, M.; Soma, K.; Dalton, G. Business case for mussel aquaculture in offshore wind farms in the North Sea. Mar. Pol. 2017, 85, 1–7. [Google Scholar] [CrossRef]
- Icely, J. Science and offshore mussel culture at Sagres, Portugal. J. Shellfish Res. 2012, 31, 299. [Google Scholar]
- Hoagland, P.; Kite-Powell, H.; Paul, W.; Hampson, G. Biological Assessment for a Blue Mussel Ocean Aquaculture Experiment in Rhode Island Sound. Technical Paper WHOI. 1998. 31p. Available online: https://hdl.handle.net/1912/9409 (accessed on 10 July 2018).
- Langan, R.; Horton, F. Design, Operation and economics of submerged longline mussel culture in the Open Ocean. Bull. Aquacul. Assoc. Can. 2003, 103, 11–20. [Google Scholar]
- Maney, E.J., Jr.; Fregeau, M.; Buttner, J.K.; Weston, S.; Lee, B. Mussel culture on long lines on Cape Ann, Massachusetts—An update. J. Shellfish Res. 2010, 29, 555, Technical Paper of the 30th Milford Aquaculture Seminar, 8–10 February 2010. [Google Scholar]
- Cicin-Sain, N.; Bunsick, S.M.; DeVoe, R.; Eichenberg, T.; Ewart, J.; Halvorson, H.; Knecht, R.; Rheault, R. Development of a Policy Framework for Offshore Marine Aquaculture in the 3–200 Mile U.S. Ocean Zone; Center for the Study of Marine Policy, University of Delaware: Newark, DE, USA, 2001. [Google Scholar]
- Fairbanks, L. Moving mussels offshore? Perceptions of offshore aquaculture policy and expansion in New England. Ocean Coast. Manag. 2016, 130, 1–12. [Google Scholar] [CrossRef]
- Helmuth, B.S.T. Intertidal mussel microclimates: Predicting the body temperature of a sessile invertebrate. Ecol. Monogr. 1998, 68, 51–71. [Google Scholar] [CrossRef]
- Comeau, L.A.; Pernet, F.; Tromblay, R.; Bates, S.S.; LeBlanc, A. Comparison of eastern oyster (Crassostrea virginica) and blue mussel (Mytilus edulis) filtration rates at low temperatures. Can. Tech. Rep. Fish. Aquat. Sci. 2008, 2810. vii + 17 p. [Google Scholar]
- Zippay, M.L.; Helmuth, B. Effects of temperature change on mussel, Mytilus. Integr. Zool. 2012, 7, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, H.S. Growth of the sea mussel. Contrib. Can. Biol. Fish. 1929, 4, 121–136. [Google Scholar] [CrossRef]
- Bayne, B.L. Marine Mussels: Their Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1976; 506p. [Google Scholar]
- Stirling, H.P.; Okumus, I. Growth and production of mussels (Mytilus edulis L.) suspended at salmon cages and shellfish farms in two Scottish sea lochs. Aquaculture 1995, 134, 193–210. [Google Scholar] [CrossRef]
- Lesser, M.P.; Witman, J.D.; Sebens, K.P. Effects of flow and seston availability on scope for growth of benthic suspension feeding invertebrates from the Gulf of Maine. Biol. Bull. 1994, 187, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, E.P.; Stillman, J.H.; Menge, B.A. Physiological community ecology: Variation in metabolic activity of ecologically important rocky intertidal invertebrates along environmental gradients. Integr. Comp. Biol. 2002, 42, 862–871. [Google Scholar] [CrossRef]
- Mizuta, D.D.; Silveira, N., Jr.; Fischer, C.E.; Lemos, D. Interannual variation in commercial oyster (Crassostrea gigas) farming in the sea (Florianópolis, Brazil, 27°44′ S; 48°33′ W) in relation to temperature, chlorophyll a and associated oceanographic conditions. Aquaculture 2010, 366–367, 105–114. [Google Scholar] [CrossRef]
- Cheney, D.; Langan, R.; Heasman, K.; Friedman, B.; Davis, J. Shellfish culture in the open ocean: Lesson learned for offshore expansion. Mar. Technol. Soc. J. 2010, 44, 55–67. [Google Scholar] [CrossRef]
- Pershing, A.J.; Alexander, M.A.; Hernandez, C.M.; Kerr, L.A.; Le Bris, A.; Mills, K.E.; Nye, J.A.; Record, M.R.; Scannell, H.A.; Scott, J.D.; et al. Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science 2015, 350, 809–812. [Google Scholar] [CrossRef]
- Fratantoni, P.; Holzwarth-Davis, T.; Taylor, M.T. Description of Oceanographic Conditions in the Northeast US Continental Shelf During 2015; Northeast Fisheries Science Center Reference Document 17-08; NOAA: Silver Spring, MD, USA, 2017; p. 36.
- Lachance, A.A.; Hennebicq, R.; Myrand, B.; Sevigny, J.M.; Kraffe, E.; Marty, Y.; Marcotte, I.; Tremblay, R. Biochemical and genetic characteristics of suspension-cultured mussels, (Mytilus edulis) in relation to byssal thread production and losses by fall-off. Aquat. Living Resour. 2011, 24, 283–293. [Google Scholar] [CrossRef][Green Version]
- Carrington, E. Seasonal variation in the attachment strength of blue mussels: Causes and consequences. Limnol. Oceanogr. 2002, 47, 1723–1733. [Google Scholar] [CrossRef]
- Lachance, A.A.; Myrand, B.; Tremblay, R.; Koutitonsky, V.; Carrington, E. Biotic and abiotic factors influencing attachment strength of blue mussel Mytilus edulis in suspended culture. Aquat. Biol. 2008, 2, 119–129. [Google Scholar] [CrossRef]
- Garner, Y.N.; Litvaitis, M.K. Effects of wave exposure, temperature and epibionte fouling on byssal thread production and growth in the blue mussel, Mytilus edulis, in the Gulf of Maine. J. Exp. Mar. Biol. Ecol. 2013, 466, 52–56. [Google Scholar] [CrossRef]
- Seguin-Heine, M.-O.; Lachance, A.A.; Genard, B.; Myrand, B.; Pellerin, C.; Marcotte, I.; Tremblay, R. Impact of open sea habitat on byssus attachment of suspension-cultured blue mussels (Mytillus edulis). Aquaculture 2014, 426–427, 189–196. [Google Scholar] [CrossRef]
- Hawkins, A.; Bayne, B. Seasonal variation in the relative utilization of carbon and nitrogen by the mussel Mytilus edulis; budget, conversion efficiencies and maintenance requirements. Mar. Ecol. Prog. Ser. 1985, 25, 181–188. [Google Scholar] [CrossRef]
- Griffiths, C.; King, J. Energy expended on growth and gonad output in the ribbed mussel Aulacomya ater. Mar. Biol. 1979, 53, 217–222. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Hilbish, T.J.; Koehn, R.K.; Newell, C.J. Temporal variation in the reproductive cycle of Mytilus edulis L. (Bivalvia, Mytilidae) from localities on the east coast of the United States. Biol. Bull. 1982, 162, 299–310. [Google Scholar] [CrossRef]
- Moeser, M.; Carrington, E. Seasonal variation in mussel byssal thread mechanics. J. Exp. Biol. 2006, 209, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.; Moeser, G.M.; Dimond, J.; Mello, J.J.; Boller, M.L. Seasonal disturbance to mussel beds: Field test of a mechanistic model predicting wave dislodgment. Limnol. Oceanogr. 2009, 54, 978–986. [Google Scholar] [CrossRef]
- Loosanoff, V.L.; Davis, H.C. Delaying spawning of lamellibranchs by low temperature. J. Mar. Res. 1951, 10, 197–202. [Google Scholar]
- Lindell, S. Hatchery and Nursery for Improved Production of Blue Mussels. In Annual Report of the Northeast Fisheries Aquaculture Center, for the Period of 2015 and 2016; United States Department of Agriculture: Washington, DC, USA, 2016; pp. 16–30. Available online: http://agresearch.umd.edu/nrac/annual-reports#overlay-context=nrac/publications-0 (accessed on 14 March 2018).
- Schneider, K.R. Heat stress in the intertidal: Comparing survival and growth of an invasive and native mussel under a variety of thermal conditions. Biol. Bull. 2008, 130, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Bayne, B.L.; Iglesias, J.I.P.; Hawkins, A.J.S.; Navarro, E.; Heral, M.; Deslous-Paoli, J.M. Feeding behaviour of the mussel, Mytilus edulis: Responses to variations in quantity and organic content of the seston. J. Mar. Biol. Assoc. UK 1993, 73, 813–829. [Google Scholar] [CrossRef]
- Galimany, E.; Ramón, M.; Ibarrola, I. Feeding behavior of the mussel Mytilus galloprovincialis (L.) in a Mediterranean estuary: A field study. Aquaculture 2011, 314, 236–243. [Google Scholar] [CrossRef]
- Cartier, S.; Pellerin, J.; Fournier, M.; Tamigneaux, E.; Girault, L.; Lemaire, N. Use of an index based on the blue mussel (Mytilus edulis and Mytilus trossulus) digestive gland weight to assess the nutrional quality of mussel farm sites. Aquaculture 2004, 241, 633–654. [Google Scholar] [CrossRef]
- Sherman, K.; Jaworski, N.A.; Smayda, T.J. (Eds.) The Northeast Shelf Ecosystem: Assessment, Sustainability and Management; Blackwell Science Inc.: Cambridge, MA, USA, 1996; p. 564. [Google Scholar]
- Lapointe, G. NROC White Paper: Overview of the Aquaculture Sector in New England; Northeast Regional Council: New York, NY, USA, 2013. [Google Scholar]
- Department of Commerce (DOC); National Oceanic and Atmospheric Administration (NOAA); National Ocean Service (NOS); Office for Coastal Management (OCM). Bathymetric Contours; NOAA’s Ocean Service, Office for Coastal Management (OCM): Charleston, SC, USA, 2013.
- Almada-Villela, P.C. The effects of reduced salinity on the shell growth of small Mytilus edulis L. J. Mar. Biol. Assoc. UK 1984, 64, 171–182. [Google Scholar] [CrossRef]
- Bøhle, B. Effects of adaptation to reduced salinity on filtration activity and growth of mussels (Mytilus edulis). J. Exp. Mar. Biol. Ecol. 1972, 10, 41–49. [Google Scholar] [CrossRef]
- Feng, H.; Vandermark, D.; Wilkin, J. Gulf of Maine salinity variation and its correlation with upstream Scotian Shelf currents at seasonal and interannual time scales. J. Geophys. Res. Oceans 2016, 121, 8585–8607. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Lüskow, F.; Pleissner, D.; Lundgreen, L.; López, M.Á.P. Effect of salinity on filtration rates of mussels Mytilus edulis with special emphasis on dwarfed mussels from the low-saline Central Baltic Sea. Helgol. Mar. Res. 2013, 67, 591–598. [Google Scholar] [CrossRef]
- Nelson, T.C. On the distribution of critical temperatures for spawning and for ciliary activity in bivalve molluscs. Science 1928, 67, 220–221. [Google Scholar] [CrossRef]
- Seidov, D.; Baranova, O.K.; Boyer, T.; Cross, S.L.; Mishonov, A.V.; Parsons, A.R. Northwest Atlantic Regional Ocean Climatology; Mishonov, A.V., Ed.; NOAA Atlas NESDIS 80, Tech.; NOAA: Silver Spring, MD, USA, 2016; p. 56. [CrossRef]
- Pondniesinski, G.S.; McAlice, B.J. Seasonality of blue mussel, Mytilus edulis L., larvae in Damariscotta river estuary, Maine, 1969–1977. Fish. Bull. 1986, 84, 995–1001. [Google Scholar]
- Almada-Villela, P.C.; Davenport, J.; Gruffydd, L.L.D. The effects of temperature on the shell growth of young Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 1982, 59, 275–288. [Google Scholar] [CrossRef]
- Newell, C.R.; Hidu, H.; McAlice, B.J.; Podniesinski, G.; Short, F.; Kindblom, L. Recruitment and commercial seed procurement of the blue mussel Mytilus edulis in Maine. J. World Aquacult. Soc. 1991, 22, 134–151. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovalelli, A. Hatchery Culture of Bivalves: A Practical Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 2004; p. 177. [Google Scholar]
- Read, K.R.H.; Cumming, K.B. Thermal tolerance of the bivalve molluscs Modiolus modiolus L., Mytilus edulis L. and Brachidontes demissus Dillwyn. Comp. Biochem. Physiol. 1967, 22, 149–155. [Google Scholar] [CrossRef]
- Engle, J.B.; Loosanoff, V.L. On season of attachment of larvae of Mytilus edulis. Limn. Ecol. 1944, 25, 433–440. [Google Scholar] [CrossRef]
- Myrand, B.; Guderley, H.; Himmelman, J.H. Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence. Mar. Ecol. Prog. Ser. 2000, 197, 193–200. [Google Scholar] [CrossRef]
- Antsulevich, A.E.; Maximovich, N.V.; Vuorinen, I. Population structure, growth and reproduction of the common mussel (Mytilus edulis L.) off the Island of Seili (SW Finland). Boreal Environ. Res. 1999, 4, 367–375. [Google Scholar]
- Heinonen, A. Reproduction of Mytilus edulis L. in Finish south-western archipelago in summer 1960. Arch. Soc. Zool. Bot. Fennicae Vanamo 1961, 16, 137–143. [Google Scholar]
- Kautsky, N. Quantitative studies on gonad cycle, fecundity, reproductive output and recruitment in a Baltic Mytilus edulis population. Mar. Biol. 1982, 68, 143–160. [Google Scholar] [CrossRef]
- Pieters, H.; Kluytmans, J.H.; Zandee, D.I.; Cadee, G.C. Tissue composition and reproduction of Mytilus edulis in relation to food availability. Neth. J. Sea Res. 1980, 14, 349–361. [Google Scholar] [CrossRef]
- Chipperfield, P.N.J. Observations on the breeding and settlement of Mytilus edulis (L.) in British waters. J. Mar. Biol. Assoc. UK 1953, 32, 449–476. [Google Scholar] [CrossRef]
- Thorarinsdottir, G.G. Gonad development, larval settlement and growth of Mytilus edulis L. in a suspended population in Hvalfjorden, south-west Iceland. Aquacult. Res. 1996, 27, 57–65. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Mortality, growth and grazing impact of a cohort of bivalve larvae, Mytilus edulis (L.). Ophelia 1981, 20, 185–192. [Google Scholar] [CrossRef]
- Rasmussen, E. Systematics and ecology of the Isefjord marine fauna (Denmark) with a survey of the eelgrass (Zostera) vegetation and its communities. Ophelia 1973, 11, 1–495. [Google Scholar] [CrossRef]
- Galimany, E.; Rose, J.M.; Dixon, M.S.; Alix, R.; Li, Y.; Wikfors, G.H. Design and use of an apparatus for quantifying bivalve suspension feeding at sea. J. Vis. Exp. 2018, 139, e58213. [Google Scholar] [CrossRef] [PubMed]
- Eckman, J.E.; Peterson, C.H.; Cahalan, J.A. Effects of flow speed, turbulence, and orientation on growth of juvenile bay scallops Argopecten irradians concentricus (Say). J. Exp. Mar. Biol. Ecol. 1989, 132, 123–140. [Google Scholar] [CrossRef]
- Newell, C.R.; Wildish, D.J.; MacDonald, B.A. The effects of velocity and seston concentration on the exhalant siphon area, valve gape and filtration rate of the mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 2001, 262, 91–111. [Google Scholar] [CrossRef]
- Hawkins, A.J.S.; Smith, R.F.M.; Bayne, B.L.; Héral, M. Novel observations underlying the fast growth of suspension-feeding in turbid environments: Mytilus edulis. Mar. Ecol. Prog. Ser. 1996, 131, 179–190. [Google Scholar] [CrossRef]
- Iglesias, J.I.P.; Pérez Camacho, A.; Navarro, E.; Labarta, U.; Beiras, R.; Hawkins, A.J.S.; Widdows, J. Microgeographic variability in feeding, absorption, and condition of mussels (Mytilus galloprovincialis Lmk.): A transplant experiment. J. Shellfish Res. 1996, 15, 673–680. [Google Scholar]
- Galimany, E.; Rose, J.M.; Dixon, M.S.; Wikfors, G.H. Quantifying feeding behavior of ribbed mussels (Geukensia demissa) in two urban sites (Long Island Sound, USA) with different seston characteristics. Estuar. Coast. 2013, 36, 1265–1273. [Google Scholar] [CrossRef]
- Cranford, P.J.; Ward, J.E.; Shumway, S.E. Bivalve filter feeding variability and limits of the aquaculture biofilter, 81–124. In Shellfish Aquaculture and the Environment; Shumway, S.E., Ed.; Wiley-Blackwell John Wiley & Sons, Inc.: West Sussex, UK, 2011; p. 507. [Google Scholar]
- Jones, H.D.; Richards, O.G.; Southern, T.A. Gill dimensions, water pumping rate and body size in the mussel Mytilus edulis L. J. Exp. Mar. Bio. Ecol. 1992, 155, 213–237. [Google Scholar] [CrossRef]
- Iglesias, J.I.P.; Urrutia, M.B.; Ibarrola, I. Measuring feeding and absorption in suspension-feeding bivalves: An appraisal of the biodeposition method. J. Exp. Mar. Biol. Ecol. 1998, 219, 71–86. [Google Scholar] [CrossRef]
- Throndsen, J. Preservation and storage. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 69–74. [Google Scholar]
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton-Methodik Mitt. Internationalen Verein Limnologie 1958, 9, 1–38. [Google Scholar]
- Li, Y.; Veilleux, D.J.; Wikfors, G.H. Particle removal by Northern bay scallops Argopecten irradians in a semi-natural setting: Applications of a flow-cytometric technique. Aquaculture 2009, 296, 237–245. [Google Scholar] [CrossRef]
- Couturier, C. Offshore mussel farming: Role of research and extension. In Proceedings of the Offshore Mussel Aquaculture Farming Workshop, Shelton, CT, USA, 7 February 2011; Available online: http://www.mbl.edu/bell/scientific-aquaculture/2011-mussel-farming-workshop/ (accessed on 15 October 2017).
- Bonardelli, J. Role of extension services to support offshore mussel culture. Shellfish Solutions A.S. In Proceedings of the Offshore Mussel Aquaculture Farming Workshop, Shelton, CT, USA, 7 February 2011; Available online: http://www.mbl.edu/bell/scientific-aquaculture/2011-mussel-farming-workshop/ (accessed on 15 October 2017).
- Varennes, E.; Hanssen, S.A.; Bonardellu, J.; Guillemette, M. Sea duck predation in mussel farms: The best nets for excluding common eiders safely and efficiently. Aquacult. Environ. Interact. 2013, 4, 31–39. [Google Scholar] [CrossRef]
- Khalaman, V.V. Fouling communities of mussel aquaculture installations in the White Sea. Russ. J. Mar. Biol. 2001, 27, 227–237. [Google Scholar]
- Watts, A.M.; Goldstien, S.J.; Hopkins, G.A. Characterizing biofouling communities on mussel farms along an environmental gradient: A step towards improved risk management. Aquacult. Environ. Interact. 2015, 8, 15–30. [Google Scholar] [CrossRef]
- Danioux, C.; Loste, C.; Paquotet, P. Offshore mollusc production in the mediterranean basin. In Options Méditerranéennes—Mediterranean Offshore Mariculture; Muir, J., Basurco, B., Eds.; Serie B, Numéro 30; Etudes et Recherches, CIHEAM, INO Reproducciones: Zaragoza, Spain, 1997; pp. 115–140. [Google Scholar]
- Gosling, E. The mussel Mytilus; ecology, physiology, genetics and culture. Dev. Aquac. Fishe. Sci. 1992, 25, 589. [Google Scholar]
- Jørgensen, C.B.; Lenses, P.S.; Risgard, H.V. Effects of temperature on the mussel pump. Mar. Ecol. Prog. Ser. 1990, 64, 89–97. [Google Scholar] [CrossRef]
- Loo, L.O. Filtration, assimilation, respiration and growth of Mytilus edulis L. at low temperatures. Ophelia 1992, 35, 123–131. [Google Scholar]
- Kittner, C.; Riisgård, H.U. Effect of temperature on filtration rate in mussel Mytilus edulis: No evidence for temperature compensation. Mar. Ecol. Prog. Ser. 2005, 305, 147–152. [Google Scholar] [CrossRef]
- Gibbs, M.T. Interactions between bivalve shellfish farms and fishery resources. Aquaculture 2004, 240, 267–296. [Google Scholar] [CrossRef]
- Duinker, A.; Haland, L.; Hovgaard, P.; Mortensen, S. Gonad development and spawning in one and two year old mussels (Mytilus edulis) from Western Norway. J. Mar. Biol. Assoc. UK 2008, 88, 1465–1473. [Google Scholar] [CrossRef]
- Comeau, L.A.; Filgueira, R.; Davidson, J.D.P.; Nadeau, A.; Guyondet, T.; Sonier, R.; Ramsay, A.; Davidson, J. Population structure and grazing capacity of cultivated mussels in Prince Edward Island, Canada. Can. Tech. Rep. Fish. Aquat. Sci. 2017, 3228. viii+23 p. [Google Scholar]
- NOAA. Foreign Trade Cumulative Data. 2018. Available online: https://www.st.nmfs.noaa.gov/commercial-fisheries/foreign-trade/index (accessed on 29 December 2018).
- Petersen, J.K.; Sejr, M.K.; Larsen, J.E.N. Clearance rates in the Artic bivalves Hiatella artica and Mya sp. Polar Biol. 2003, 26, 334–341. [Google Scholar]
- Ibarra, A.M.; Ascencio-Michel, R.; Ramírez, J.L.; Manzano-Sarabia, M.; Rodríguez-Jaramillo, C. Performance of diploid and triploid Crassostrea gigas (Thunberg, 1793) grown in tropical versus temperate natural environmental conditions. J. Shellfish Res. 2017, 36, 119–139. [Google Scholar] [CrossRef]
- Cranford, P.J.; Hill, P.S. Seasonal variation in food utilization by the suspension-feeding bivalves molluscs Mytilus edulis and Placopecten magellanicus. Mar. Ecol. Prog. Ser. 1999, 190, 223–239. [Google Scholar] [CrossRef]
- Figueiras, F.G.; Labarta, U.; Fernández Reiriz, M.J. Coastal upwelling, primary production and mussel growth in the Rías Baixas of Galicia. Hydrobiologia 2002, 484, 121–131. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Kittner, C.; Seerup, D.F. Regulation of the opening state and filtration rate in filter-feeding bilvalves (Cardium edule, Mytilus edulis, Mya arenaria) in response to low algal concentration. J. Exp. Mar. Biol. Ecol. 2003, 284, 105–127. [Google Scholar] [CrossRef]
- Gosling, E. Marine Bivalve Molluscs, 2nd ed.; Willey Blackwell: Hoboken, NJ, USA, 2015; p. 524. [Google Scholar]
- Riisgård, H.U.; Egede, P.P.; Saavedra, I.B. Feeding behavior of the mussel, Mytilus edulis: New observations, with a minireview of the current knowledge. J. Mar. Biol. 2011, 2011, 312459. [Google Scholar] [CrossRef]
- Newell, C.R.; Langdon, R.I. Mechanisms and physiology of larval and adult feeding. 185–229. In The Eastern Oyster Crassostrea Virginica; Kennedy, V.S., Newell, R.I.E., Eble, A.F., Eds.; Maryland Sea Grant College, University of Maryland System: College Park, MD, USA, 1996; p. 734. [Google Scholar]
- Irisarri, J.; Fernández-Reiriz, M.J.; Robinson, S.M.C.; Cranford, P.J.; Labarta, U. Absorption efficiency of mussels Mytilus edulis and Mytilus galloproviancialis cultured under Integrated Multi-Trophic Aquaculture conditions in the Bay of Fundy (Canada) and Ría Ares-Betanzos (Spain). Aquaculture 2013, 388–391, 182–192. [Google Scholar] [CrossRef]
- Riisgård, H.U. Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar. Ecol. Prog. Ser. 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Cucci, T.L.; Shumway, S.E.; Brown, W.S.; Newell, C.R. Using phytoplankton and flow cytometry to analyze grazing by marine organisms. Cytometry 1989, 10, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Møhlenberg, F.; Riisgård, H.U. Filtration rate, using a new indirect technique, in thirteen species of suspension feeding bivalves. Mar. Biol. 1979, 54, 143–148. [Google Scholar] [CrossRef]
- Silverman, H.; Lynn, J.W.; Beninger, P.G.; Dietz, T.H. The role of latero-frontal cirri in particle capture by the gills of Mytilus edulis. Biol. Bull. 1999, 197, 368–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strohmeier, T.; Strand, O.; Alunno-Bruscia, M.; Duinker, A.; Cranford, P.J. Variability in particle retention efficiency by the mussel Mytilus edulis. J. Exp. Mar. Biol. Ecol. 2012, 412, 96–102. [Google Scholar] [CrossRef]
- Wright, R.T.; Coffin, R.B.; Ersing, C.P.; Pearson, D. Field and laboratory measurements of bivalve filtration of natural marine bacterioplankton. Limnol. Oceanogr. 1982, 27, 91–98. [Google Scholar] [CrossRef]
- Cranford, P.J.; Li, W.; Strand, Ø.; Strohmeier, T. Phytoplankton Depletion by Mussel Aquaculture: High Resolution Mapping, Ecosystem Modeling and Potential Indicators of Ecological Carrying Capacity. ICES CM Document 2008/H:12. 2008. 5p. Available online: www.ices.df/products/CMKdocs/CM-2008/H/H1208.pdf (accessed on 15 January 2018).
- Lutz-Collins, V.O.; Quijón, P.A.; Davidson, J. Blue mussel fouling communities: Polychaete composition in relation to mussel stocking density and seasonality of mussel deployment and sampling. Aquac. Res. 2009, 40, 1789–1792. [Google Scholar] [CrossRef]
- CINEMAR. Open Ocean Aquaculture Demonstration Project. Annual Progress Report for Calendar Year 2001; The Cooperative Institute for New England Mariculture and Fisheries (CINEMAR): Durham, NH, USA, 2002. [Google Scholar]
- CINEMAR. Open Ocean Aquaculture Demonstration Project. Annual Progress Report for Calendar Year 2002; The Cooperative institute for New England Mariculture and Fisheries (CINEMAR): Durham, NH, USA, 2003. [Google Scholar]
- Bayne, B.L. Growth and the delay of metamorphosis of the larvae of Mytilus edulis L. Ophelia 1965, 2, 1–47. [Google Scholar] [CrossRef]







| Physical Effects | Period | Study Region | Temperature (°C) | Reference |
|---|---|---|---|---|
| Filtration rate and growth is significantly lowered | <5 | Bayne [37]; Almada-Vilella et al. [71]; Comeau et al. [34] | ||
| Acclimating optimum | 10–16 | Newell et al. [72]; Helm et al. [73] | ||
| Optimum growth | 10–20 | Coulthard [36]; Stirling & Okumus [38] | ||
| Limit for optimum physiological performance | 20 | Bayne [37] | ||
| Growth lowered; mortalities | >20 | Read & Cumming [74]; Almada-Vilella et al. [71] | ||
| High byssus strength | Winter | Rhode Island, New Hampshire | <6 | Carrington [46]; Garner & Litivaitis [48] |
| Low byssus strength | Summer | Rhode Island | 18 | Carrington [46] |
| Summer | New Hampshire | 13.3 | Garner & Litivaitis [48] | |
| USA | ||||
| Spawning | Late May to June (1969–1977) | Damariscotta Estuary, Maine | 10–12 | Pondniesinski & McAlice [70] |
| Spawning | June | Maine | 10–12.5 | Newell et al. 72] |
| Spawning | Late April to June | Stony Brook, New York | 11–15 | Newell et al. [52] |
| Spawning | August–October | Shinnecock, New York | 16–22 | Newell et al. [52] |
| Spawning | May | Milford, Connecticut | 15–16 | Engle & Loosanoff [75] |
| Spawning | CANADA | |||
| Spawning | Mid June–Late July | Great Entry Lagoon, Canada | 10.3–20.7 | Myrand et al. [76] |
| Spawning | Mid June Late August | Open Sea, Canada | 10–15 | Myrand et al. [76] |
| Spawning | EUROPE | |||
| Spawning | 26 July–2 August 1996 | Finland | 14–17 | Antsulevich et al. [77] |
| Spawning | May to July 1960 | Finland | 12–15 | Heinonen [78] |
| Spawning | 15-May | Sweden | 8.5 | Kautsky [79] |
| Spawning | 30-May | Sweden | 9.5 | Kautsky [79] |
| Spawning | 5 April to 25 April | Holland | 6–8 | Pieters et al. [80] |
| Spawning | Mid-April to end of May | England | 9–12.5 | Chipperfield [81] |
| Spawning | Middle July to August | Iceland | 10–12 | Thorarinsdottir [82] |
| Spawning | Early May | Norway | 8 | Bohle [65] |
| Spawning | May | Denmark | 7–16 | Jørgensen [83] |
| Spawning | May–June | Denmark | 13–14 | Rasmussen [84] |
| Stations (Approx.) | Station | Physical Location (Related Area) | Description | |
|---|---|---|---|---|
| Latitude (N) | Longitude (W) | |||
| 40.625 | −72.375 | 1 | Outside Shinnecock Inlet (LI) | Intended multi-trophic aquaculture |
| 41.125 | −71.375 | 2 | Southwest of Block Island (RI) | Intended wind-farm co-siting |
| 41.125 | −70.875 | 3 | Southwest Martha’s Vineyard (RI) | Past trial |
| 42.125 | −70.125 | 4 | North of Cape Cod (MA) | Area selected in the present study |
| 42.625 | −70.625 | 5 | North of Cape Ann (MA) | Ongoing trial |
| 43.125 | −70.375 | 6 | Offshore New Hampshire (NH) | Ongoing commercial mariculture |
| Term, Units | Explanation (Galimany et al. [85]) | Calculation (according to biodeposition methods in Galimany et al. [59] and Iglesias et al. [93]) |
| Clearance rate (CR), L·h−1 | Volume of water cleared of particles per unit of time | (mg inorganic matter from both feces and pseudofeces per unit of time (mg·h−1))/(mg inorganic matter (PIM; mg·L−1) in the water) |
| Filtration rate (FR), mg·h−1 | Mass of particles cleared from the water per unit of time | CR × TPM (mg·L−1) in the water |
| Rejection Rate (RR), % | Percentage of particles filtered but rejected | [(total rejection rate mg·h−1)/(total filtration rate (mg·h−1)] × 100 |
| Organic ingestion rate (OIR), mg·h−1 | Amount of particulate organic matter ingested per unit of time | (CR × POM (mg·L−1) in the water)—(rejection rate of organic matter (mg·h−1)) |
| Absorption rate (AR), mg·h−1 | Amount of ingested particulate matter that is absorbed in the mussels’ digestive system | OIR—(egestion rate of organic matter) |
| Absorption efficiency (AE), % | Percentage of particulate matter ingested and retained | (AR/OIR) × 100 |
| Selection efficiency (SE), fraction | Organic food selected from the total particulates in the water | 1 − [(organic fraction with pseudofeces)/(organic fraction within total particles available in the water)] |
| Depth (m) | Summer Season Minus Annual Mean (T, °C) | North Area (T ± s.d. a, °C) | South Area (T ± s.d. a, °C) |
|---|---|---|---|
| 0 | 5.8 | 16.12 ± 1.75 | 19.48 ± 1.98 |
| 5 | 5.7 | 15.80 ± 1.75 | 19.12 ± 1.99 |
| 10 | 5.4 | 14.95 ± 1.80 | 18.24 ± 2.24 |
| 15 | 4.8 | 13.62 ± 1.75 | 16.94 ± 2.51 |
| 20 | 4.1 | 12.26 ± 1.91 | 15.67 ± 2.70 |
| 25 | 3.7 | 11.07 ± 1.94 | 14.48 ± 2.70 |
| Sampling Date | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring (May) | Environmental conditions | T (°C) | DO (mg/L) | Sal | Chl a b (µg/L) | TPM (mg/L) | POM (mg/L) | PIM (mg/L) | f (ratio) |
| 5.68 ± 0.09 | 11.08 ± 0.70 | 29.77 ± 0.08 | 2.08 ± 0.24 | 1.25 ± 0.38 | 0.88 ± 0.18 | 0.37 ± 0.22 | 0.70 | ||
| Mussel characteristics | Length (mm) | CI (%) | GTT (h) | ||||||
| 54.7± 3.50 | 31.67 ± 3.23 | 1.5 | |||||||
| Summer (August) | Environmental conditions | T (°C) | DO (mg/L) | Sal | Chl a b (µg/L) | TPM (mg/L) | POM (mg/L) | PIM (mg/L) | f (ratio) |
| 10.51 ± n.a. | 7.25 ± n.a. | 31.04 ± n.a. | 2.09 ± 0.06 | 1.19 ± 0.45 | 0.86 ± 0.19 | 0.37± 0.34 | 0.72 | ||
| Mussel characteristics | Length (mm) | CI (%) | GTT (h) | ||||||
| 69.0 ± 3.64 | 26.84 ± 6.41 | 1.5 | |||||||
| Size Class (µm) | Particle Abundance | Ratio | |
|---|---|---|---|
| Phytoplankton (cell/mL) | Non-Phytoplankton (cell/mL) | ||
| 2–5 | 360 ± 37 | 672 ± 224 | 0.54 |
| 5–20 | 805 ± 217 | 611 ± 285 | 1.32 |
| >20 | 118 ± 57 | 327 ± 60 | 0.36 |
| Season | CR (L/h) * | FR (mg/h) | RR (%) | OIR (mg/h) * | AR (mg/h) * | AE (%) * | SE |
|---|---|---|---|---|---|---|---|
| Spring (May) | 1.15 ± 0.90 | 0.92 ± 0.72 | 21.67 ± 10.80 | 0.96 ± 0.74 | 0.82 ± 0.66 | 87.18 ± 24.13 | 0.91 ± 0.66 |
| Summer (August) | 0.51 ± 0.31 | 0.49 ± 0.30 | 15.37 ± 7.66 | 0.43 ± 0.26 | 0.20 ± 0.22 | 36.15 ± 21.63 | 0.94 ± 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuta, D.D.; Wikfors, G.H. Depth Selection and In Situ Validation for Offshore Mussel Aquaculture in Northeast United States Federal Waters. J. Mar. Sci. Eng. 2019, 7, 293. https://doi.org/10.3390/jmse7090293
Mizuta DD, Wikfors GH. Depth Selection and In Situ Validation for Offshore Mussel Aquaculture in Northeast United States Federal Waters. Journal of Marine Science and Engineering. 2019; 7(9):293. https://doi.org/10.3390/jmse7090293
Chicago/Turabian StyleMizuta, Darien Danielle, and Gary H. Wikfors. 2019. "Depth Selection and In Situ Validation for Offshore Mussel Aquaculture in Northeast United States Federal Waters" Journal of Marine Science and Engineering 7, no. 9: 293. https://doi.org/10.3390/jmse7090293
APA StyleMizuta, D. D., & Wikfors, G. H. (2019). Depth Selection and In Situ Validation for Offshore Mussel Aquaculture in Northeast United States Federal Waters. Journal of Marine Science and Engineering, 7(9), 293. https://doi.org/10.3390/jmse7090293




