Coral Reef Community Changes in Karimunjawa National Park, Indonesia: Assessing the Efficacy of Management in the Face of Local and Global Stressors
Abstract
1. Introduction
1.1. Karimunjawa National Park
1.2. Threats and Management
1.2.1. Local Pressures: Fishing, Aquaculture and Tourism
Fishing
Aquaculture
Tourism
1.2.2. Global Pressures: Climate Change
1.3. The Challenge: Using Monitoring Data to Understand the Effects of Local vs. Global Stressors on Complex Coral Reef Communities
1.4. Aims
2. Materials and Methods
2.1. Ecological Surveys
2.2. Photo Quadrat Processing
2.3. Bleaching Data
2.4. Statistical Analyses
3. Results
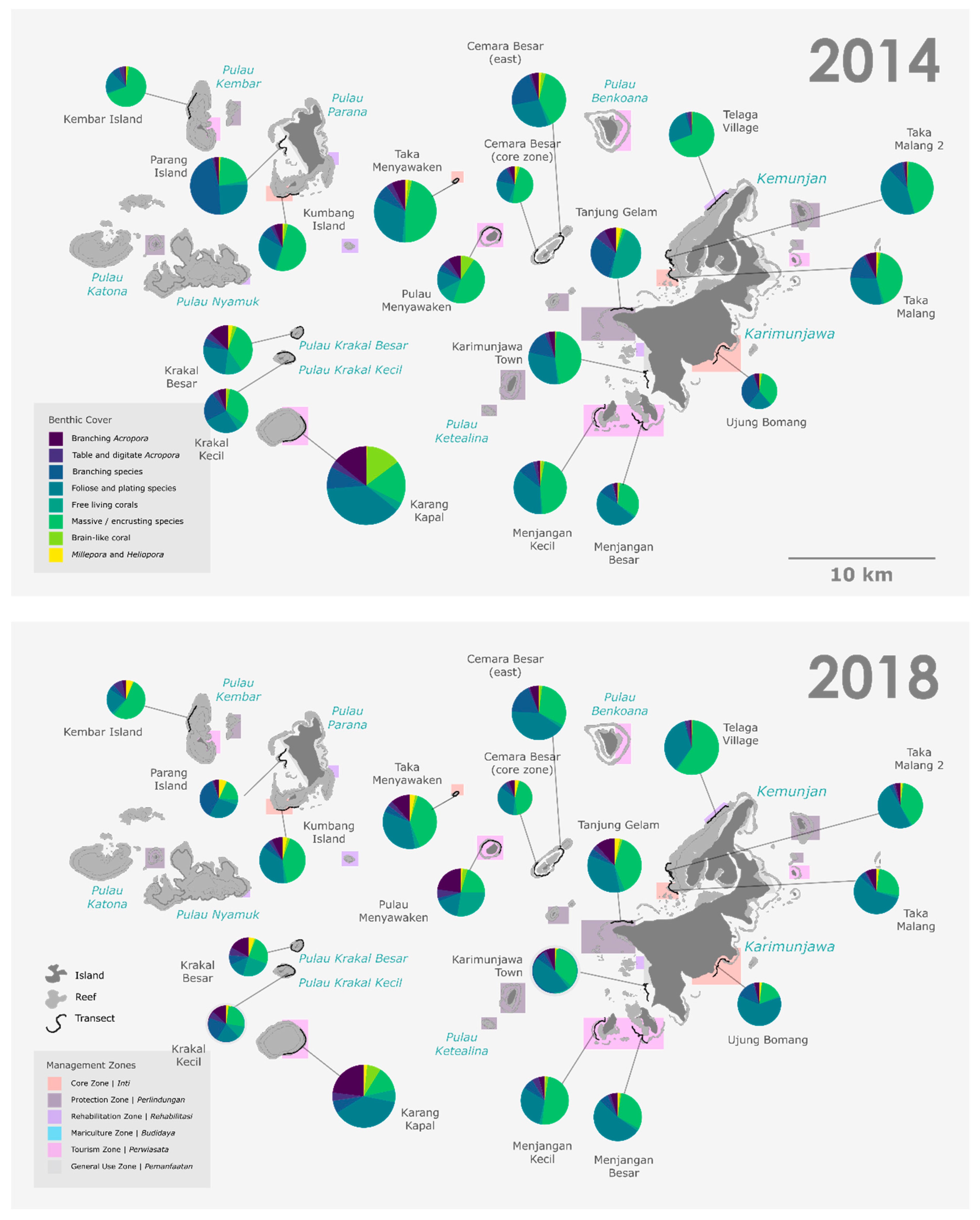
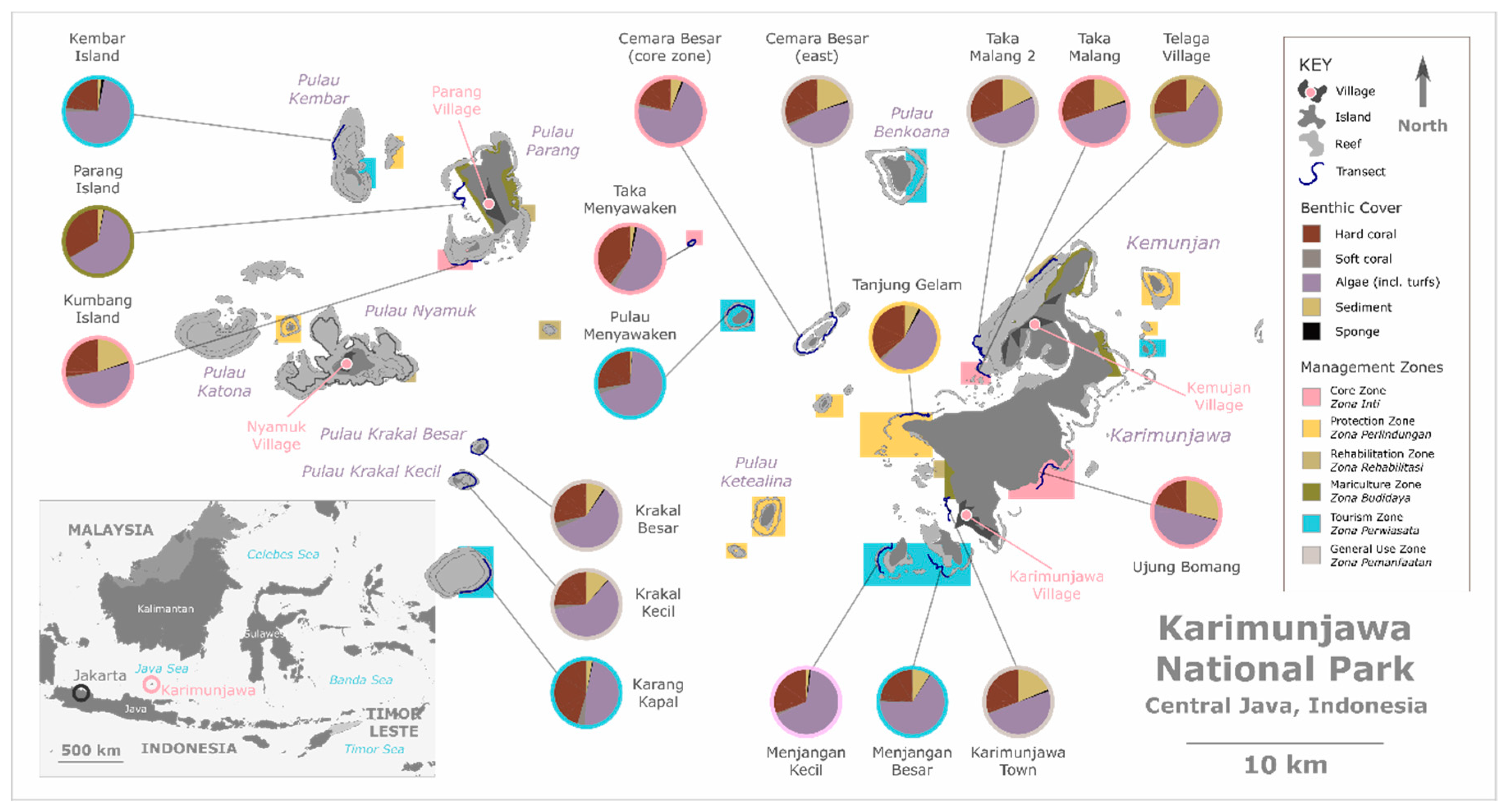
3.1. Spatial Variability in Benthic Composition
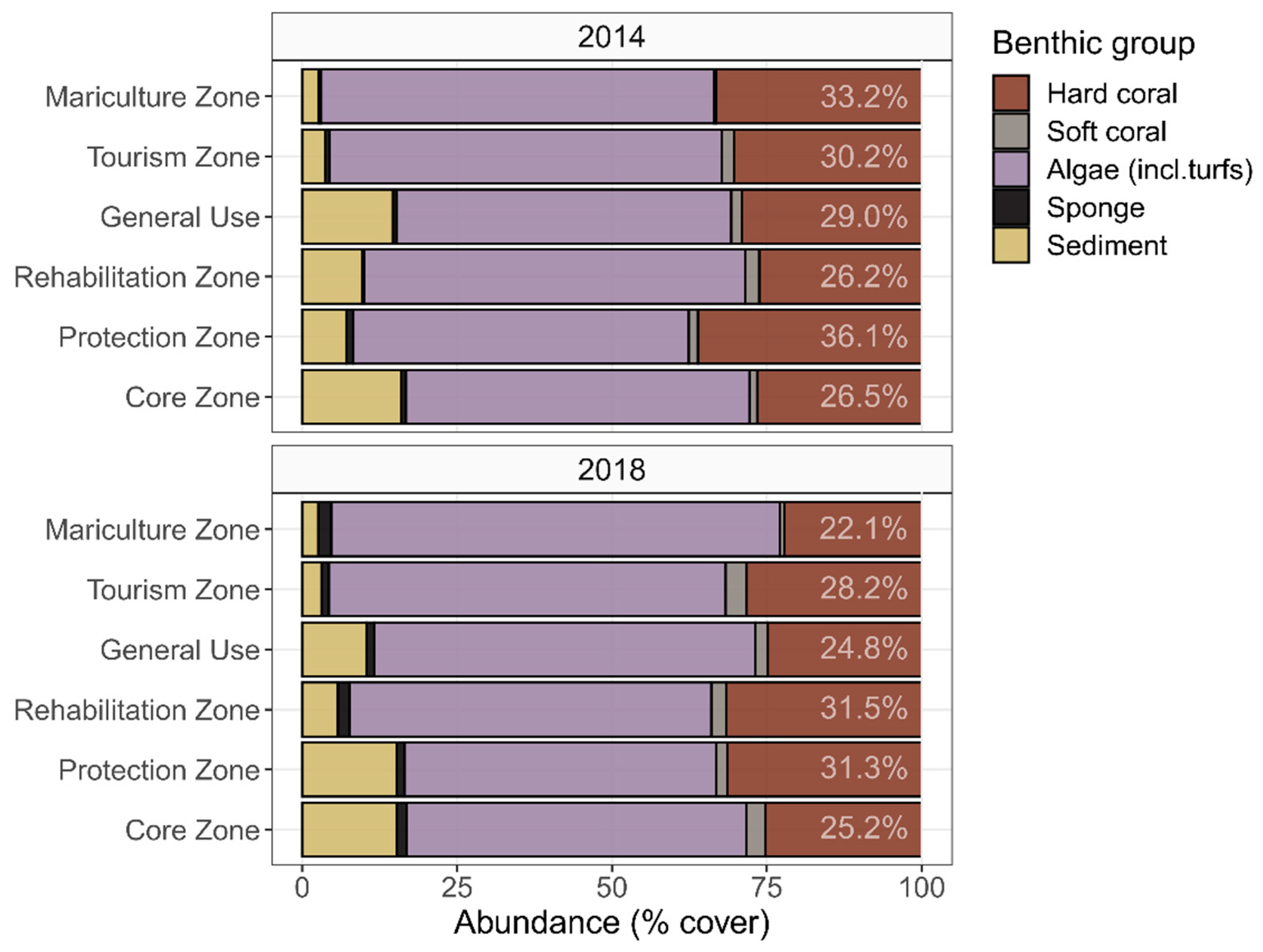
3.2. Temporal Variability in Benthic Composition
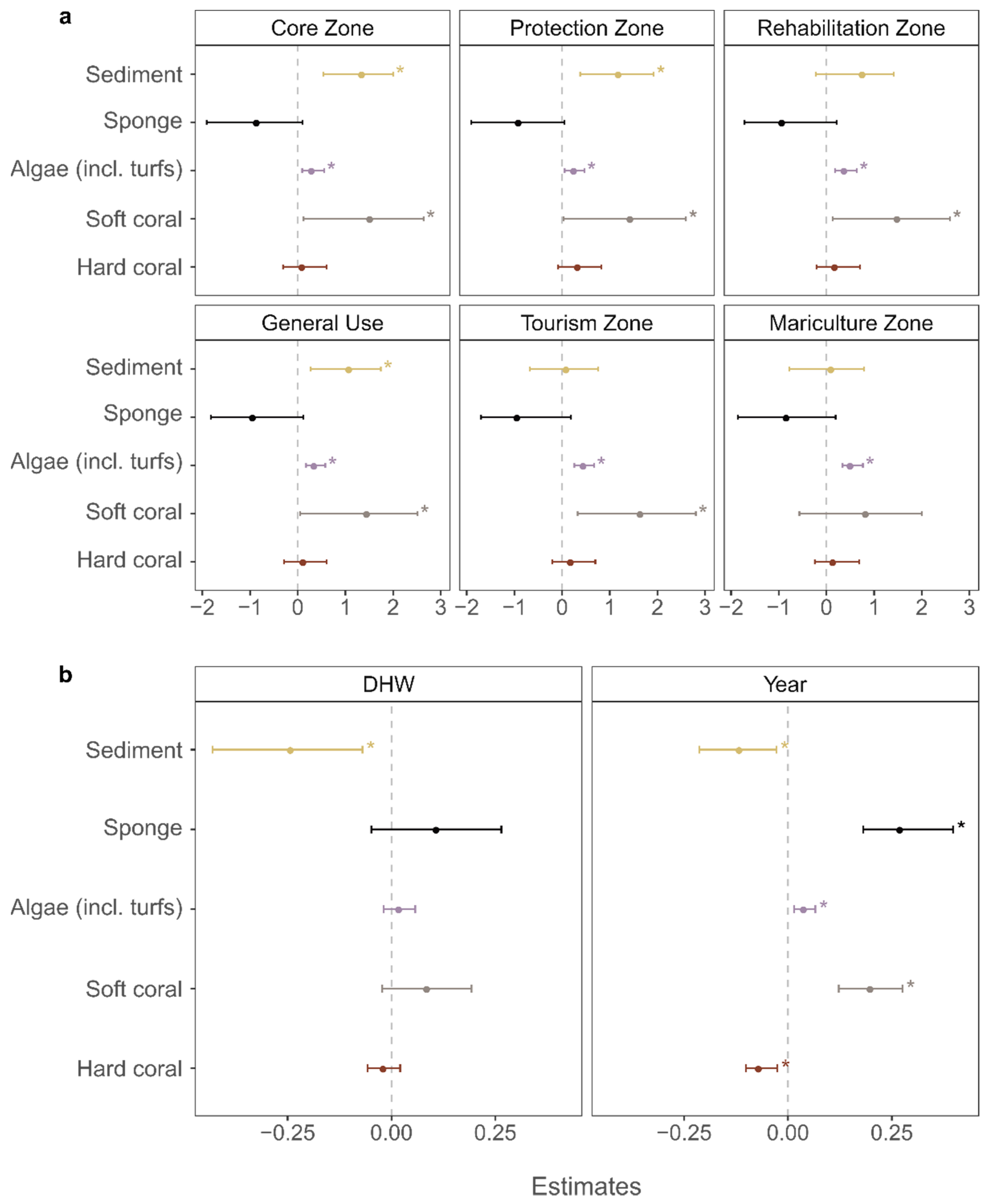
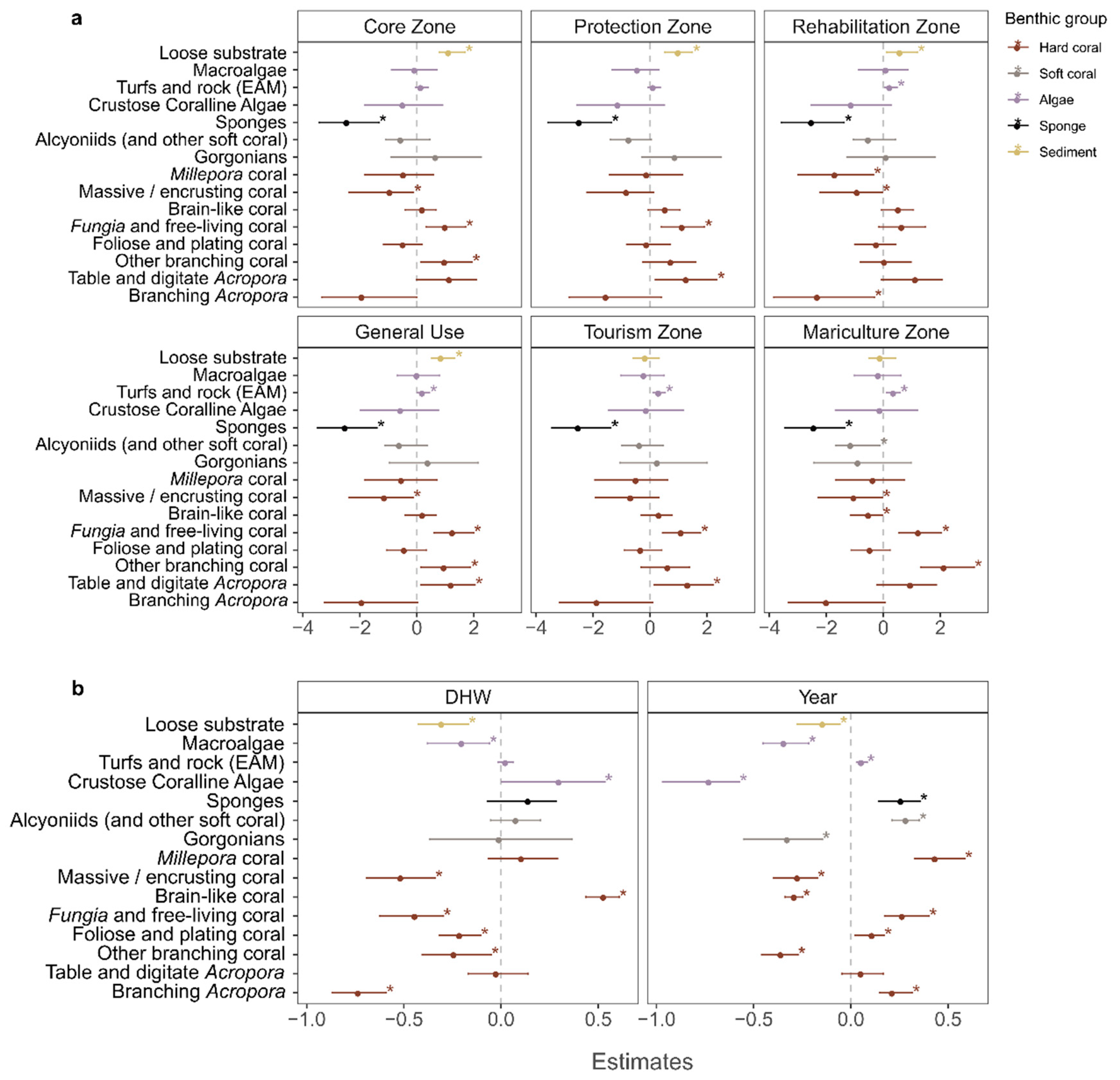
3.3. Global Pressures: Effect of Ocean Warming
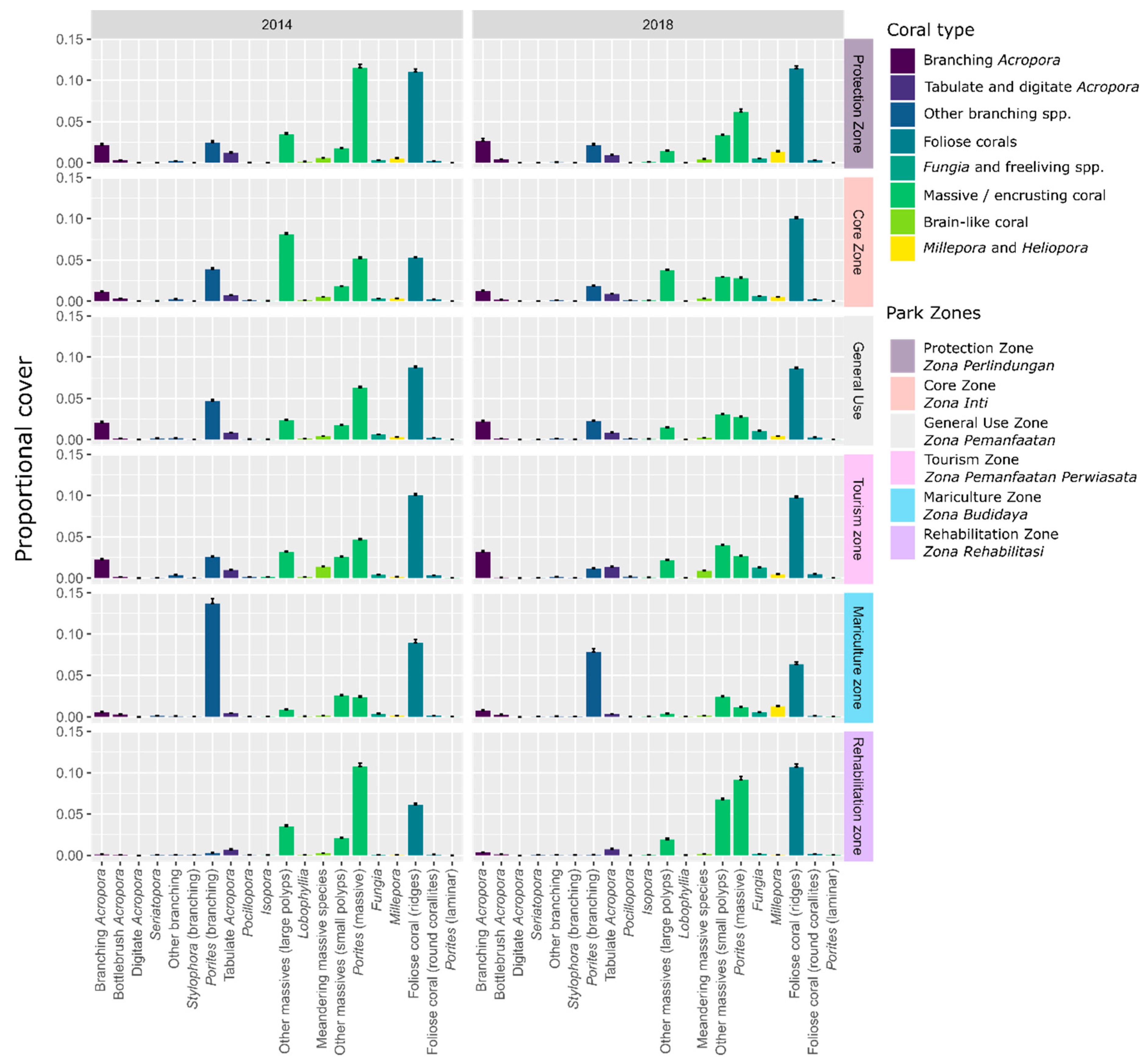
3.4. Local Stressors: Effect of Management Zone
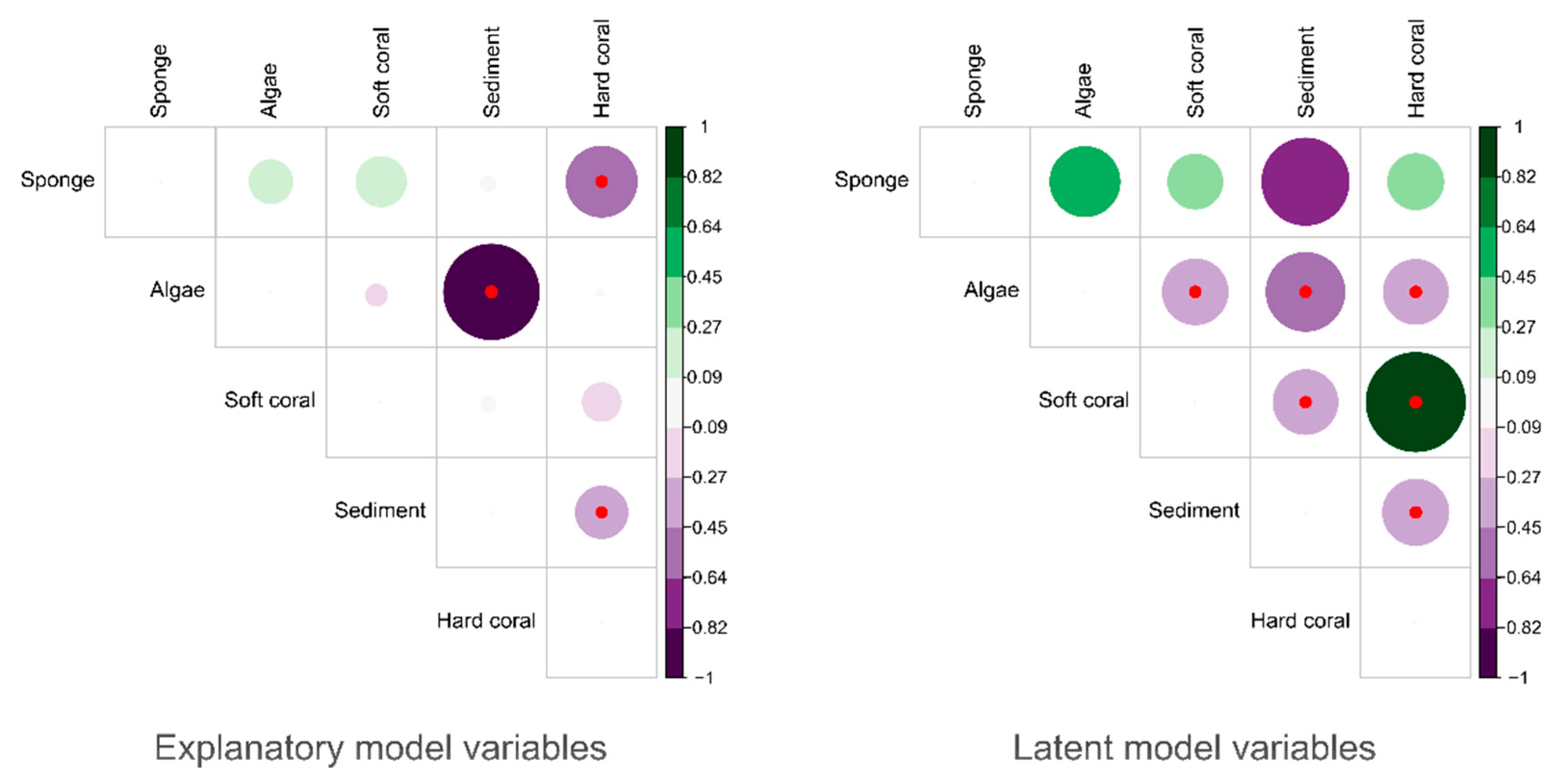
3.5. Community Co-Occurrence Patterns
4. Discussion
4.1. Spatial Variability: Status of Karimunjawa’s Coral Reef Benthic Composition across the Park
Testing Spatial Variability: Effect of Management Zone
4.2. Temporal Variation: Trends in Karimunjawa’s Coral Reef Benthic Composition between 2014 and 2018
4.3. Impact of Global Climate Change
4.4. Impact of Local Stressors
4.4.1. Changes in Fishing Pressure
4.4.2. Declining Water Quality?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site Information | 2014 | 2018 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Zone | Transect Start | Transect End | Quadrats | Hard Coral | Soft Coral | Algae | Sponge | Sediment | Quadrats | Hard Coral | Soft Coral | Algae | Sponge | Sediment |
| Tanjung Gelam | Protection Zone | −5.83641, 110.4099 | −5.83579, 110.4199 | 872 | 0.36 | 0.01 | 0.54 | 0.01 | 0.07 | 1022 | 0.31 | 0.02 | 0.50 | 0.01 | 0.15 |
| Taka Malang | Core Zone | −5.82016, 110.4453 | −5.81611, 110.4408 | 848 | 0.30 | 0.01 | 0.49 | 0.01 | 0.19 | 808 | 0.26 | 0.02 | 0.48 | 0.02 | 0.23 |
| Taka Malang 2 | General Use | −5.81281, 110.4429 | −5.80416, 110.4411 | 887 | 0.30 | 0.00 | 0.51 | 0.01 | 0.18 | 1057 | 0.26 | 0.02 | 0.63 | 0.01 | 0.07 |
| Cemara Besar East | General Use | −5.80417, 110.379 | −5.79646, 110.3811 | 908 | 0.31 | 0.02 | 0.47 | 0.01 | 0.20 | 987 | 0.31 | 0.02 | 0.50 | 0.01 | 0.16 |
| Cemara Besar MDC Site | Core Zone | −5.80364, 110.3699 | −5.81044, 110.3745 | 1006 | 0.21 | 0.01 | 0.71 | 0.01 | 0.06 | 921 | 0.20 | 0.03 | 0.68 | 0.03 | 0.06 |
| Taka Menyawakan | Core Zone | −5.7657, 110.327 | −5.76774, 110.3256 | 861 | 0.40 | 0.02 | 0.55 | 0.01 | 0.02 | 848 | 0.30 | 0.08 | 0.59 | 0.01 | 0.02 |
| Menjangan Kecil MDC | Tourism Zone | −5.88842, 110.4063 | −5.89342, 110.4005 | 929 | 0.31 | 0.01 | 0.66 | 0.01 | 0.01 | 1040 | 0.28 | 0.03 | 0.67 | 0.01 | 0.01 |
| Menjangan Besar MDC | Tourism Zone | −5.89317, 110.4212 | −5.90024, 110.427 | 1055 | 0.24 | 0.01 | 0.66 | 0.00 | 0.09 | 748 | 0.28 | 0.04 | 0.60 | 0.01 | 0.07 |
| Karimunjawa Town | General Use | −5.87799, 110.429 | −5.86981, 110.4283 | 916 | 0.31 | 0.01 | 0.49 | 0.01 | 0.19 | 866 | 0.25 | 0.02 | 0.53 | 0.01 | 0.19 |
| Kumbang Island | Core Zone | −5.77328, 110.2406 | −5.77505, 110.2287 | 892 | 0.27 | 0.01 | 0.51 | 0.01 | 0.20 | 668 | 0.27 | 0.02 | 0.55 | 0.01 | 0.16 |
| Kembar Island | Tourism Zone | −5.73295, 110.1812 | −5.72017, 110.1849 | 1097 | 0.23 | 0.02 | 0.72 | 0.01 | 0.02 | 768 | 0.22 | 0.04 | 0.70 | 0.02 | 0.01 |
| Parang Island | Mariculture Zone | −5.75195, 110.2333 | −5.74226, 110.2337 | 914 | 0.33 | 0.00 | 0.63 | 0.00 | 0.03 | 966 | 0.22 | 0.01 | 0.72 | 0.02 | 0.03 |
| Ujung Bomang | Core Zone | −5.85618, 110.4712 | −5.86506, 110.4651 | 902 | 0.21 | 0.01 | 0.49 | 0.00 | 0.29 | 1012 | 0.25 | 0.02 | 0.45 | 0.01 | 0.27 |
| Telaga Village | Rehabilitation Zone | −5.78234, 110.4616 | −5.77334, 110.4722 | 1027 | 0.26 | 0.02 | 0.62 | 0.00 | 0.10 | 752 | 0.32 | 0.02 | 0.58 | 0.02 | 0.06 |
| Pulau Menyawakan | Tourism Zone | −5.79194, 110.3451 | −5.79273, 110.348 | 186 | 0.27 | 0.03 | 0.69 | 0.00 | 0.01 | 807 | 0.28 | 0.03 | 0.66 | 0.01 | 0.02 |
| Karang Kapal | Tourism Zone | −5.89661, 110.2433 | −5.90763, 110.2321 | 986 | 0.45 | 0.04 | 0.48 | 0.00 | 0.03 | 708 | 0.36 | 0.02 | 0.58 | 0.01 | 0.02 |
| Krakal Kecil | General Use | −5.86028, 110.229 | −5.86547, 110.2335 | 927 | 0.26 | 0.02 | 0.61 | 0.00 | 0.11 | 920 | 0.21 | 0.01 | 0.70 | 0.01 | 0.06 |
| Krakal Besar | General Use | −5.84614, 110.2393 | −5.84823, 110.237 | 1011 | 0.28 | 0.03 | 0.58 | 0.01 | 0.09 | 1049 | 0.23 | 0.03 | 0.66 | 0.02 | 0.07 |
| Mean | 0.30 | 0.02 | 0.58 | 0.01 | 0.10 | 0.27 | 0.03 | 0.60 | 0.01 | 0.09 | |||||
| StDev | 0.06 | 0.01 | 0.08 | 0.00 | 0.08 | 0.04 | 0.02 | 0.09 | 0.01 | 0.08 | |||||
| Benthic Group | Benthic Sub-Group | Short Descriptor | Full DescriptorIncluding Pooled Sub-Groups Available in the Full Downloadable Dataset |
|---|---|---|---|
| Hard coral | ACR_BRA | Branching Acropora | Branching coral species from the Family Acroporidae, including bottlebrush (BRA_BOT_Ac) and branching (BRA_ARB_Ac) morphologies |
| Hard coral | ACR_TCD | Table and digitate Acropora | Other morphologies from the Family Acroporidae, including corymbose, tabular, plate (BRA_TAB-Ac) and digitate (BRA_DIG_Ac) morphologies. |
| Hard coral | BRA_nACR | Other branching coral | Non-Acroporid branching species. This category pools Pocillopora (BRA_VER_Po), Stylophora (BRA_RND_St), Anacropora and Echninopora (BRA_OTH), branching Porites (BRA_SMO_Po) and fine branching species such as Seriatopora (BRA_FIN_Se) |
| Hard coral | MSE | Massive to encrusting coral | Massive, submassive and encrusting (“MASE”) coral species with rounded corallites, including those with small to almost invisible corallites, such as Pavona, Psammocora, Coscinaraea, Gardineroseris (MASE_SML_O) or larger rounded corallites such as Diploastrea; Dipsastraea; Favites; Astrea; Galaxea; Goniopora; Astreopora (MASE_LRG_O), Isopora (MASE_LRG_I) and Porites with submassive/massive morphologies (MASE_SMO_P) |
| Hard coral | MSEM | Brain-like coral | Brain corals: massive, submassive and encrusting (“MASE”) coral species with corallites forming meandering ridges or valleys, such as Platygyra, Leptoria and Goniastrea (MASE_MEA_O) and Lobophyllia (MASE_MEA_L) |
| Hard coral | FREE | Free-living coral | Free living species, typically Fungia (NON_FREE) |
| Hard coral | FLP | Foliose coral | Hard corals with foliose morphologies, including those with visible relief structures (TFP_RND_A1) and those with visible rounded corallites (TFP_RND_A2) |
| Hard coral | NON_HERM | Non-scleractinian coral | Non-scleractinian hermatypic corals, mainly the hydrocoral Millepora (NON_MIL) and blue coral Helipora (NON_HEL) |
| Soft Coral * | GORG | Gorgonians | Gorgonian corals including families of sea fan, plumes and whips belonging to the sub-ordersHolaxonia and Scleraxonia (GORG) |
| Soft Coral * | OTH-SF | Alyciionids and other soft coral | Leathery soft corals from the Family Alycyoniidae, such as Lobophytum and Sarcophyton (SINV_SFC_A) and other non-Alcyoniid species, e.g., Xeniidae; Neptheidae; Tubipora; Briareum (SINV_SFC_E). |
| Sponge | SPONG | Sponges | Sponges, including branching and rope forms (SINV_SPO_E), fan-shaped sponges (SINV_SPO_F), massive to encrusting forms (SINV_SPOM) and hollow cups, barrels and tubs (SINV_SPO_V). |
| Algae | MACRO | Macroalgae | Macroalgae, includes all algae > 1 cm in height, including globular algae such as caulerpa (MACR_GLOB), filamentous forms (MACR_Fil_A), sheeting forms like Ulva (MACR_Fol_S), leathery macrophytes (MACR_LTH_O), foliose forms (either branched, MACR_Fol_B, feathery MACR_Fol_P, fan shaped MACR_Fol_F or other MACR_Fol_O), calcifying algae Halimeda (MACR_Cal_H) and other calcifying species (MACR_Cal_O), and Padina (MACR_Cal_P). This group also includes cyanobacteria found smothering dead coral (CYANO_DHC), rubble (CAL_CCA_RB) and rock (CAL_CCA_Sub) as well as any other algae not fitting into these categories (ALGAE_OTH). |
| Algae | EAM | Epilithic algal matrix | Turfs and biofilms covering dead hard coral (EAM_DHC), rubble (EAM_RB), rock or other substrate (EAM_Sub). |
| Algae | CCA | Crustose coralline algae | Crustose coralline algae, including forms encrusting dead hard coral (CAL_CCA_DC) found on substrate (CAL_CCA_RB) and rubble (CAL_CCA_RB). |
| Sediment | NON_COLONIZABLE | Loose substrate | Loose non-living substrate, including rubble (LSUB_RUB), sand (LSUB_SAND) and sediment (LSUB_SEDI). |
References
- Baum, G.; Januar, H.I.; Ferse, S.C.A.; Kunzmann, A. Local and regional impacts of pollution on coral reefs along the Thousand Islands north of the megacity Jakarta, Indonesia. PLoS ONE 2015, 10, e0138271. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.; Ravilious, C.; Green, E. World Atlas of Coral Reefs; University of California Press: Berkeley, CA, USA, 2001; p. 436. [Google Scholar]
- Pendleton, L.; Comte, A.; Langdon, C.; Ekstrom, J.A.; Cooley, S.R.; Suatoni, L.; Beck, M.W.; Brander, L.M.; Burke, L.; Cinner, J.E.; et al. Coral reefs and people in a high-CO2 world: Where can science make a difference to people? PLoS ONE 2016, 11, e0164699. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011; ISBN 978-1-56973-762-0. [Google Scholar]
- Pranato, I.B. Indonesia-Coral Reef Rehabilitation and Management Program-Coral Triangle Initiative (COREMAP-CTI); Wolrd Bank Group: Washington, DC, USA, 2015. [Google Scholar]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 global-scale coral bleaching event: Insights and impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M. Chapter 3: Impacts of 1.5 °C global warming on natural and human systems. In Special Report on Global Warming of 1.5 °C; Intergovermental Panel on Climate Change: Incheon, Korea, 2018. [Google Scholar]
- Hoegh-Guldberg, O.; Kennedy, E.V.; Beyer, H.L.; McClennen, C.; Possingham, H.P. Securing a long-term future for coral reefs. Trends Ecol. Evolut. 2018, 33, 936–944. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.L.; Wisshak, M.; Form, A.U.; Carricart-Ganivet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 2013, 23, 912–918. [Google Scholar] [CrossRef]
- Beger, M.; McGowan, J.; Treml, E.A.; Green, A.L.; White, A.T.; Wolff, N.H.; Klein, C.J.; Mumby, P.J.; Possingham, H.P. Integrating regional conservation priorities for multiple objectives into national policy. Nat. Commun. 2015, 6, 8208. [Google Scholar] [CrossRef]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef]
- Campbell, S.J. Rebuilding Management Effectiveness in Karimunjawa National Park, Indonesia; NOAA Final Report; NOAA: Silver Spring, MD, USA, 2006. [Google Scholar]
- Asaad, I.; Lundquist, C.J.; Erdmann, M.V.; Van Hooidonk, R.; Costello, M.J. Designating spatial priorities for marine biodiversity conservation in the Coral Triangle. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- UNEP-WCMC. Global Distribution of Warm-Water Coral Reefs, Compiled from Multiple Sources; UNEP World Conservation Monitoring Centre: Cambridge, UK, 2010. [Google Scholar]
- Campbell, S.J.; Kartawijaya, T.; Yulianto, I.; Prasetia, R.; Clifton, J. Co-management approaches and incentives improve management effectiveness in the Karimunjawa National Park, Indonesia. Mar. Pol. 2013, 41, 72–79. [Google Scholar] [CrossRef]
- Allen Coral Atlas. Imagery, Maps and Monitoring of the Worlds Tropical Coral Reefs; Zenodo Repository. Available online: www.allencoralatlas.org (accessed on 20 September 2020).
- Solihuddin, T.; Utami, D.A.; Salim, H.L.; Prihantono, J. Sedimentary environment of a modern carbonate platform of Karimunjawa islands, Central Java. Indones. J. Geosci. 2019, 6, 57–72. [Google Scholar] [CrossRef]
- Edinger, E.N.; Kolasa, J.; Risk, M.J. Biogeographic variation in coral species diversity on coral reefs in three regions of Indonesia. Divers. Distrib. 2000, 6, 113–127. [Google Scholar] [CrossRef]
- Hafsaridewi, R.; Sulistiono, F.A.; Sutrisno, D.; Koeshendrajana, S. Resource management in the Karimunjawa Islands, Central Java of Indonesia, through DPSIR approach. Adv. Environ. Sci. 2018, 10, 7–22. [Google Scholar]
- Bejarano, S.; Pardede, S.; Campbell, S.J.; Hoey, A.S.; Ferse, S.C.A. Herbivorous fish rise as a destructive fishing practice falls in an Indonesian marine national park. Ecol. Appl. 2019, 29, e01981. [Google Scholar] [CrossRef]
- Yuliana, E.; Fahrudin, A.; Boer, M.; Kamal, M.M.; Pardede, S.T. The effectiveness of the zoning system in the management of reef fisheries in the marine protected area of Karimunjawa National Park, Indonesia. AACl Bioflux 2016, 9, 483–497. [Google Scholar]
- Ardiwijaya, R.; Baird, A.; Kartawijaya, T.; Campbell, S. Changes in Reef Fish Biomass in Karimunjawa National Park: A Test of the Effectiveness of Government Gazetted Marine Parks in Indonesia. In Proceedings of the International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–9 July 2008. [Google Scholar]
- Taruc, S.A.K. Resilience Studies of an Indonesian Coral Reef: Ecological and Social Assessments In Karimunjawa National Park; University of Queensland: Brisbane, Australia, 2011. [Google Scholar]
- Maynard, J.; Anthony, K.; Afatta, S.; Anggraini, L.; Haryanti, D.; Ambariyanto, A. Rock anchoring in Karimun Jawa, Indonesia: Ecological impacts and management implications. Pac. Conserv. Biol. 2008, 14, 242–243. [Google Scholar] [CrossRef][Green Version]
- Marnane, M.J.; Ardiwijaya, R.L.; Wibowo, J.; Pardede, S.; Mukminin, A.; Herdiana, Y. Study on Muroami Fishing Activity in Karimunjawa Islands; Wildlife Conservation Society-Marine Program Report: Bogor, Indonesia, 2004. [Google Scholar]
- Purwanti, F. Tourism and conservation management options for Karimunjawa Marine National Park (Case study and reviews). J. Coast. Develop. 2001, 4, 99–104. [Google Scholar]
- Holmes, K.E.; Edinger, E.N.; Hariyadi, S.; Limmon, G.V.; Risk, M.J. Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Mar. Pollut. Bull. 2000, 40, 606–617. [Google Scholar] [CrossRef]
- Purwaningsih, R. Fishery Supporting Industry Planning for Karimunjawa Jepara Sea Farming. In Proceedings of the International Conference of Management Science, Yogyakarta, Indonesia, 10 March 2016. [Google Scholar]
- Sabdono, A.; Radjasa, O.K.; Trianto, A.; Sarjito, S.; Munasik, M.; Wijayanti, D.P. Preliminary study of the effect of nutrient enrichment, released by marine floating cages, on the coral disease outbreak in Karimunjawa, Indonesia. Reg. Stud. Mar. Sci. 2019, 30, 100704. [Google Scholar] [CrossRef]
- Maynard, J.A.; Anthony, K.R.N.; Afatta, S.; Dahl-Tacconi, N.; Hoegh-Guldberg, O.V.E. Making a model meaningful to coral reef managers in a developing nation: A case study of overfishing and rock anchoring in Indonesia. Conserv. Biol. 2010, 24, 1316–1326. [Google Scholar] [CrossRef]
- Baskara, K.A.; Hendarto, M.; Susilowati, I. Economic valuation of marine protected area of Karimunjawa, Jepara, Indonesia. AACL Bioflux 2017, 10, 1554–1568. [Google Scholar]
- Beyer, H.L.; Kennedy, E.V.; Beger, M.; Chen, C.A.; Cinner, J.E.; Darling, E.S.; Eakin, C.M.; Gates, R.D.; Heron, S.F.; Knowlton, N.; et al. Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 2018, 11, e12587. [Google Scholar] [CrossRef]
- Sea Temperature Info. Karimunjawa Sea Water Temperature, World Sea Water Temperatures. Available online: https://seatemperature.info/karimunjawa-water-temperature.html (accessed on 20 September 2020).
- Pardede, S.; Tarigan, S.A.R.; Setiawan, F.; Muttaqin, E.; Muttaqin, A.; Muhidin, D. Laporan Teknis: Monitoring ekosistem terumbu karang Taman Nasional Karimunjawa 2016; Wildlife Conservation Society-Marine Program Report: Bogor, Indonesia, 2016. [Google Scholar]
- Peterson, E.E.; Santos-Fernández, E.; Chen, C.; Clifford, S.; Vercelloni, J.; Pearse, A.; Brown, R.; Christensen, B.; James, A.; Anthony, K.; et al. Monitoring through many eyes: Integrating disparate datasets to improve monitoring of the Great Barrier Reef. Environ. Modell. Softw. 2020, 124, 104557. [Google Scholar] [CrossRef]
- Perkins, N.R.; Foster, S.D.; Hill, N.A.; Barrett, N.S. Image subsampling and point scoring approaches for large-scale marine benthic monitoring programs. Estuar. Coast. Shelf Sci. 2016, 176, 36–46. [Google Scholar] [CrossRef]
- Darling, E.S.; McClanahan, T.R.; Côté, I.M. Life histories predict coral community disassembly under multiple stressors. Glob. Chang. Biol. 2013, 19, 1930–1940. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Hughes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S.; et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Warton, D.I.; Blanchet, F.G.; O’Hara, R.B.; Ovaskainen, O.; Taskinen, S.; Walker, S.C.; Hui, F.K.C. So many variables: Joint modeling in community ecology. Trends Ecol. Evolut. 2015, 30, 766–779. [Google Scholar] [CrossRef]
- González-Rivero, M.; Bongaerts, P.; Beijbom, O.; Pizarro, O.; Friedman, A.; Rodriguez-Ramirez, A.; Upcroft, B.; Laffoley, D.; Kline, D.; Bailhache, C.; et al. The Catlin Seaview Survey–kilometre-scale seascape assessment, and monitoring of coral reef ecosystems. Aquat. Conserv. Mar. Freshwater Ecosyst. 2014, 24, 184–198. [Google Scholar] [CrossRef]
- Cadman, C.A. Indonesia-Coral Reef Rehabilitation and Management Program-Coral Triangle Initiative (COREMAP-CTI); P127813-Implementation Status Results Report Sequence 06; Asian Development Bank: Mandaluyong, Philippines, 2013. [Google Scholar]
- González-Rivero, M.; Rodriguez-Ramirez, A.; Beijbom, O.; Dalton, P.; Kennedy, E.V.; Neal, B.P.; Vercelloni, J.; Bongaerts, P.; Ganase, A.; Bryant, D.E.P.; et al. Seaview Survey Photo-quadrat and Image Classification Dataset; University of Queensland Research Data Collection: Brisbane, Australia, 2019. [Google Scholar] [CrossRef]
- González-Rivero, M.; Beijbom, O.; Rodriguez-Ramirez, A.; Holtrop, T.; González-Marrero, Y.; Ganase, A.; Roelfsema, C.; Phinn, S.; Hoegh-Guldberg, O. Scaling up ecological measurements of coral reefs using semi-automated field image collection and analysis. Remote Sens. 2016, 8, 30. [Google Scholar] [CrossRef]
- González-Rivero, M.; Beijbom, O.; Rodriguez-Ramirez, A.; Bryant, D.E.P.; Ganase, A.; Gonzalez-Marrero, Y.; Herrera-Reveles, A.; Kennedy, E.V.; Kim, C.J.S.; Lopez-Marcano, S.; et al. Monitoring of coral reefs using Artificial Intelligence: A feasible and cost-effective approach. Remote Sens. 2020, 12, 489. [Google Scholar] [CrossRef]
- Althaus, F.; Hill, N.; Ferrari, R.; Edwards, L.; Przeslawski, R.; Schönberg, C.H.L.; Stuart-Smith, R.; Barrett, N.; Edgar, G.; Colquhoun, J.; et al. A Standardised Vocabulary for Identifying Benthic Biota and Substrata from Underwater Imagery: The CATAMI classification scheme. PLoS ONE 2015, 10, e0141039. [Google Scholar] [CrossRef]
- Wallace, C.C. Staghorn Corals of the World: A Revision of the Coral Genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) Worldwide, with Emphasis on Morphology, Phylogeny and Biogeography; Carden, C.W., Ed.; CSIRO Publishing: Clayton, Australia, 1999. [Google Scholar]
- Heron, S.F.; Maynard, J.A.; van Hooidonk, R.; Eakin, C.M. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef]
- Liu, G.; Eakin, C.M.; Chen, M.; Kumar, A.; De La Cour, J.L.; Heron, S.F.; Geiger, E.F.; Skirving, W.J.; Tirak, K.V.; Strong, A.E. Predicting heat stress to inform reef management: NOAA Coral Reef Watch’s 4-month coral bleaching outlook. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- NOAA Coral Reef Watch. NOAA Coral Reef Watch Daily Global 5-km Satellite Virtual Station Time Series Data; NOAA Coral Reef Watch: College Park, MD, USA, 2013. Available online: http://coralreefwatch.noaa.gov (accessed on 15 October 2018.).
- Vercelloni, J.; Liquet, B.; Kennedy, E.V.; González-Rivero, M.; Caley, M.J.; Peterson, E.E.; Puotinen, M.; Hoegh-Guldberg, O.; Mengersen, K. Forecasting intensifying disturbance effects on coral reefs. Glob. Chang. Biol. 2020, 26. [Google Scholar] [CrossRef]
- Hui, F.K.C. Boral-Bayesian ordination and regression analysis of multivariate abundance data in R. Methods Ecol. Evolut. 2016, 7, 744–750. [Google Scholar] [CrossRef]
- Campbell, S.J.; Hoey, A.S.; Maynard, J.; Kartawijaya, T.; Cinner, J.; Graham, N.A.J.; Baird, A.H. Weak compliance undermines the success of no-take zones in a large government-controlled marine protected area. PLoS ONE 2012, 7, e50074. [Google Scholar] [CrossRef]
- Supriyanto, R.A.; Martani, I.B. Laporan Pelaksanaan Kegiatan: Monitoring Terumbu Karang dan Ikan Balai Taman Nasional Karimunjawa Tahun 2015; Direktorat Jenderal Perlindungan Hutan dan Konservasi Alam: Semarang, Indonesia, 2015. [Google Scholar]
- Bryant, D.E.P.; Rodriguez-Ramirez, A.; Phinn, S.; González-Rivero, M.; Brown, K.T.; Neal, B.P.; Hoegh-Guldberg, O.; Dove, S. Comparison of two photographic methodologies for collecting and analyzing the condition of coral reef ecosystems. Ecosphere 2017, 8, e01971. [Google Scholar] [CrossRef]
- Priyanto, F. The Impact of National Park Zoning against Livelihood Strategy of Compressor’s Fisherman. (Case in Village of Karimunjawa, Jepara Regency, Central Java). Ph.D. Thesis, University of Western Australia, Crawley, Australia, 2011. [Google Scholar]
- Karimunjawa, B.T.N. Penataan Zonasi Taman Nasional Karimunjawa Kabupaten Jepara Provnsi jawa Tengah; Direktorat Jenderal Perlindungan Hutan dan Konservasi Alam: Semarang, Indonesia, 2004. [Google Scholar]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr. Biol. 2014, 24, 1000–1005. [Google Scholar] [CrossRef]
- Harris, D.L.; Rovere, A.; Casella, E.; Power, H.; Canavesio, R.; Collin, A.; Pomeroy, A.; Webster, J.M.; Parravicini, V. Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Sci. Adv. 2018, 4, eaao4350. [Google Scholar] [CrossRef] [PubMed]
- Madduppa, H.; Schupp, P.J.; Faisal, M.R.; Sastria, M.Y.; Thoms, C. Persistent outbreaks of the “black disease” sponge Terpios hoshinota in Indonesian coral reefs. Mar. Biodivers. 2017, 47, 149–151. [Google Scholar] [CrossRef]
- Rutzler, K.; Musik, K. Terpios hoshinota, a new cyanobacteriosponge threatening Pacific reefs. Sci. Mar. 1993, 4, 395–403. [Google Scholar]
- Shi, Q.; Liu, G.H.; Yan, H.Q.; Zhang, H.L. Black Disease (Terpios hoshinota): A Probable Cause for the Rapid Coral Mortality at the Northern Reef of Yongxing Island in the South China Sea. AMBIO 2012, 41, 446–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raymundo, L.J.; Rosell, K.B.; Reboton, C.T.; Kaczmarsky, L. Coral diseases on Philippine reefs: Genus Porites is a dominant host. Dis. Aquat. Organ. 2005, 64, 181–191. [Google Scholar] [CrossRef][Green Version]
- Madin, E.M.P.; Darling, E.S.; Hardt, M.J. Emerging technologies and coral reef conservation: Opportunities, challenges, and moving forward. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
| Core Zone | Protection Zone | Tourism Zone | Mariculture Zone | Rehabilitation Zone | General Use Zone | |
|---|---|---|---|---|---|---|
| Area (km2) | 4.5 | 26 | 27.3 | 13.1 | 0.7 | 1003 |
| Fishing | × | × | P | × | ✓ | ✓ |
| Research | p | p | P | p | p | p |
| Education | p | p | P | ✓ | ✓ | ✓ |
| Boat transit | × * | ✓ | ✓ | ✓ | ✓ | ✓ |
| Anchoring | × * | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tourism | × | × | ✓ | ✓ | ✓ | ✓ |
| Restoration | × | × | ✓ | ✓ | ✓ | ✓ |
| Traditional use | p | p | P | ✓ | ✓ | ✓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, E.V.; Vercelloni, J.; Neal, B.P.; Ambariyanto; Bryant, D.E.P.; Ganase, A.; Gartrell, P.; Brown, K.; Kim, C.J.S.; Hudatwi, M.; et al. Coral Reef Community Changes in Karimunjawa National Park, Indonesia: Assessing the Efficacy of Management in the Face of Local and Global Stressors. J. Mar. Sci. Eng. 2020, 8, 760. https://doi.org/10.3390/jmse8100760
Kennedy EV, Vercelloni J, Neal BP, Ambariyanto, Bryant DEP, Ganase A, Gartrell P, Brown K, Kim CJS, Hudatwi M, et al. Coral Reef Community Changes in Karimunjawa National Park, Indonesia: Assessing the Efficacy of Management in the Face of Local and Global Stressors. Journal of Marine Science and Engineering. 2020; 8(10):760. https://doi.org/10.3390/jmse8100760
Chicago/Turabian StyleKennedy, Emma V, Julie Vercelloni, Benjamin P Neal, Ambariyanto, Dominic E.P. Bryant, Anjani Ganase, Patrick Gartrell, Kristen Brown, Catherine J.S. Kim, Mu’alimah Hudatwi, and et al. 2020. "Coral Reef Community Changes in Karimunjawa National Park, Indonesia: Assessing the Efficacy of Management in the Face of Local and Global Stressors" Journal of Marine Science and Engineering 8, no. 10: 760. https://doi.org/10.3390/jmse8100760
APA StyleKennedy, E. V., Vercelloni, J., Neal, B. P., Ambariyanto, Bryant, D. E. P., Ganase, A., Gartrell, P., Brown, K., Kim, C. J. S., Hudatwi, M., Hadi, A., Prabowo, A., Prihatinningsih, P., Haryanta, S., Markey, K., Green, S., Dalton, P., Lopez-Marcano, S., Rodriguez-Ramirez, A., ... Hoegh-Guldberg, O. (2020). Coral Reef Community Changes in Karimunjawa National Park, Indonesia: Assessing the Efficacy of Management in the Face of Local and Global Stressors. Journal of Marine Science and Engineering, 8(10), 760. https://doi.org/10.3390/jmse8100760






