Benthic Species Distribution Linked to Morphological Features of a Barred Coast
Abstract
:1. Introduction
2. Study Area and Data Collection
2.1. Study Sites
2.2. Data Collection Techniques
3. Data Analysis and Methods
3.1. Definition of Morphological Features
3.2. Characterization of the Morphological Features
3.3. Benthic Species Data
3.4. Relationship between Benthic Species and Morphologic Features
4. Results
4.1. Shape Characteristics of the Morphological Features
4.2. Sediment Characteristics Related to the Morphological Features
4.3. Macrobenthic Species in the Near Shore
4.4. Total Abundance, Biomass, Number of Species, and Diversity of the Benthic Fauna
4.5. Species Composition Correlated with Environmental Parameters
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luijendijk, A.; Hagenaars, G.; Ranasinghe, R.; Baart, F.; Donchyts, G.; Aarninkhof, S. The State of the World’s Beaches. Sci. Rep. 2018, 8, 6641. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Duong, T.M.; Uhlenbrook, S.; Roelvink, D.; Stive, M. Climate-change impact assessment for inlet-interrupted coastlines. Nat. Clim. Chang. 2013, 3, 83–87. [Google Scholar] [CrossRef]
- Hamm, L.; Capobianco, M.; Dette, H.; Lechuga, A.; Spanhoff, R.; Stive, M.J.F. A summary of European experience with shore nourishment. Coast. Eng. 2002, 47, 237–264. [Google Scholar] [CrossRef]
- Hanson, H.; Brampton, A.; Capobianco, M.; Dette, H.H.; Hamm, L. Beach nourishment projects, practices, and objectives—A European overview. Coast. Eng. 2002, 47, 81–111. [Google Scholar] [CrossRef]
- Radermacher, M.; de Schipper, M.A.; Price, T.D.; Huisman, B.J.A.; Aarninkhof, S.G.J.; Reniers, A.J.H.M. Behaviour of subtidal sandbars in response to nourishments. Geomorphology 2018, 313, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Grunnet, N.M.; Ruessink, B.G. Morphodynamic response of nearshore bars to a shoreface nourishment. Coast. Eng. 2005, 52, 119–137. [Google Scholar] [CrossRef]
- Reise, K. Sediment mediated species interactions in coastal waters. J. Sea Res. 2002, 48, 127–141. [Google Scholar] [CrossRef]
- Widdows, J.; Brinsley, M. Impact of biotic and abiotic processes on sediment dynamics and the consequences to the structure and functioning of the intertidal zone. J. Sea Res. 2002, 48, 143–156. [Google Scholar] [CrossRef]
- Reiss, H.; Birchenough, S.; Borja, A.; Buhl-Mortensen, L.; Craeymeersch, J.; Dannheim, J.; Darr, A.; Galparsoro, I.; Gogina, M.; Neumann, H.; et al. Benthos distribution modelling and its relevance for marine ecosystem management. ICES J. Mar. Sci. 2015, 72, 297–315. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.; Elliott, M. Ecology of Marine Sediments, 2nd ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Herman, P.M.J.; Ysebaert, T. Multi-scale analysis of species-environment relationships. Mar. Ecol. Prog. Ser. 2005, 302, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Rachor, E. The ICES North Sea Benthos Project 2000: North Sea Benthic Communities by 2000; ICES: Copenhagen, Denmark, 2007. [Google Scholar]
- Aldridge, J.N.; Bergman, M.J.N.; Bolam, T.; Craeymeersch, J.A.; Degraer, S.; Duineveld, G.C.A.; Eggleton, J.D.; Goethals, P.; Hillewaert, H.; Irion, G.; et al. Structure and Dynamics of the North Sea benthos; Rees, H.L., Eggleton, J.D., Rachor, E., Vanden Berghe, E., Eds.; ICES No. 2; ICES Cooperative Research Report; ICES: Copenhagen, Denmark, 2007; ISBN 87-7482-058-3. [Google Scholar]
- Rachor, E.; Reiss, H.; Degraer, S.; Duineveld, G.C.A.; van Hoey, G.; Lavaleye, M.; Willems, W.; Rees, H.L. Structure, distribution, and characterizing species of North Sea macro-zoobenthos communities in 2000. In Structure and Dynamics of the North Sea Benthos; ICES: Copenhagen, Denmark, 2003; pp. 46–59. [Google Scholar]
- Reiss, H.; Degraer, S.; Duineveld, G.C.A.; Kröncke, I.; Aldridge, J.; Craeymeersch, J.A.; Eggleton, J.D.; Hillewaert, H.; Lavaleye, M.S.S.; Moll, A.; et al. Spatial patterns of infauna, epifauna, and demersal fish communities in the North Sea. ICES J. Mar. Sci. 2010, 67, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Van Hoey, G.; Degraer, S.; Vincx, M. Macrobenthic community structure of soft-bottom sediments at the Belgian Continental Shelf. Estuar. Coast. Shelf Sci. 2004, 59, 599–613. [Google Scholar] [CrossRef] [Green Version]
- Baptist, M.J.; van Dalfsen, J.; Weber, A.; Passchier, S.; van Heteren, S. The distribution of macrozoobenthos in the southern North Sea in relation to meso-scale bedforms. Estuar. Coast. Shelf Sci. 2006, 68, 538–546. [Google Scholar] [CrossRef]
- Damveld, J.H.; van der Reijden, K.J.; Cheng, C.; Koop, L.; Haaksma, L.R.; Walsh, C.A.J.; Soetaert, K.; Borsje, B.W.; Govers, L.L.; Roos, P.C.; et al. Video Transects Reveal That Tidal Sand Waves Affect the Spatial Distribution of Benthic Organisms and Sand Ripples. Geophys. Res. Lett. 2018, 45, 11.837–11.846. [Google Scholar] [CrossRef] [Green Version]
- de Jong, M.F.; Baptist, M.J.; Lindeboom, H.J.; Hoekstra, P. Relationships between macrozoobenthos and habitat characteristics in an intensively used area of the Dutch coastal zone. ICES J. Mar. Sci. 2015, 72, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- van Dijk, T.A.G.P.; van Dalfsen, J.A.; Van Lancker, V.; van Overmeeren, R.A.; van Heteren, S.; Doornenbal, P.J. Benthic Habitat Variations over Tidal Ridges, North Sea, the Netherlands. In Seafloor Geomorphology as Benthic Habitat; Elsevier: Amsterdam, The Netherlands, 2012; pp. 241–249. ISBN 9780123851406. [Google Scholar]
- Markert, E.; Kröncke, I.; Kubicki, A. Small scale morphodynamics of shoreface-connected ridges and their impact on benthic macrofauna. J. Sea Res. 2015, 99, 47–55. [Google Scholar] [CrossRef]
- Janssen, G.M.; Mulder, S. Zonation of macrofauna across sandy beaches and surf zones along the Dutch coast. Oceanologia 2005, 47, 265–282. [Google Scholar]
- Ysebaert, T.; Herman, P.M.J.J. Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Mar. Ecol. Prog. Ser. 2002, 244, 105–124. [Google Scholar] [CrossRef]
- Walstra, D.J.R.; Reniers, A.J.H.M.; Ranasinghe, R.; Roelvink, J.A.; Ruessink, B.G. On bar growth and decay during interannual net offshore migration. Coast. Eng. 2012, 60, 190–200. [Google Scholar] [CrossRef]
- Battjes, J.A. Surf similarity. Coast. Eng. Proc. 1974, 1(14), 26. [Google Scholar] [CrossRef]
- van der Zanden, J. Sand Transport Processes in the Surf and Swash Zones; University of Twente: Enschede, The Netherlands, 2016. [Google Scholar]
- Van der Zanden, J.; Van der A, D.A.; O’Donoghue, T.; Hurther, D.; Caceres, I.; Thorne, P.D.; Van der Werf, J.J.; Hulscher, S.J.M.H.; Ribberink, J.S. Suspended and bedload transport in the surf zone: Implications for sand transport models. Coast. Eng. Proc. 2017, 1, 30. [Google Scholar] [CrossRef] [Green Version]
- Komar, P.D. Beach Processes and Sedimentation; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1997; ISBN 978-0137549382. [Google Scholar]
- van Enckevort, I.M.J.; Ruessink, B.G. Video observations of nearshore bar behaviour. Part 1: Alongshore uniform variability. Cont. Shelf Res. 2003, 23, 501–512. [Google Scholar] [CrossRef]
- Wijnberg, K.M.; Kroon, A. Barred beaches. Geomorphology 2002, 48, 103–120. [Google Scholar] [CrossRef]
- Walstra, D.J.R. On the anatomy of nearshore sandbars. In A Systematic Exposition of Inter-Annual Sandbar Dynamics; Delft University of Technology: Delft, The Netherlands, 2016. [Google Scholar]
- Ojeda, E.; Ruessink, B.G.; Guillen, J. Morphodynamic response of a two-barred beach to a shoreface nourishment. Coast. Eng. 2008, 55, 1185–1196. [Google Scholar] [CrossRef]
- Wijnberg, K.M.; Terwindt, J.H.J. Extracting decadal morphological behaviour from high-resolution, long-term bathymetric surveys along the Holland coast using eigenfunction analysis. Mar. Geol. 1995, 126, 301–330. [Google Scholar] [CrossRef]
- McLachlan, A.; Brown, A.C. The Ecology of Sandy Shores; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Rijkswaterstaat. Sedimentatlas Waddenzee; RIKZ: Haren, The Netherlands, 1999. [Google Scholar]
- Vanagt, T.; van de Moortel, L.; Heusinkveld, J.; Vanden Eede, S.; van Steenbrugge, L.; van Hoey, G.; Vincx, M. Veldcampagne Ecologie Ameland 2010; eCOAST: Ostend, Belgium, 2011. [Google Scholar]
- Verduin, E.C.; Leewis, L.; Templeman, D. Veldcampagne Ameland 2011. In Onderzoek Naar de Ecologische Effecten Van Zandsuppleties Op Macrobenthos, Epibenthos en Demersale Vis Op Ameland; Grontmij: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Faasse, M.; Lock, K.; Vanagt, T. Labrapport Benthos Ameland. In Najaar 2012; eCoast: Oostende, Belgium, 2013. [Google Scholar]
- Faasse, M.; Vanagt, T. Labrapport Strand-en Vooroeverbenthos Ameland. In Najaar 2013; eCOAST: Oostend, Belgium, 2014. [Google Scholar]
- Verduin, E.; de Vos, L. Analyseverslag To Analyses Ameland & Schiermonnikoog; 2014-8 To tbv KPP B&O Kust Ecologie; Grontmij: Amsterdam, The Natherlands, 2014. [Google Scholar]
- Ricciardi, A.; Bourget, E. Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol. Prog. Ser. 1998, 163, 245–251. [Google Scholar] [CrossRef] [Green Version]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; ISBN 0-387-98957-9. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: New York, NY, USA; Highland Statistics Ltd.: Newburgh, NY, USA, 2009. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Package Nlme: Linear and Nonlinear Mixed Effects Models; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Zuur, A.K.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007; ISBN 9780387459677. [Google Scholar]
- Lenth, R. Pakage Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the Spatial Component of Ecological Variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Dufrene, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Janssen, G.M.; Kleef, H.; Mulder, S.; Tydeman, P. Pilot assessment of depth related distribution of macrofauna in surf zone along Dutch coast and its implications for coastal management. Mar. Ecol. 2008, 29, 186–194. [Google Scholar] [CrossRef]
- Degraer, S.; Wittoeck, J.; Appeltans, W.; Cooreman, K.; Deprez, T.; Hillewaert, H.; Hostens, K.; Mees, J.; Vanden Berghe, E.; Vincx, M. The Macrobenthos Atlas of the Belgian Part of the North Sea; Belgian Science Policy: Brussels, Belgium, 2006. [Google Scholar]
- Witbaard, R.; Bergman, M.J.N.; van Weerlee, E.; Duineveld, G.C.A. An estimation of the effects of Ensis directus on the transport and burial of silt in the near-shore Dutch coastal zone of the North Sea. J. Sea Res. 2017, 127, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Breine, N.T.; De Backer, A.; Van Colen, C.; Moens, T.; Hostens, K.; Van Hoey, G. Structural and functional diversity of soft-bottom macrobenthic communities in the Southern North Sea. Estuar. Coast. Shelf Sci. 2018, 214, 173–184. [Google Scholar] [CrossRef]
- van Dalfsen, J.A. Inventarisatie Brandingszone; IMARES: Wageningen, The Netherlands, 2009. [Google Scholar]
- van Dalfsen, J.A.; Essink, K. Risk Analysis of Coastal Nourishment Techniques in The Netherlands; RIKZ: Haren, The Netherlands, 1997. [Google Scholar]
- Kröncke, I.; Becker, L.R.; Badewien, T.H.; Bartholomä, A.; Schulz, A.-C.; Zielinski, O. Near- and Offshore Macrofauna Communities and Their Physical Environment in a South-Eastern North Sea Sandy Beach System. Front. Mar. Sci. 2018, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rabaut, M.; Guilini, K.; Van Hoey, G.; Vincx, M.; Degraer, S. A bio-engineered soft-bottom environment: The impact of Lanice conchilega on the benthic species-specific densities and community structure. Estuar. Coast. Shelf Sci. 2007, 75, 525–536. [Google Scholar] [CrossRef] [Green Version]
- van Scheppingen, Y.; Groenewold, A. De Ruimtelijke Verspreiding Van Het Benthos in de Zuidelijke Noordzee; de Nederlandse Kustzone Overzicht 1988–1989; Rijkswaterstaat Directie Noordzee: Rijswijk, The Netherlands, 1990. [Google Scholar]
- Verduin, E.C.; Templeman, D.; van Moorsel, G.W.N.M. The Macrobenthic Fauna Monitoring in the Dutch Sector of the North Sea, MWTL 2010 and a Comparison with Previous Data; Grontmij: Amsterdam, The Natherlands, 2012. [Google Scholar]
- Heip, C.; Basford, D.; Craeymeersch, J.A.; Dewarumez, J.M.; Dörjes, J.; de Wilde, P.; Duineveld, G.; Eleftheriou, A.; Herman, P.J.; Niermann, U.; et al. Trends in biomass, density and diversity of North Sea macrofauna. ICES J. Mar. Sci 1992, 49, 13–22. [Google Scholar] [CrossRef]
- Armonies, W.; Reise, K. Faunal diversity across a sandy shore. Mar. Ecol. Prog. Ser. 2000, 196, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Degraer, S.; Volckaert, A.; Vincx, M. Macrobenthic zonation patterns along a morphodynamical continuum of macrotidal, low tide bar/rip and ultra-dissipative sandy beaches. Estuar. Coast. Shelf Sci. 2003, 56, 459–468. [Google Scholar] [CrossRef]
- Speybroeck, J.; Bonte, D.; Courtens, W.; Gheskiere, T.; Grootaert, P.; Maelfait, J.; Provoost, S.; Sabbe, K.; Stienen, E.W.M.; van Lancker, V.; et al. The Belgian sandy beach ecosystem: A review. Mar. Ecol. 2008, 29, 171–185. [Google Scholar] [CrossRef]
- Leewis, L.; van Bodegom, P.M.; Rozema, J.; Janssen, G.M. Does beach nourishment have long-term effects on intertidal macroinvertebrate species abundance? Estuar. Coast. Shelf Sci. 2012, 113, 172–181. [Google Scholar] [CrossRef]
- Dauer, D.M. Functional morphology and feeding behavior of Scolelepis squamata (Polychaeta: Spionidae). Mar. Biol. 1983, 77, 279–285. [Google Scholar] [CrossRef]
- d’Udekem d’Acoz, C. The genus Bathyporeia Lindström, 1855, in western Europe (Crustacea: Amphipoda: Pontoporeiidae). Zool. Verh. 2004, 348, 3–162. [Google Scholar]
- Holtmann, S.E.; Groenewold, A.; Schrader, K.H.M.; Asjes, J.; Craeymeersch, J.A.; Duineveld, G.C.A.; van Bostelen, A.J.; van der Meer, J. Atlas of the Zoobenthos of the Dutch Continental Shelf; Ministry of Transport, Public Works and Water Management, Directorate-General of Public Works and Water Management, North Sea Directorate: Rijswijk, The Netherlands, 1996.
- Fiege, D.; Licher, F.; Mackie, A.S.Y. A partial review of the European Magelonidae (Annelida: Polychaeta): Magelona mirabilis redefined and M. johnstoni sp. nov. distinguished. J. Mar. Biol. Assoc. UK 2000, 80, 215–234. [Google Scholar] [CrossRef]
- Witbaard, R.; Duineveld, G.C.A.; Bergman, M.J.N.; Witte, H.I.J.; Groot, L.; Rozemeijer, M.J.C. The growth and dynamics of Ensis directus in the near-shore Dutch coastal zone of the North Sea. J. Sea Res. 2015, 95, 95–105. [Google Scholar] [CrossRef] [Green Version]










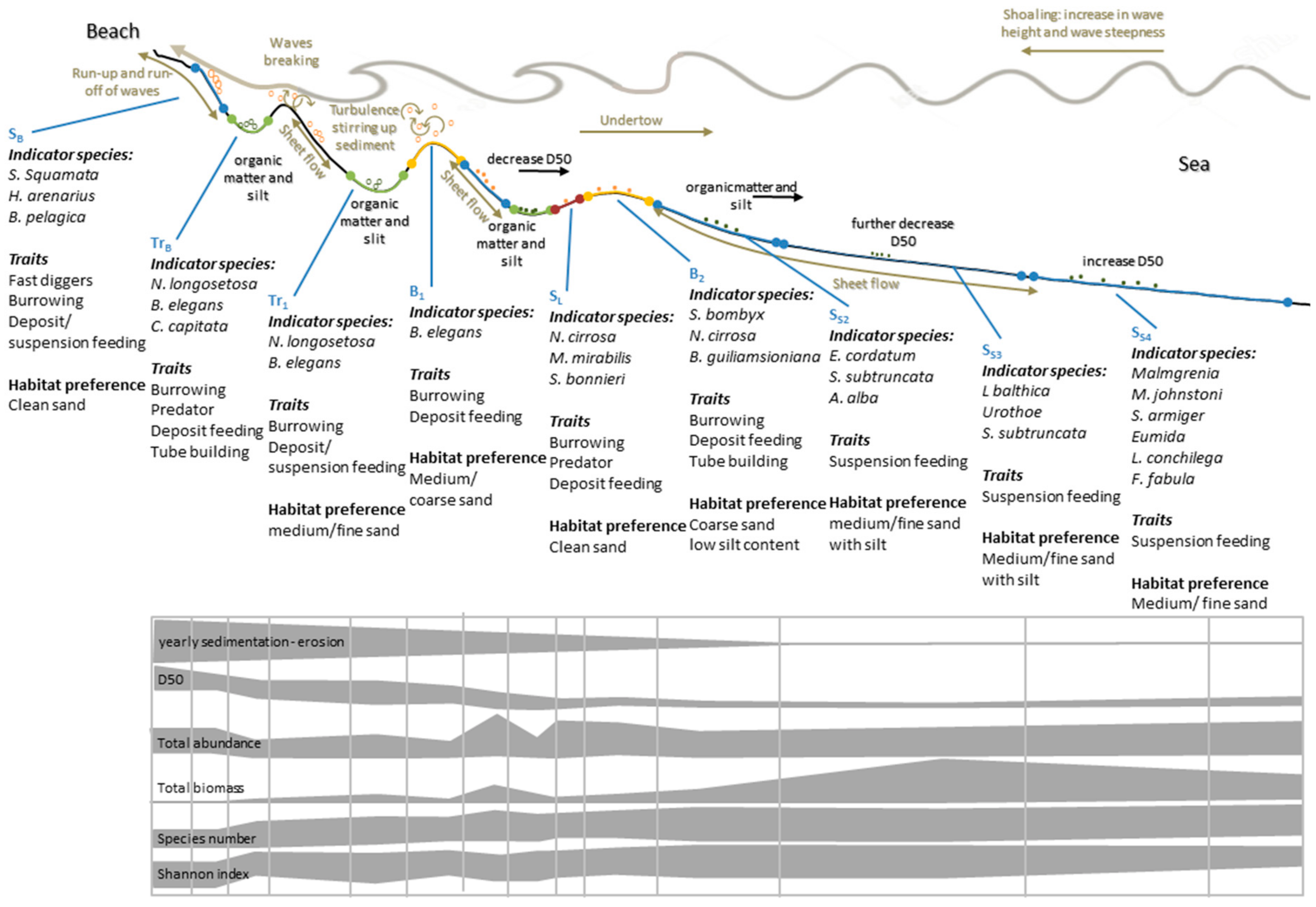
| Code | Description | Definition | No. Samples |
|---|---|---|---|
| SB | Beach slope | The low waterline including 75% of the slope. | n1 = 75, n2 = 32 |
| TrB | Beach trough | The depression close to the beach including 25% of the upward slopes. | n1 = 20, n2 = 8 |
| Tr1 | First trough | The depression including 25% of the upward slopes. | n1 = 57, n2 = 23 |
| B1 | First bar crest | The bar crest, including 25% of the downward slopes. | n1 = 77, n2 = 31 |
| SS1 | Seaward slope first bar crest | The seaward slope of the first bar crest, 25%–75%. | n1 = 76, n2 = 25 |
| Tr2 | Second trough | The depression including 25% of the upward slopes. | n1 = 68, n2 = 21 |
| SL | Landward slope second trough | The landward slope of the second bar crest 25%–75%. | n1 = 54, n2 = 19 |
| B2 | Second bar crest | The bar crest, including 25% of the downward slopes. | n1 = 69, n2 = 31 |
| SS2 | Seaward slope second bar crest | The seaward slope of the second bar crest up to 8m water depth at Ameland and 6m water depth at Schiermonnikoog | n1 = 106, n2 = 33 |
| SS3 | Lower part seaward slope | The seaward slope from 8 m to 10 m water depth at Ameland, and 6m to 7 m water depth at Schiermonnikoog | n1 = 78, n2 = 33 |
| SS4 | Gentle slope | From the lower part of the seaward slope up to 3.5 km offshore. | n1 = 86, n2 = 38 |
| MED50 | MEMud | ||||||||||
| exp. var. | est. | std.e | variance | exp. var. | est. | std.e | variance | ||||
| profH | 2.11 | * | 0.68 | σ1 | 2.75 | profH | −0.12 | *** | 0.01 | σ1 | 0.10 |
| relH | 2.65 | 1.47 | σ2 | 2.11 | relH | −0.23 | *** | 0.05 | σ2 | 0.06 | |
| SB | 15.07 | *** | 3.01 | σ3 | 6.87 | Y2010 + | −0.43 | ** | 0.13 | σ3 | 0.27 |
| TrB | 7.88 | 4.44 | Y2011 | 0.002 | 0.16 | ||||||
| Tr1 | 8.61 | * | 3.58 | w2010 | 1.0 | Y2012 | −2.91 | 0.38 | w2010 | 1.0 | |
| B1 + | 189.75 | *** | 3.72 | w2011 | 1.7 | Y2013 | −2.88 | 0.36 | w2011 | 1.5 | |
| SS1 | −7.55 | * | 3.97 | w2012 | 1.4 | Y2014 | −3.45 | 0.44 | w2012 | 10.2 | |
| Tr2 | −7.25 | * | 3.62 | w2013 | 1.2 | w2013 | 9.4 | ||||
| SL | −10.45 | ** | 3.07 | w2014 | 2.9 | w2014 | 6.9 | ||||
| B2 | −11.68 | *** | 2.69 | ||||||||
| SS2 | −14.92 | *** | 2.95 | ||||||||
| SS3 | −10.48 | * | 3.81 | ||||||||
| SS4 | −1.01 | 4.49 | |||||||||
| MEOM | |||||||||||
| exp. var. | est. | std.e | variance | ||||||||
| profH | −0.05 | *** | 0.01 | σ1 | 0.06 | ||||||
| relH | −0.08 | *** | 0.02 | σ2 | 0.00 | ||||||
| Y2010* | −0.83 | *** | 0.07 | σ3 | 0.16 | ||||||
| Y2011 | −0.11 | 0.09 | |||||||||
| Y2012 | −0.22 | 0.09 | w2010 | 1.0 | |||||||
| Y2013 | 0.36 | 0.09 | w2011 | 1.6 | |||||||
| Y2014 | 0.32 | 0.10 | w2012 | 1.1 | |||||||
| w2013 | 0.8 | ||||||||||
| w2014 | 1.4 | ||||||||||
| MEAB | MEBM | ||||||||||
| expl var. | est. | std.e | variance | expl var. | est. | std.e | variance | ||||
| profH | −0.25 | *** | 0.04 | σ1 | 0.85 | profH | −0.58 | *** | 0.06 | σ1 | 0.89 |
| relH | −0.14 | * | 0.07 | σ2 | 0.24 | relH | −0.32 | * | 0.12 | σ2 | 0.32 |
| Ln(mud) | 0.057 | * | 0.02 | σ3 | 0.88 | Ln(mud) | 0.13 | ** | 0.03 | σ3 | 1.36 |
| Ln(slope) | −0.16 | ** | 0.04 | SB | −0.62 | * | 0.23 | ||||
| SB | 0.18 | 0.15 | w2010 | 1.0 | TrB | 0.50 | 0.33 | w2010 | 1.0 | ||
| TrB | −0.24 | 0.20 | w2011 | 1.0 | Tr1 | 0.58 | * | 0.27 | w2011 | 0.8 | |
| Tr1 | 0.28 | 0.16 | w2012 | 0.6 | B1 + | −1.58 | * | 0.50 | w2012 | 1.2 | |
| B1 + | 5.63 | *** | 0.43 | w2013 | 0.7 | SS1 | 0.76 | ** | 0.22 | w2013 | 0.7 |
| SS1 | 0.52 | ** | 0.14 | w2014 | 0.7 | Tr2 | 0.12 | 0.27 | w2014 | 0.8 | |
| Tr2 | −0.08 | 0.17 | SL | −0.23 | 0.23 | ||||||
| SL | −0.05 | 0.14 | B2 | −0.16 | 0.21 | ||||||
| B2 | −0.32 | * | 0.13 | SS2 | −0.08 | 0.22 | |||||
| SS2 | −0.39 | * | 0.14 | SS3 | 0.09 | 0.29 | |||||
| SS3 | −0.61 | ** | 0.18 | SS4 | −1.28 | ** | 0.34 | ||||
| SS4 | −0.94 | *** | 0.23 | ||||||||
| MESN | MEH | ||||||||||
| expl var. | est. | std.e | variance | expl var. | est. | std.e | variance | ||||
| profH | −1.13 | *** | 0.15 | σ1 | 2.73 | profH | −0.04 | * | 0.02 | σ1 | 0.19 |
| SE | 0.78 | ** | 0.21 | σ2 | 1.18 | Ln(mud) | −0.06 | *** | 0.01 | σ2 | 0.08 |
| SB | −2.0 | * | 0.60 | σ3 | 2.82 | Ln(slope) | 0.10 | ** | 0.03 | σ3 | 0.43 |
| TrB | 0.18 | 0.73 | SB | −0.32 | ** | 0.09 | |||||
| Tr1 | −0.53 | 0.52 | w2010 | 1.0 | TrB | −0.02 | 0.12 | w2010 | 1.0 | ||
| B1 + | 5.88 | *** | 1.43 | w2011 | 1.0 | Tr1 | −0.20 | * | 0.08 | w2011 | 1.2 |
| SS1 | 0.78 | 0.46 | w2012 | 0.8 | B1 + | 1.23 | *** | 0.13 | w2012 | 0.9 | |
| Tr2 | −0.79 | 0.51 | w2013 | 1.1 | SS1 | −0.19 | * | 0.08 | w2013 | 1.1 | |
| SL | 0.11 | 0.54 | w2014 | 1.2 | Tr2 | −0.01 | 0.08 | w2014 | 0.8 | ||
| B2 | −0.60 | 0.54 | SL | −0.04 | 0.08 | ||||||
| SS2 | −0.59 | 0.54 | B2 | 0.17 | * | 0.08 | |||||
| SS3 | −1.33 | * | 0.67 | SS2 | 0.15 | 0.08 | |||||
| SS4 | −2.26 | * | 0.88 | SS3 | 0.26 | * | 0.10 | ||||
| SS4 | 0.24 | * | 0.12 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzhauer, H.; Borsje, B.W.; van Dalfsen, J.A.; Wijnberg, K.M.; Hulscher, S.J.M.H.; Herman, P.M.J. Benthic Species Distribution Linked to Morphological Features of a Barred Coast. J. Mar. Sci. Eng. 2020, 8, 16. https://doi.org/10.3390/jmse8010016
Holzhauer H, Borsje BW, van Dalfsen JA, Wijnberg KM, Hulscher SJMH, Herman PMJ. Benthic Species Distribution Linked to Morphological Features of a Barred Coast. Journal of Marine Science and Engineering. 2020; 8(1):16. https://doi.org/10.3390/jmse8010016
Chicago/Turabian StyleHolzhauer, Harriëtte, Bas W. Borsje, Jan A. van Dalfsen, Kathelijne M. Wijnberg, Suzanne J.M.H. Hulscher, and Peter M.J. Herman. 2020. "Benthic Species Distribution Linked to Morphological Features of a Barred Coast" Journal of Marine Science and Engineering 8, no. 1: 16. https://doi.org/10.3390/jmse8010016
APA StyleHolzhauer, H., Borsje, B. W., van Dalfsen, J. A., Wijnberg, K. M., Hulscher, S. J. M. H., & Herman, P. M. J. (2020). Benthic Species Distribution Linked to Morphological Features of a Barred Coast. Journal of Marine Science and Engineering, 8(1), 16. https://doi.org/10.3390/jmse8010016








