Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis
Abstract
1. Introduction
2. Allocation of Carbon Stocks
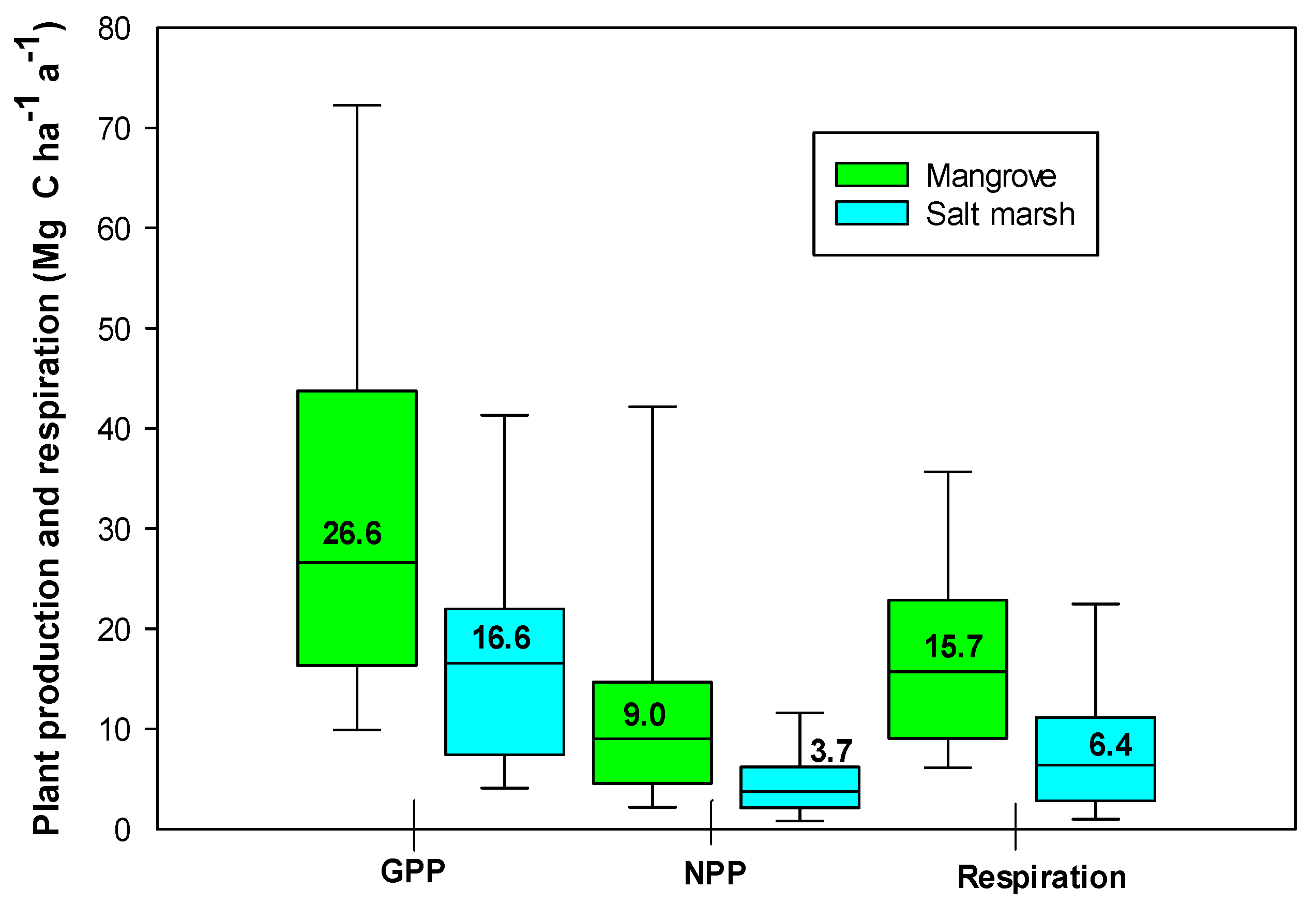
3. Primary Production and Plant Respiration
4. Soil Carbon Biogeochemistry
4.1. Soil-Air/Water Fluxes
4.2. Soil DIC Production
4.3. CORG Burial in Soils
5. Carbon Biogeochemistry in Tidal Waters
5.1. CO2 and CH4 Emissions
5.2. Carbon Export
6. Whole-Ecosystem Carbon Mass Balance
7. Data Refinements and Future Needs
- Clarity of their global area, as areal estimates vary greatly;
- Estimates of ecosystem GPP, NPP and R, using eddy covariance technology;
- Estimates of belowground root production;
- Quantification and extrapolation of algal mat production and carbon fixation of nitrogen fixers on tree stems, downed wood and plant debris (litter; leaves);
- Quantification of allochthonous inputs from marine and terrestrial sources, such as adjacent seagrass beds, other estuary producers and advection from offshore;
- Estimates of DIC, DOC and CH4 exchange between these wetlands and adjacent waters;
- Estimates of CO2 and CH4 release from associated tidal creeks and waterways;
- Estimates of benthic microalgal GPP;
- Estimates of soil C stocks and fluxes deeper than 50 cm, especially in salt marshes;
- Reconciling rates of soil respiration at the surface and in subsurface deposits and their linkage;
- Quantification of groundwater export of dissolved carbon in relation to porewater advection of mineralized carbon within deep mangrove and marsh soils;
- Quantification of the fate of roots and their productivity;
- Understanding of CORG differences between within-site and between-site locations (e.g., differences with intertidal position);
- Clarification of links among roots, litter and the soil CORG pool in relation to mineralization rates;
- Clarification of the contributions of litter, dead plants, wood and the soil CORG pool to carbon burial.
8. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Coastal Ecosystem Processes; CRC Press: Boca Raton, FL, USA, 1998; pp. 43–92. [Google Scholar]
- Tiner, R.W.; Milton, G.R. Estuarine marsh: An overview. In The Wetland Book. II. Distribution, Description and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 55–72. [Google Scholar]
- Cragg, S.M.; Friess, D.A.; Gillis, L.G.; Trevathan-Tackett, S.M.; Terrett, O.M.; Watts, J.E.M.; Distel, D.L.; Dupree, P. Vascular plants are globally significant contributors to marine carbon fluxes and sinks. Annu. Rev. Mar. Sci. 2020, 12, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Blue Carbon: Coastal Sequestration for Climate Change Mitigation; Springer Briefs in Climate Studies; Springer Science: Cham, Switzerland, 2018. [Google Scholar]
- Johnson, B.J.; Lovelock, C.E.; Herr, D. Climate regulation: Salt marshes and blue carbon. In The Wetland Book. I. Structure, Function, Management, and Methods; Finlayson, C.M., Everard, M., Irvine, K., McInnes, R.J., Middleton, B.A., van Dam, A.A., Davidson, M.C., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 1185–1196. [Google Scholar]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Scince 2020, 2, 67. [Google Scholar] [CrossRef]
- Couto, T.D.T.C. Carbon Budget in a Temperate Estuary Salt Marsh. Influence of Temperature Increase in Carbon Sequestration. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Couto, T.; Duarte, B.; Caçador, I.; Baeta, A.; Marques, J.C. Salt marsh plants carbon storage in a temperate Atlantic estuary illustrated by a stable isotopic analysis-based approach. Ecol. Ind. 2013, 32, 305–311. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J.; Lubberts, R.K.; van de Plassche, O. Organic carbon isotope systematics of coastal marshes. Estuar. Coast. Shelf Sci. 1997, 45, 681–687. [Google Scholar] [CrossRef]
- Więski, K.; Guo, H.; Craft, C.B.; Pennings, S.C. Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia coast. Estuaries Coasts 2010, 33, 161–169. [Google Scholar] [CrossRef]
- Tripathee, R.; Schäfer, K.V.R. Above-and belowground biomass allocation in four dominant salt marsh species of the eastern United States. Wetlands 2014, 35, 21–30. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Moyer, R.P. Plant size metrics and organic carbon content of Florida salt marsh vegetation. Wetl. Ecol. Manag. 2017, 25, 443–455. [Google Scholar] [CrossRef]
- Jones, J.A.; Cherry, J.A.; McKee, K.L. Species and tissue type regulate long-term decomposition of brackish marsh plants grown under elevated CO2 conditions. Estuar. Coast. Shelf Sci. 2016, 169, 38–45. [Google Scholar] [CrossRef]
- Keefe, C.W.; Boynton, W.R. Standing crop of salt marshes surrounding Chincoteague Bay, Maryland-Virginia. Chesap. Sci. 1973, 14, 117–123. [Google Scholar] [CrossRef]
- Benner, R.; Fogel, M.L.; Sprague, E.K. Diagenesis of belowground biomass of Spartina alterniflora in salt-marsh sediments. Limnol. Oceanogr. 1991, 36, 1358–1374. [Google Scholar] [CrossRef]
- Carey, J.C.; Moran, S.B.; Kelly, R.P.; Kolker, A.S.; Fulweiler, R.W. The declining role of organic matter in New England salt marshes. Estuaries Coasts 2017, 40, 626–639. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Buresh, R.J.; Patrick, W.H., Jr. Relationship of soil properties to standing crop biomass of Spartina alterniflora in a Louisiana marsh. Estuar. Coast. Mar. Sci. 1979, 8, 477–487. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, X.; Wang, W.; Zhao, Q.; Zhang, S. Soil organic carbon contents and stocks in coastal salt marshes with Spartina alterniflora following an invasion chronosequence in the Yellow River delta, China. Chin. Geogra Sci. 2018, 28, 374–385. [Google Scholar] [CrossRef]
- Schäfer, K.V.R.; Tripathee, R.; Artigas, F.; Morin, T.H.; Bohrer, G. Carbon dioxide fluxes of an urban tidal marsh in the Hudson-Raritan estuary. J. Geophys. Res. Biogeosci. 2014, 119, 2065–2081. [Google Scholar] [CrossRef]
- Santini, N.S.; Lovelock, C.E.; Hua, Q.; Zawadzki, A.; Mazumder, D.; Mercer, T.R.; Muñoz-Rojas, M.; Hardwick, S.A.; Madala, B.S.; Cornwell, W.; et al. Natural and regenerated saltmarshes exhibit similar soil and belowground organic carbon stocks, root production and soil respiration. Ecosystems 2019, 22, 1803–1822. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Pezechki, S.R. The role of soil organic carbon in maintaining surface elevation in rapidly subsiding U.S. Gulf of Mexico coastal marshes. Water Air Soil Pollut. 2003, 3, 167–179. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Bernardino, A.F.; Ferreira, T.O.; Giovannoni, L.R.; de O. Gomes, L.E.; Romero, D.J.; Jimenez, L.C.Z.; Ruiz, F. Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol. Lett. 2018, 14, 20180208. [Google Scholar] [CrossRef]
- An, S.-U.; Cho, H.; Jung, U.-J.; Kim, B.; Lee, H.; Hyun, J.-H. Invasive Spartina anglica greatly alters the rates and pathways of organic carbon oxidation and associated microbial communities in an intertidal wetland of the Han River estuary, Yellow Sea. Front. Mar. Sci. 2020, 7, 59. [Google Scholar] [CrossRef]
- Saunders, C.J.; Megonigal, J.P.; Reynolds, J.F. Comparison of belowground biomass in C3- and C4-dominated mixed communities in a Chesapeake Bay brackish marsh. Plant Soil 2006, 280, 305–322. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, J.; Xue, L.; Craft, C.B. Distribution of organic carbon storage in different salt-marsh plant communities: A case study at the Yangtze estuary. Estuar. Coast. Shelf Sci. 2020, 243, 106900. [Google Scholar] [CrossRef]
- Connor, R.; Chmura, G.L. Dynamics of above- and belowground organic matter in a high latitude macrotidal saltmarsh. Mar. Ecol. Prog. Ser. 2000, 204, 101–110. [Google Scholar] [CrossRef]
- Castillo, J.M.; Rubio-Casal, A.E.; Figueroa, E. Cordgrass biomass in coastal marshes. In Source: Biomass; Momba, M., Bux, F., Eds.; Intechiopen: Sciyo, Croatia, 2010; pp. 1–26. [Google Scholar]
- Doughty, C.L.; Cavanaugh, K.C. Mapping coastal wetland biomass from high resolution unmanned aerial vehicle (UAV) imagery. Remote Sens. 2019, 11, 540. [Google Scholar] [CrossRef]
- Sousa, A.I.; Santos, D.B.; da Silva, E.F.; Sousa, L.P.; Cleary, D.F.R.; Soares, A.M.V.M.; Lillebø, A.I. ‘Blue carbon’ and nutrient stocks of salt marshes at a temperate coastal lagoon (Ria de Aveiro, Portugal). Sci. Rep. 2017, 7, 41225. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.B.; Ballanti, L.; Thomas, N.; Nguyen, D.; Holmquist, J.R.; Simard, M.; Windham-Myers, L. A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. ISPRS J. Photogram. Remote Sens. 2018, 139, 255–271. [Google Scholar] [CrossRef]
- Moore, G.E.; Burdick, D.M.; Peter, C.R.; Keirstead, D.R. Belowground biomass of Phragmites australis in coastal marshes. Northeast. Nat. 2012, 19, 611–626. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Unger, V. Geomorphic influences on the contribution of vegetation to soil C accumulation and accretion in Spartina alterniflora marshes. Biogeosciences 2018, 15, 379–397. [Google Scholar] [CrossRef]
- Brown, C.E.; Rajkaran, A. Biomass partitioning in an endemic southern African salt marsh species Salicornia tegetaria (Chenopodiaceae). Afr. J. Aquat. Sci. 2020, 45. [Google Scholar] [CrossRef]
- Chastain, S.G.; Kohfeld, K.; Pellatt, M.G. Carbon stocks and accumulation rates in salt marshes of the Pacific coast of Canada. Biogeosci. Discuss. 2018. [Google Scholar] [CrossRef]
- Elschot, K.; Bakker, J.P.; Temmerman, S.; van de Koppel, J.; Bouma, T.J. Ecosystem engineering by large grazers enhances carbon stocks in a tidal salt marsh. Mar. Ecol. Prog. Ser. 2015, 537, 9–21. [Google Scholar] [CrossRef]
- Kelleway, J.J.; Saintilan, N.; Macreadie, P.I.; Skilbeck, C.G.; Zawadzki, A.; Ralph, P.J. Seventy years of continuous encroachment substantially increases ‘blue carbon’ capacity as mangroves replace intertidal salt marshes. Glob. Chang. Biol. 2016, 22, 1097–1109. [Google Scholar] [CrossRef]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, J.P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Radabaugh, K.R.; Moyer, R.P.; Chappel, A.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Smoak, J.M. Coastal blue carbon assessment of mangroves, salt marshes, and salt barrens in Tampa Bay, Florida, USA. Estuar. Coasts 2018, 41, 1496–1510. [Google Scholar] [CrossRef]
- Dontis, E.E.; Radabaugh, K.R.; Chappel, A.R.; Russo, C.E.; Moyer, R.P. Carbon storage increases with site age as created salt marshes transition to mangrove forests in Tampa Bay, Florida (USA). Estuar. Coasts 2020. [Google Scholar] [CrossRef]
- Yando, E.S.; Osland, M.J.; Willis, J.M.; Day, R.H.; Krauss, K.W.; Hester, M.W. Salt marsh-mangrove ecotones: Using structural gradients to investigate the effects of woody plant encroachment on plant-soil interactions and ecosystem carbon pools. J. Ecol. 2016, 104, 1020–1031. [Google Scholar] [CrossRef]
- Bulmer, R.H.; Stephenson, F.; Jones, H.F.E.; Townsend, M.; Hillman, J.R.; Schwendenmann, L.; Lundquist, C.J. Blue carbon stocks and cross-habitat subsidies. Front. Mar. Sci. 2020, 7, 380. [Google Scholar] [CrossRef]
- Cartaxana, P.; Catarino, F. Allocation of nitrogen and carbon in an estuarine salt marsh in Portugal. J. Coast. Conserv. 1997, 3, 27–34. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Rathore, A.P.; Jha, B. Aboveground, belowground biomass and nutrient pool in Salicornia brachiata at coastal area of India: Interactive effects of soil characteristics. Ecol. Res. 2018, 33, 1207–1218. [Google Scholar] [CrossRef]
- Burden, A.; Garbutt, A.; Evans, C.D. Effect of restoration on saltmarsh carbon accumulation in eastern England. Biol. Lett. 2019, 15, 20180773. [Google Scholar] [CrossRef]
- Martinetto, P.; Montemayor, D.I.; Alberti, J.; Costa, C.S.B.; Iribarne, O. Crab bioturbation and herbivory may account for variability in carbon sequestration and stocks in South West Atlantic salt marshes. Front. Mar. Sci. 2016, 3, 122. [Google Scholar] [CrossRef]
- Kulawardhana, R.W.; Feagin, R.A.; Popescu, S.C.; Boutton, T.W.; Yeager, K.M.; Bianchi, T.S. The role of elevation, relative sea-level history, and vegetation transition in determining carbon distribution in Spartina alterniflora dominated salt marshes. Estuar. Coast. Shelf Sci. 2015, 154, 48–57. [Google Scholar] [CrossRef]
- Owers, C.J.; Rogers, K.; Woodroffe, C.D. Spatial variation of above-ground carbon storage in temperate coastal wetlands. Estuar. Coast. Shelf Sci. 2018, 210, 55–67. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Mcowen, C.J.; Weatherdon, L.V.; Van Bochove, J.-W.; Sullivan, E.; Blyth, S.; Zockler, C.; Stanwell-Smith, D.; Kingston, N.; Martin, C.S.; Spalding, M.; et al. A global map of saltmarshes. Biodivers. Data J. 2017, 5, e11764. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Connolly, R.M.; Otero, X.L.; Marchand, C.; Ferreira, T.O.; Rivera-Monroy, V.H. Biogeochemical cycles: Global approaches and perspectives. In Mangrove Ecosystems: A Global Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 163–209. [Google Scholar]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycl. 2003, 17. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Twilley, R.R.; Castañeda-Moya, E.; Rivera-Monroy, V.H.; Rovai, A. Productivity and carbon dynamics in mangrove wetlands. In Mangrove Ecosystems: A Global Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–162. [Google Scholar]
- Chmura, G.L. Tidal salt marshes. In The Management of Natural Coastal Carbon Sinks; Larroley, D.D., Grimsditch, G., Eds.; IUCN: Gland, Switzerland, 2009; pp. 5–11. [Google Scholar]
- Blum, L.K. Spartina alterniflora root dynamics in a Virginia marsh. Mar. Ecol. Prog. Ser. 1993, 102, 169–178. [Google Scholar] [CrossRef]
- Howes, B.L.; Dacey, J.W.H.; Teal, J.M. Annual carbon mineralization and belowground production of Spartina alterniflora in a New England salt marsh. Ecology 1985, 66, 595–605. [Google Scholar] [CrossRef]
- Roman, C.T.; Daiber, F.C. Aboveground and belowground primary production dynamics of two Delaware Bay tidal marshes. Bull. Torrey Bot. Club 1984, 111, 34–41. [Google Scholar] [CrossRef]
- Feiijtel, T.C.; DeLaune, R.D.; Patrick, W.H., Jr. Carbon flow in coastal Louisiana. Mar. Ecol. Prog. Ser. 1985, 24, 255–260. [Google Scholar] [CrossRef]
- Forbrich, I.; Giblin, A.E. Marsh-atmosphere CO2 exchange in a New England salt marsh. J. Geophys. Res. Biogeosci. 2015, 120, 1825–1838. [Google Scholar] [CrossRef]
- Kathilankal, J.C.; Mozdzer, T.J.; Fuentes, J.D.; D’Odorico, P.; McGlathery, K.J.; Zieman, J.C. Tidal influences on carbon assimilation by a salt marsh. Environ. Res. Lett. 2008, 3, 044010. [Google Scholar] [CrossRef]
- Schubauer, J.P.; Hopkinson, C.S. Above-and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnol. Oceanogr. 1984, 29, 1052–1065. [Google Scholar] [CrossRef]
- Groenendijk, A.M.; Vink-Lievaart, M.A. Primary production and biomass on a Dutch salt marsh: Emphasis on the below-ground component. Vegetatio 1987, 70, 21–27. [Google Scholar]
- Hemminga, M.A.; Huiskes, A.H.L.; Steegstra, M.; van Soelen, J. Assessment of carbon allocation and biomass production in a natural stand of the salt marsh plant Spartina anglica using 13C. Mar. Ecol. Prog. Ser. 1996, 130, 169–178. [Google Scholar] [CrossRef]
- Xi, M.; Zhang, X.; Kong, F.; Li, Y.; Sui, X.; Wang, X. CO2 exchange under different vegetation covers in a coastal wetland of Jiaozhou Bay, China. Ecol. Eng. 2019, 137, 26–33. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Murrell, M.C.; Amacker, K.S.; Harper, J.W.; Philipps, S.; Woodrey, M.S. Seasonal and inter-annual patterns in primary production, respiration, and net ecosystem metabolism in three estuaries in the northeast Gulf of Mexico. Estuaries Coasts 2014, 37, S222–S241. [Google Scholar] [CrossRef]
- Wilson, B.J.; Mortazavi, B.; Kiene, R.P. Spatial and temporal variability in carbon dioxide and methane exchange at three coastal marshes along a salinity gradient in a northern Gulf of Mexico estuary. Biogeochemistry 2015, 123, 329–347. [Google Scholar] [CrossRef]
- Sousa, A.I.; Lillebø, A.I.; Pardal, M.A.; Caçador, I. Productivity and nutrient cycling in salt marshes: Contribution to ecosystem health. Estuar. Coast. Shelf Sci. 2010, 87, 640–646. [Google Scholar] [CrossRef]
- Castillo, J.M.; Leira-Doce, P.; Rubio-Casal, A.E.; Figueroa, E. Spatial and temporal variations in aboveground and belowground biomass of Spartina maritima (small cordgrass) in created and natural marshes. Estuar. Coast. Shelf Sci. 2008, 78, 819–826. [Google Scholar] [CrossRef]
- Abdul-Aziz, O.I.; Ishtiaq, K.S.; Tang, J.; Moseman-Valtierra, S.; Kroeger, K.D.; Gonneea, M.E.; Mora, J.; Morkeski, K. Environmental controls, emergent scaling, and predictions of greenhouse gas (GHG) fluxes in coastal salt marshes. J. Geophys. Res. Biogeosci. 2018, 123, 2234–2256. [Google Scholar] [CrossRef]
- Da Cunha Lana, P.; Guiss, C.; Disarό, S.T. Seasonal variation of biomass and production dynamics for above- and belowground components of a Spartina alterniflora marsh in the euhaline sector of Paranaguá Bay (SE Brazil). Estuar. Coast. Shelf Sci. 1991, 32, 231–241. [Google Scholar] [CrossRef]
- Krauss, K.W.; Holm, G.O., Jr.; Perez, B.C.; McWhorter, D.E.; Cormier, N.; Moss, R.F.; Johnson, D.J.; Neubauer, S.C.; Raynie, R.C. Component greenhouse gas fluxes and radiative balance from two deltaic marshes in Louisiana: Pairing chamber techniques and eddy covariance. J. Geophys. Res. Biogeosci. 2016, 121, 1503–1521. [Google Scholar] [CrossRef]
- Scarton, F.; Day, J.W.; Rismondo, A. Primary production and decomposition of Sarcocornia fruticosa (L.) Scott and Phragmites australis Trin. Ex Steudel in the Po delta, Italy. Estuaries 2002, 25, 325–336. [Google Scholar] [CrossRef]
- Neves, J.P.; Ferreira, L.F.; Simões, M.P.; Gazarini, L.C. Primary production and nutrient content in two salt marsh species, Atriplex portulacoides L. and Limoniastrum monopetalum L., in southern Portugal. Estuaries Coasts 2007, 30, 459–468. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Liu, F.; Zhang, Y.; Liu, C.; Lin, G. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: A meta-analysis of eddy covariance data. Glob. Chang. Biol. 2017, 23, 1180–1198. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Long, S.P.; Mason, C.F. Net primary production, decomposition and export of Spartina anglica on a Suffolk salt marsh. J. Ecol. 1986, 74, 647–662. [Google Scholar] [CrossRef]
- Morris, J.T.; Jensen, A. The carbon balance of grazed and non-grazed Spartina anglica saltmarshes at Skallingen, Denmark. J. Ecol. 1998, 86, 229–242. [Google Scholar] [CrossRef]
- Drake, B.G.; Read, M. Carbon dioxide assimilation, photosynthetic efficiency, and respiration of a Chesapeake Bay salt marsh. J. Ecol. 1981, 69, 405–423. [Google Scholar] [CrossRef]
- Costa, C.S.B. Production ecology of Scirpus maritimus in southern Brazil. Cienc. Cult. 1998, 50, 273–280. [Google Scholar]
- Rivera-Monroy, V.H.; Elliton, C.; Narra, S.; Meselhe, E.; Zhao, X.; White, E.; Sasser, C.E.; Visser, J.M.; Meng, X.; Wang, H.; et al. Wetland biomass and productivity in coastal Louisiana: Base line data (1976-2015) and knowledge gaps for the development of spatially explicit models for ecosystem restoration and rehabilitation initiatives. Water 2019, 11, 2054. [Google Scholar] [CrossRef]
- Stagg, C.L.; Schoolmaster, D.R., Jr.; Piazza, S.C.; Snedden, G.; Steyer, G.D.; Fischenich, C.J.; McComas, R.W. A landscape-scale assessment of above-and belowground primary production in coastal wetlands: Implications for climate change-induced community shifts. Estuaries Coasts 2017, 40, 856–879. [Google Scholar] [CrossRef]
- Darby, F.A.; Turner, R.E. Below- and aboveground Spartina alterinflora production in a Louisiana salt marsh. Estuaries Coasts 2008, 31, 223–231. [Google Scholar] [CrossRef]
- Ge, Z.-M.; Guo, H.-Q.; Zhao, B.; Zhang, L.-Q. Plant invasion impacts on the gross and net primary production of the salt marsh on eastern coast of China: Insights from leaf to ecosystem. J. Geophys. Res. Biogeosci. 2015, 120, 169–186. [Google Scholar] [CrossRef]
- Curcό, A.; Ibàñez, C.; Day, J.W.; Prat, N. Net primary production and decomposition of salt marshes of the Ebre delta (Catalonia, Spain). Estuaries 2002, 25, 309–324. [Google Scholar] [CrossRef]
- Van de Broek, M.; Temmerman, S.; Merckx, R.; Govers, G. Controls on soil organic carbon stocks in tidal marshes long an estuarine salinity gradient. Biogeosciences 2016, 13, 6611–6624. [Google Scholar] [CrossRef]
- Gabriel, B.C.; de la Cruz, A.A. Species composition, standing stock, and net primary production of a salt marsh community in Mississippi. Chesap. Sci. 1974, 15, 72–77. [Google Scholar] [CrossRef]
- Tobias, C.; Neubauer, S.C. Salt marsh biogeochemistry —An overview. In Coastal Wetlands: An Integrated Ecosystem Approach; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Brinson, M.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 445–492. [Google Scholar]
- Selisker, D.M.; Gallagher, J.L.; Burdick, D.M.; Mutz, L.A. The regulation of ecosystem functions by ecotypic variation in the dominant plant: A Spartina alteriflora salt-marsh case study. J. Ecol. 2002, 90, 1–11. [Google Scholar] [CrossRef]
- Van Raalte, C.D.; Valiela, I.; Teal, J.M. Production of epibenthic salt marsh algae: Light and nutrient limitation. Limnol. Oceanogr. 1976, 21, 862–872. [Google Scholar]
- Baas, P.; Hester, M.W.; Joye, S.B. Benthic primary production and nitrogen cycling in Spartina alterniflora marshes: Effect of restoration after acute dieback. Biogeochemistry 2014, 117, 511–524. [Google Scholar] [CrossRef]
- Zedler, J.B. Algal mat productivity: Comparisons in a salt marsh. Estuaries 1980, 3, 122–131. [Google Scholar] [CrossRef]
- Chalmers, A.G.; Wiegert, R.G.; Wolf, P.L. Carbon balance in a salt marsh: Interactions of diffusive export, tidal deposition, and rainfall-caused erosion. Estuar. Coast. Shelf Sci. 1985, 21, 757–771. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Currin, C.A. Community structure and functional dynamics of benthic microalgae in salt marshes. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 81–106. [Google Scholar]
- Miller, W.D.; Neubauer, S.C.; Anderson, I.C. Effects of sea level induced disturbances on high salt marsh metabolism. Estuaries 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling in the world’s mangrove ecosystems revisited: Significance of non-steady state diagenesis and subsurface linkages between the forest floor and the coastal ocean. Forests 2020, 11, 977. [Google Scholar] [CrossRef]
- Burden, A.; Garbutt, R.A.; Evans, C.D.; Jones, D.L.; Cooper, D.M. Carbon sequestration and biogeochemical cycling in a saltmarsh subject to coastal managed realignment. Estuar. Coast. Shelf Sci. 2013, 120, 12–20. [Google Scholar] [CrossRef]
- Al-Haj, A.N.; Fulweiler, R.W. A synthesis of methane emissions from shallow vegetated coastal ecosystems. Glob. Chang. Biol. 2020, 26, 2988–3005. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhary, D.R.; Lee, J.; Byun, C.; Ding, W.; Kwon, B.-O.; Khim, J.S.; Kang, H. Microbial mechanism for enhanced methane emission in deep soil layer of Phragmites-introduced tidal marsh. Environ. Int. 2020, 134, 105251. [Google Scholar] [CrossRef]
- Olsson, L.; Ye, S.; Yu, X.; Wei, M.; Krauss, K.W.; Brix, H. Factors influencing CO2 and CH4 emissions from coastal wetlands in the Liaohe delta, northeast China. Biogeosci. Discuss. 2015, 12, 3469–3503. [Google Scholar] [CrossRef]
- Smith, C.J.; DeLaune, R.D.; Patrick, W.H., Jr. Carbon dioxide emission and carbon accumulation in coastal wetlands. Estuar. Coast. Shelf Sci. 1983, 17, 21–29. [Google Scholar] [CrossRef]
- Wigand, C.; Brennan, P.; Stolt, M.; Holt, M.; Ryba, S. Soil respiration rates in coastal marshes subject to increasing watershed nitrogen loads in southern New England, USA. Wetlands 2009, 29, 952–963. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, W.; Liu, D.; Kang, H.; Freeman, C.; Xiang, J.; Lin, Y. Exotic Spartina alterniflora invasion alters ecosystem-atmosphere exchange of CH4 and N2O and carbon sequestration in a coastal salt marsh in China. Glob. Chang. Biol. 2015, 21, 1567–1580. [Google Scholar] [CrossRef]
- Ye, S.; Krauss, K.W.; Brix, H.; Wei, M.; Olsson, L.; Yu, X.; Ma, X.; Wang, J.; Yuan, H.; Zhao, G.; et al. Inter-annual variability of area-scaled gaseous carbon emissions from wetland soils in the Liaohe delta, China. PLoS ONE 2016, 11, e0160612. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Yang, L.; Sun, Z. Emissions of biogenic sulfur gases (H2S, CO2) from Phragmites australis coastal marsh in the Yellow River estuary of China. Chin. Geogr. Sci. 2016, 26, 770–778. [Google Scholar] [CrossRef]
- Otani, S.; Endo, T. CO2 flux in tidal flats and salt marshes. In Blue Carbon in Shallow Coastal Ecosystems; Kuwae, T., Hori, M., Eds.; Springer: Singapore, 2019; pp. 223–250. [Google Scholar]
- Gribsholt, B.; Kristensen, E. Benthic metabolism and sulfur cycling along an inundation gradient in a tidal Spartina anglica salt marsh. Limnol. Oceangr. 2003, 48, 2151–2162. [Google Scholar] [CrossRef]
- Gribsholt, B.; Kostka, J.E.; Kristensen, E. Impact of fiddler crabs and plant roots on sediment biogeochemistry in a Georgia saltmarsh. Mar. Ecol. Prog. Ser. 2003, 259, 237–251. [Google Scholar] [CrossRef]
- Hamersley, M.R.; Howes, B.L. Contribution of denitrification to nitrogen, carbon, and oxygen cycling in tidal creek sediments of a New England salt marsh. Mar. Ecol. Prog. Ser. 2003, 262, 55–69. [Google Scholar] [CrossRef]
- Magenheimer, J.F.; Moore, T.R.; Chmura, G.L.; Daoust, R.J. Methane and carbon dioxide flux from a macrotidal salt marsh, Bay of Fundy, New Brunswick. Estuaries 1996, 19, 139–145. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Smith, A.C.; Kostka, J.E. Relative contributions of sulfate-and iron (III) reduction to organic matter mineralization and process controls in contrasting habitats of the Georgia saltmarsh. Appl. Geochem. 2007, 22, 2637–2651. [Google Scholar] [CrossRef]
- Yamochi, S.; Tanaka, T.; Otani, Y.; Endo, T. Effects of light, temperature and ground water level on the CO2 flux of the sediment in the high water temperate seasons at the artificial north salt marsh of Osaka Nanko bird sanctuary, Japan. Ecol. Eng. 2017, 98, 330–338. [Google Scholar] [CrossRef]
- Kostka, J.E.; Gribsholt, B.; Petrie, E.; Dalton, D.; Skelton, H.; Kristensen, K. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnol. Oceanogr. 2002, 47, 230–240. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Hollibaugh, J.T.; Bano, N.; Haskins, J. Effects of upwelling on short-term variability in microbial and biogeochemical processes in estuarine sediments from Elkhorn Slough, CA, USA. Aquat. Micro Ecol. 2010, 58, 261–271. [Google Scholar] [CrossRef]
- Wei, S.; Han, G.; Chu, X.; Song, W.; He, W.; Xia, J.; Wu, H. Effect of tidal flooding on ecosystem CO2 and CH4 fluxes in a salt marsh in the Yellow River delta. Estuar. Coast. Shelf Sci. 2020, 232, 106512. [Google Scholar] [CrossRef]
- Morris, J.T.; Whiting, G.J. Emission of gaseous carbon dioxide from salt-marsh sediments and its relation to other carbon losses. Estuaries 1986, 9, 9–19. [Google Scholar] [CrossRef]
- Lewis, D.B.; Brown, J.A.; Jimenez, K.L. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuar. Coast. Shelf Sci. 2014, 139, 11–19. [Google Scholar] [CrossRef]
- Tong, C.; Wang, W.-Q.; Zeng, C.-S.; Marrs, R. Methane (CH4) emission from a tidal marsh in the Min River estuary, southeast China. J. Environ. Sci. Health 2010, 45, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Huang, J.-F.; Zhu, W.-F.; Tong, C. Impacts of increasing salinity and inundation on rates and pathways of organic carbon mineralization in tidal wetlands: A review. Hydrobiologia 2019, 827, 31–49. [Google Scholar] [CrossRef]
- Kostka, J.E.; Roychoudhury, A.; Van Cappellen, P. Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochemistry 2002, 60, 49–76. [Google Scholar] [CrossRef]
- Mok, J.-S.; Cho, H.-Y.; Hyun, J.-H. Rates of anaerobic carbon mineralization and sulfate reduction in association with bioturbation in the intertidal mudflat of Ganghwa, Korea. Sea 2005, 10, 38–46. [Google Scholar]
- Smith, A.C. The Impacts of Macrobenthos on the Rates and Pathways of Organic Matter Mineralization in Two Coastal Marine Ecosystems of the Southeastern United States. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2004. [Google Scholar]
- van de Velde, S.J.; Hidalgo-Martinez, S.; Callebaut, I.; Antler, G.; Rebecca, K.; Leermakers, M.; Meysman, F.J.R. Burrowing fauna mediate alternative stable states in the redox cycling of salt marsh sediments. Geochim. Cosmochim. Acta 2020, 276, 31–49. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, D. Seasonal and spatial variability of sediment oxygen fluxes in the Beobsan intertidal flat of Taean Bay, mid-western Korean Peninsula. Geosci. J. 2007, 11, 323–329. [Google Scholar] [CrossRef]
- Howes, B.L.; Dacey, J.W.H.; King, G.M. Carbon flow through oxygen and sulfate reduction pathways in salt marsh sediments. Limnol. Oceangr. 1984, 29, 1037–1051. [Google Scholar] [CrossRef]
- Morris, J.T.; Bradley, P.M. Effects of nutrient loading on the carbon balance of coastal wetland sediments. Limnol. Oceanogr. 1999, 44, 699–702. [Google Scholar] [CrossRef]
- Zhong, Q.; Du, Q.; Gong, J.; Zhang, C.; Wang, K. Effects of in situ experimental air warming on the soil respiration in a coastal salt marsh reclaimed for agriculture. Plant Soil 2013, 371, 487–502. [Google Scholar] [CrossRef]
- Simpson, L.T.; Osbourne, T.Z.; Feller, I.C. Wetland soil CO2 efflux along a latitudinal gradient of spatial and temporal complexity. Estuaries Coasts 2019, 42, 45–54. [Google Scholar] [CrossRef]
- Wang, J. Carbon Dioxide and Methane Emissions from a California Salt Marsh. Master’s Thesis, University of California, Santa Barbara, CA, USA, 2018. [Google Scholar]
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity influence on methane emissions from tidal marshes. Wetlands 2011, 31, 831–842. [Google Scholar] [CrossRef]
- Moseman-Valtierra, S.; Abdul-Aziz, O.I.; Tang, J.; Ishtiaq, K.S.; Morkeski, K.; Mora, J.; Quinn, R.K.; Martin, G.M.; Egan, K.; Brannon, E.Q.; et al. Carbon dioxide fluxes reflect plant zonation and belowground biomass in a coastal marsh. Ecosphere 2016, 7, e01560. [Google Scholar] [CrossRef]
- Chmura, G.L.; Kellman, L.; van Ardenne, L.; Guntenspergen, G.R. Greenhouse gas fluxes from salt marshes exposed to chronic nutrient enrichment. PLoS ONE 2016, 11, e0149937. [Google Scholar] [CrossRef] [PubMed]
- Holm, G.O., Jr.; Perez, B.C.; McWhorter, D.E.; Krauss, K.W.; Johnson, D.J.; Raynie, R.C.; Killebrew, C.J. Ecosystem level methane fluxes from tidal freshwater and brackish marshes of the Mississippi River delta: Implications for coastal wetland carbon projects. Wetlands 2016, 36, 401–413. [Google Scholar] [CrossRef]
- Kelley, C.A.; Martens, C.S.; Chanton, J.P. Variations in sedimentary carbon remineralization rates in the White Oak River estuary, North Carolina. Limnol. Oceanogr. 1990, 35, 372–383. [Google Scholar] [CrossRef]
- Xu, X.; Fu, G.; Zou, X.; Ge, C.; Zhao, Z. Diurnal variations of carbon dioxide, methane, and nitrous oxide fluxes from invasive Spartina alterniflora dominated coastal wetland in northern Jiangsu Province. Acta Oceanol. Sin. 2017, 36, 105–113. [Google Scholar] [CrossRef]
- Hu, M.; Ren, H.; Ren, P.; Li, J.; Wilson, B.J.; Tong, C. Response of gaseous carbon emissions to low-level salinity increase in tidal marsh ecosystem of the Min River estuary, southeastern China. J. Environ. Sci. 2017, 52, 210–222. [Google Scholar]
- Hu, M.; Wilson, B.J.; Sun, Z.; Ren, P.; Tong, C. Effects of the addition of nitrogen and sulfate on CH4 and CO2 emissions, soil, and pore water chemistry in a high marsh of the Min River estuary in southeastern China. Sci. Total Environ. 2017, 579, 292–304. [Google Scholar] [CrossRef]
- Song, H.; Liu, X. Anthropogenic effects on fluxes of ecosystem respiration and methane in the Yellow River estuary, China. Wetlands 2016, 36, 113–123. [Google Scholar] [CrossRef]
- Zhang, L.H.; Song, L.P.; Zhang, L.W.; Shao, H.B. Diurnal dynamics of CH4, CO2 and N2O fluxes in the saline-alkaline soils of the Yellow River delta, China. Plant Biosyst. 2015, 149, 797–805. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Mok, J.-S.; Cho, H.-Y.; Kim, S.-H.; Lee, K.S.; Kostka, J.E. Rapid organic matter mineralization coupled to iron cycling in intertidal mud flats of the Han River estuary, Yellow Sea. Biogeochemistry 2009, 92, 231–245. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.H.; Eyre, B.D. Methane emissions partially offset “blue carbon” burial in mangroves. Sci. Adv. 2018, 4, eaao4985. [Google Scholar] [CrossRef]
- Gazeau, F.; Gattuso, J.-P.; Middelburg, J.J.; Brion, N.; Schiettecatte, L.-S.; Frankignoulle, M.; Borges, A.V. Planktonic and whole system metabolism in a nutrient-rich estuary (the Scheldt estuary). Estuaries 2005, 28, 868–883. [Google Scholar] [CrossRef]
- Cai, W.-J.; Pomeroy, L.R.; Moran, M.A.; Wang, Y. Oxygen and carbon dioxide mass balance for the estuarine-intertidal marsh complex of five rivers in the southeastern U.S. Limnol. Oceanogr. 1999, 44, 639–649. [Google Scholar] [CrossRef]
- Geoghegan, E.K.; Caplan, J.S.; Leech, F.N.; Weber, P.E.; Bauer, C.E.; Mozdzer, T.J. Nitrogen enrichment alters carbon fluxes in a New England salt marsh. Ecosyst. Health Sustain. 2018, 4, 277–287. [Google Scholar] [CrossRef]
- Hopkinson, C.S., Jr. Patterns of organic carbon exchange between coastal ecosystems: The mass balance approach in salt marsh ecosystems. In Coastal-Offshore Ecosystem Interactions; Jansson, B.-O., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 122–154. [Google Scholar]
- Yang, W.-B.; Yuan, C.S.; Tong, C.; Yang, P.; Yang, L.; Huang, B.-Q. Diurnal variation of CO2, CH4, and N2O emission fluxes continuously monitored in-situ in three environmental habitats in a subtropical estuarine wetland. Mar. Pollut. Bull. 2017, 119, 289–298. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Harriss, R.C.; Sebacher, D.I. Methane flux from coastal salt marshes. J. Geophys. Res. 1985, 90, 5710–5720. [Google Scholar] [CrossRef]
- Trifunovic, B.; Vázquez-Lule, A.; Capooci, M.; Seyfferth, A.L.; Moffat, C.; Vargas, R. Carbon dioxide and methane emissions from a temperate salt marsh tidal creek. J. Geophys. Res. Biogeosci. 2020, 125. [Google Scholar] [CrossRef]
- Mayen, J. Spatial and Temporal Variations in pCO2 and Atmospheric CO2 exchanges in a Temperate Salt Marsh System. Master’s Thesis, Université de Pau et des Pays de L’Adour, Pau, France, 2020. [Google Scholar]
- Huertas, I.E.; Flecha, S.; Perez, F.F.; de la Paz, M. Spatio-temporal variability and controls on methane and nitrous oxide in the Guadalquivir estuary, southwestern Europe. Aquat. Sci. 2018, 80, 29. [Google Scholar] [CrossRef]
- Matoušů, A.; Osudar, R.; Šimek, K.; Bussmann, I. Methane distribution and methane oxidation in the water column of the Elbe estuary, Germany. Aquat. Sci. 2017, 79, 443–458. [Google Scholar] [CrossRef]
- Daniel, I.; DeGrandpre, M.; Farías, L. Greenhouse gas emissions from the Tubul-Raqui estuary (central Chile 36°S). Estuar. Coast. Shelf Sci. 2013, 134, 31–44. [Google Scholar] [CrossRef]
- Huertas, I.E.; de la Paz, F.F.; Navarro, G.; Flecha, S. Methane emissions from the salt marshes of Doñana wetlands: Spatio-temporal variability and controlling factors. Front. Ecol. Evol. 2019, 7, 32. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Cabeçadas, G.; Mateus, M.D. Inorganic carbon distribution and CO2 fluxes in a large European estuary (Tagas, Portugal). Sci. Rep. 2017, 7, 7376. [Google Scholar] [CrossRef]
- Maher, D.T.; Call, M.; Santos, I.R.; Sanders, C.J. Beyond burial: Lateral exchange is a significant atmospheric carbon sink in mangrove forests. Biol. Lett. 2018, 14, 20180200. [Google Scholar] [CrossRef]
- Ray, R.; Baum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef]
- Call, M.; Sanders, C.J.; Macklin, P.A.; Santos, I.R.; Maher, D.T. Carbon outwelling and emissions from two contrasting mangrove creeks during the monsoon storm season in Palau, Micronesia. Estuar. Coast. Shelf Sci. 2019, 218, 340–348. [Google Scholar] [CrossRef]
- Jennerjahn, T.C.; Ittekkot, V. Relevance of mangroves from the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 2002, 89, 23–30. [Google Scholar] [CrossRef]
- Ray, R.; Thouzeau, G.; Walcker, R.; Vantrepotte, V.; Gleixner, G.; Morvan, S.; Devesa, J.; Michaud, E. Mangrove-derived organic and inorganic carbon exchanges between the Sinnamary estuarine system (French Guiana, South America) and Atlantic Ocean. J. Geophys. Res. Biogeosci. 2020, 125. [Google Scholar] [CrossRef]
- Adame, M.F.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Schielbel, H.N.; Gardner, G.B.; Wang, X.; Peri, F.; Chen, R.F. Seasonal export of dissolved organic matter from a New England salt marsh. J. Coast. Res. 2017, 34, 939–954. [Google Scholar]
- Forja, J.M.; Ortega, T.; Ponce, R.; de la Paz, M.; Rubio, J.A.; Gόmez-Parra, A. Tidal transport of inorganic carbon and nutrients in a coastal salt marsh (Bay of Cádiz, SW Spain). Cienc. Mar. 2003, 29, 469–481. [Google Scholar] [CrossRef]
- Laffaille, P.; Brosse, S.; Feuteun, E.; Baisez, A.; Leeefeuvre, J.-C. Role of fish communities in particulate organic matter fluxes between salt marshes and coastal marine waters in the Mont Saint-Michel bay. Hydrobiologia 1998, 373–374, 121–133. [Google Scholar] [CrossRef]
- Das, A.; Justic, D.; Swenson, E.; Turner, R.E.; Inoue, M.; Park, D. Coastal land loss and hypoxia: The ‘outwelling’ hypothesis revisited. Environ. Res. Lett. 2011, 6, 025001. [Google Scholar] [CrossRef]
- Duarte, B.; Valentin, J.M.; Dias, J.M.; Silva, H.; Marques, J.C.; Caçador, I. Modelling sea level rise (SLR) impacts on salt marsh detrital outwelling C and N exports from an estuarine coastal lagoon to the ocean (Ria de Aveiro, Portugal). Ecol. Modell. 2014, 289, 36–44. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, K.E.; Cole, J.J. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnol. Oceanogr. 2000, 45, 1707–1717. [Google Scholar] [CrossRef]
- Cai, W.-J.; Wiebe, W.J.; Wang, Y.; Sheldon, J.E. Intertidal marsh as a source of dissolved inorganic carbon and a sink of nitrate in the Satilla River-estuarine complex in the southeastern U.S. Limnol. Oceanogr. 2000, 45, 1743–1752. [Google Scholar] [CrossRef]
- Cai, W.-J.; Wang, Y. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnol. Oceanogr. 1998, 43, 657–668. [Google Scholar] [CrossRef]
- Joesoef, A.; Kirchman, D.L.; Sommerfield, C.K.; Cai, W.-J. Seasonal variability of the inorganic carbon system in a large coastal plain estuary. Biogeosciences 2017, 14, 4949–4963. [Google Scholar] [CrossRef]
- Taylor, D.I.; Allanson, B.R. Organic carbon fluxes between a high marsh and estuary, and the inapplicability of the outwelling hypothesis. Mar. Ecol. Prog. Ser. 1995, 120, 263–270. [Google Scholar] [CrossRef]
- Wang, Z.A.; Cai, W.-J. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): A marsh CO2 pump. Limnol. Oceanogr. 2004, 49, 341–354. [Google Scholar] [CrossRef]
- Cai, W.-J.; Wang, Z.A.; Wang, Y. The role of marsh-dominated heterotrophic continental margins in transport of CO2 between the atmosphere, the land-sea interface, and the ocean. Geophys. Res. Lett. 2003, 30, 1849. [Google Scholar] [CrossRef]
- Winter, P.E.D.; Schlacher, T.A.; Baird, D. Carbon flux between an estuary and the ocean: A case for outwelling. Hydrobiologia 1996, 337, 123–132. [Google Scholar] [CrossRef]
- Wang, Z.A.; Kroeger, K.D.; Ganju, N.K.; Gonneea, M.E.; Chu, S.N. Intertidal salt marshes as an important source of inorganic carbon to the coastal ocean. Limnol. Oceanogr. 2016, 61, 1916–1931. [Google Scholar] [CrossRef]
- Cai, W.J. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu. Rev. Mar. Sci. 2011, 3, 123–145. [Google Scholar] [CrossRef]
- Wang, S.R.; Di Iorio, D.; Cai, W.-J.; Hopkinson, C.S. Inorganic carbon and oxygen dynamics in a marsh-dominated estuary. Limnol. Oceangr. 2017, 63, 47–71. [Google Scholar] [CrossRef]
- Childers, D.L.; Day, J.W., Jr.; McKellar, H.N., Jr. Twenty more years of marsh and estuarine flux studies: Revisiting Nixon (1980). In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 391–423. [Google Scholar]
- Gardner, L.R.; Kjerfve, B. Tidal fluxes of nutrients and suspended sediments at the North Inlet-Winyah Bay National Estuarine Research Reserve. Estuar. Coast. Shelf Sci. 2006, 70, 682–692. [Google Scholar] [CrossRef]
- Osburn, C.L.; Mikan, M.P.; Etheridge, J.R.; Burchell, M.R.; Birgand, F. Seasonal variation in the quality of dissolved and particulate organic matter exchanged between a salt marsh and its adjacent estuary. J. Geophys. Res. Biogeosci. 2015, 120, 1430–1449. [Google Scholar] [CrossRef]
- Childers, D.L.; McKellar, H.N.; Dame, R.F.; Sklar, F.H.; Blood, E.R. A dynamics nutrient budget of subsystem interactions in a salt marsh estuary. Estuar. Coast. Shelf Sci. 1993, 36, 105–131. [Google Scholar] [CrossRef]
- Roman, C.T.; Daiber, F.C. Organic carbon flux through a Delaware Bay salt marsh: Tidal exchange, particle size distribution, and storms. Mar. Ecol. Prog. Ser. 1989, 54, 149–156. [Google Scholar] [CrossRef]
- Wolaver, T.G.; Spurrier, J.D. Carbon transport between a euhaline vegetated marsh in South Carolina and the adjacent tidal creek: Contributions via tidal inundation, runoff and seepage. Mar. Ecol. Prog. Ser. 1988, 42, 53–62. [Google Scholar] [CrossRef]
- Dankers, N.; Binsbergen, M.; Zegers, K.; Laane, R.; van der Loeff, M.R. Transportation of water, particulate and dissolved organic and inorganic matter between a salt marsh and the Ems-Dollard estuary, The Netherlands. Estuar. Coast. Shelf Sci. 1984, 19, 143–165. [Google Scholar] [CrossRef]
- Santos, I.R.; Maher, D.T.; Larkin, R.; Webb, J.R.; Sanders, C.J. Carbon outwelling and outgassing vs. burial in an estuarine tidal creek surrounded by mangrove and saltmarsh wetlands. Limnol. Oceanogr. 2019, 64, 996–1013. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Ouyang, Z.; Xie, X.; Guo, H.; Gu, C.; Xiao, X.; Ge, Z.; Peng, C.; Zhao, B. Multi-scale temporal variation of methane flux and its controls in a subtropical tidal salt marsh in eastern China. Biogeochemistry 2018, 137, 163–179. [Google Scholar] [CrossRef]
- Sadat-Noori, M.; Maher, D.T.; Santos, I.R. Groundwater discharge as a source of dissolved carbon and greenhouse gases in a subtropical estuary. Estuaries Coasts 2016, 39, 639–656. [Google Scholar] [CrossRef]
- Deborde, J.; Anschutz, P.; Guérin, F.; Poirier, D.; Marty, D.; Boucher, G.; Thouzeau, G.; Canton, M.; Abril, G. Methane sources, sinks and fluxes in a temperate tidal lagoon: The Arcachon lagoon (SW France). Estuar. Coast. Shelf Sci. 2010, 89, 256–266. [Google Scholar] [CrossRef]
- Upstill-Goddard, R.C.; Barnes, J.; Frost, T.; Punshon, S.; Owens, N.J.P. Methane in the southern North Sea: Low-salinity inputs, estuarine removal, and atmospheric flux. Glob. Biogeochem. Cycle 2000, 14, 1205–1217. [Google Scholar] [CrossRef]
- Weston, N.B.; Neubauer, S.C.; Velinsky, D.J.; Vile, M.A. Net ecosystem carbon exchange and the greenhouse gas balance of tidal marshes along an estuarine salinity gradient. Biogeochemistry 2014, 120, 163–189. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Ouyang, X.; Lee, S.Y.; Connolly, R.M. The role of root decomposition in global mangrove and saltmarsh carbon budgets. Earth Sci. Rev. 2017, 166, 53–63. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Schulz, K.G.; Call, M.; Jacobsen, G.E.; Sanders, C.J. Blue carbon oxidation revealed by radiogenic and stable isotopes in a mangrove system. Geophys. Res. 2017, 44, 4889–4896. [Google Scholar] [CrossRef]
- Ward, N.D.; Morrison, E.S.; Liu, Y.; Rivas-Ubach, A.; Osborne, T.Z.; Ogram, A.V.; Bianchi, T.S. Marine microbial community responses related to wetland carbon mobilization in the coastal zone. Limnol. Oceangr. Lett. 2019, 4, 25–33. [Google Scholar] [CrossRef]
- Huang, T.-H.; Fu, Y.-H.; Pan, P.Y.; Chen, C.-T.A. Fluvial carbon fluxes in tropical rivers. Curr. Opin. Environ. Sustain. 2012, 4, 162–169. [Google Scholar] [CrossRef]
- Chen, C.-T.-A.; Huang, T.-H.; Chen, Y.-C.; Bai, Y.; He, X.; Kang, Y. Air-sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 2013, 10, 6509–6544. [Google Scholar] [CrossRef]
- Bauer, J.E.; Cai, W.-J.; Raymond, P.A.; Bianchi, T.S.; Hopkinson, C.S.; Regnier, P.A.G. The changing carbon cycle of the coastal ocean. Nature 2013, 504, 61–70. [Google Scholar] [CrossRef]
- McKenzie, L.J.; Nordlund, L.M.; Jones, B.L.; Cullen- Unsworth, L.C.; Roelfsema, C.; Unsworth, R.K.F. The global distribution of seagrass meadows. Environ. Res. Lett. 2020, 15, 074041. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nature Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Murrell, M.C.; Caffrey, J.M.; Marcovich, D.T.; Beck, M.W.; Jarvis, B.M.; Hagy, J.D., III. Seasonal oxygen dynamics in a warm temperate estuary: Effects of hydrologic variability on measurements of primary production, respiration, and net metabolism. Estuaries Coasts 2018, 41, 690–707. [Google Scholar] [CrossRef]
- Egea, L.G.; Jiménez-Ramos, R.; Hernández, I.; Brun, F.G. Effect of in situ short-term temperature increase on carbon metabolism and dissolved organic carbon (DOC) fluxes in a community dominated by the seagrass Cymodocea nodosa. PLoS ONE 2019, 14, e0210386. [Google Scholar] [CrossRef]
- Koopmans, D.; Holtappels, M.; Chennu, A.; Weber, M.; de Beer, D. The response of seagrass (Posidonia oceanica) meadow metabolism to CO2 levels and hydrodynamic exchange determined with aquatic eddy covariance. Biogeosci. Discuss. 2018. [Google Scholar] [CrossRef]
- Ganguly, D.; Singh, G.; Ramachandran, P.; Selvam, A.P.; Banderjee, K.; Ramachandran, R. Seagrass metabolism and carbon dynamics in a tropical coastal embayment. Ambio 2017, 46, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Cardini, U.; van Hoytema, N.; Bednarz, V.N.; Al-Rshaidat, M.M.D.; Wild, C. N2 fixation and primary productivity in a Red Sea Halophila stipulacea meadow exposed to seasonality. Limnol. Oceanogr. 2017, 63, 786–798. [Google Scholar] [CrossRef]
- Olivé, I.; Silva, J.; Costa, M.M.; Santos, R. Estimating seagrass community metabolism using benthic chambers: The effect of incubation time. Estuaries Coasts 2016, 39, 138–144. [Google Scholar] [CrossRef]
- Long, M.H.; Berg, P.; McGlathery, K.J.; Zieman, J.C. Sub-tropical seagrass ecosystem metabolism measured by eddy covariance. Mar. Ecol. Prog. Ser. 2015, 529, 75–90. [Google Scholar] [CrossRef]
- Berg, P.; Delgard, M.L.; Polsenaere, P.; McGlathery, K.J.; Doney, S.C.; Berger, A.C. Dynamics of benthic metabolism, O2, and pCO2 in a temperate seagrass meadow. Limnol. Oceanogr. 2019, 64, 2586–2604. [Google Scholar] [CrossRef]
- Koopmans, D.; Holtappels, M.; Chennu, A.; Weber, M.; de Beer, D. High net primary production of Mediterranean seagrass (Posidonia oceanica) meadows determined with aquatic eddy covariance. Front. Mar. Sci. 2020, 7, 118. [Google Scholar] [CrossRef]
- Anton, A.; Baldry, K.; Coker, D.J.; Duarte, C.M. Drivers of the low metabolic rates of seagrass meadows in the Red Sea. Front. Mar. Sci. 2020, 7, 69. [Google Scholar] [CrossRef]
- Burkholz, C.; Duarte, C.M.; Garcias-Bonet, N. Thermal dependence of seagrass ecosystem metabolism in the Red Sea. Mar. Ecol. Prog. Ser. 2019, 614, 79–90. [Google Scholar] [CrossRef]
- Champenois, W.; Borges, A.V. Inter-annual variations over a decade of primary production of the seagrass Posidonia oceanica. Limnol. Oceanogr. 2018, 64, 32–45. [Google Scholar] [CrossRef]
- Long, M.H.; Berg, P.; Falter, J.L. Seagrass metabolism across a productivity gradient using the eddy covariance, Eulerian control volume, and biomass addition techniques. J. Geophys. Res. Oceans 2015, 120, 3624–3639. [Google Scholar] [CrossRef]
- Berger, A.C.; Berg, P.; McGlathery, K.J.; Delgard, M.L. Long-term trends and resilience of seagrass metabolism: A decadal aquatic eddy covariance study. Limnol. Oceanogr. 2020, 65, 1423–1438. [Google Scholar] [CrossRef]
- Ikawa, H.; Oechel, W.C. Temporal variations in air-sea CO2 exchange near large kelp beds near San Diego, California. J. Geophys Res. Oceans 2015, 120, 50–63. [Google Scholar] [CrossRef]
- Towle, D.W.; Pearse, J.S. Production of the giant kelp, Macrocystis, estimated by in situ incorporation of 14C in polyethylene bags. Limnol. Oceanogr. 1973, 18, 155–159. [Google Scholar] [CrossRef]
- Bensoussan, N.; Gattuso, J.-P. Community primary production and calcification in a NW Mediterranean ecosystem dominated by calcareous macroalgae. Mar. Ecol. Prog. Ser. 2007, 334, 37–45. [Google Scholar] [CrossRef][Green Version]
- Migné, A.; Ouisse, V.; Hubas, C.; Davoult, D. Freshwater seepages and ephemeral macroalgae proliferation in an intertidal bay: II. Effect on benthic biomass and metabolism. Estuar. Coast. Shelf Sci. 2011, 92, 161–168. [Google Scholar] [CrossRef]
- Golléty, C.; Migné, A.; Davoult, D. Benthic metabolism on a sheltered rocky shore: Role of the canopy in the carbon budget. J. Phycol. 2008, 44, 1146–1153. [Google Scholar] [CrossRef]
- Rovelli, L.; Attard, K.M.; Cárdenas, C.A.; Glud, R.N. Benthic primary production and respiration of shallow rocky habitats: A case study from South Bay (Doumer Island, Western Antarctic Peninsula). Polar Biol. 2019, 42, 1459–1474. [Google Scholar] [CrossRef]
- Hubas, C.; Davoult, D. Does seasonal proliferation of Enteromorpha sp. Affect the annual benthic metabolism of a small macrotidal estuary? (Roscoff Aber Bay, France). Estuar. Coast. Shelf Sci. 2006, 70, 287–296. [Google Scholar] [CrossRef]
- Naumann, M.S.; Jantzen, C.; Haas, A.F.; Iglesias-Prieto, R.; Wild, C. Benthic primary production budget of a Caribbean reef lagoon (Puerto Morelos, Mexico). PLoS ONE 2013, 8, e82923. [Google Scholar] [CrossRef]
- Miller, R.J.; Reed, D.C.; Brzezinski, M.A. Partitioning of primary production among giant kelp (Macrocystis pyrifera), understory macroalgae, and phytoplankton on a temperate reef. Limnol. Oceangr. 2011, 56, 119–132. [Google Scholar] [CrossRef]
- Stuhldreier, I.; Sánchez-Noguera, C.; Roth, F.; Cortés, J.; Rixen, T.; Wild, C. Upwelling increases net primary production of corals and reef-wide gross primary production along the Pacific coast of Costa Rica. Front. Mar. Sci. 2015, 2, 113. [Google Scholar] [CrossRef]
- Tait, L.W.; Schiel, D.R. Primary productivity of intertidal macroalgal assemblages: Comparison of laboratory and in situ photorespirometry. Mar. Ecol. Prog. Ser. 2010, 416, 115–125. [Google Scholar] [CrossRef]
- Giordano, J.C.P.; Brush, M.J.; Anderson, I.C. Ecosystem metabolism in shallow coastal lagoons: Patterns and partitioning of planktonic, benthic, and integrated community rates. Mar. Ecol. Prog. Ser. 2012, 458, 21–38. [Google Scholar] [CrossRef]
- Attard, K.M.; Glud, R.N.; McGinnis, D.F.; Rysgaard, S. Seasonal rates of benthic primary production in a Greenland fjord measured by aquatic eddy correlation. Limnol. Oceanogr. 2014, 59, 1555–1569. [Google Scholar] [CrossRef]
- Ruiz-Halpern, S.; Vaquer-Sunyer, R.; Duarte, C.M. Annual benthic metabolism and organic carbon fluxes in a semi-enclosed Mediterranean bay dominated by the macroalgae Caulerpa prolifera. Front. Mar. Sci. 2014, 1, 67. [Google Scholar] [CrossRef]
- Attard, K.M.; Rodil, I.F.; Berg, P.; Norkko, J.; Norkko, A.; Glud, R.N. Seasonal metabolism and carbon export of a key coastal habitat: The perennial canopy-forming macroalga Fucus vesiculosus. Limnol. Oceangr. 2018, 64, 149–164. [Google Scholar] [CrossRef]
- Apostolaki, E.T.; Holmer, M.; Marbà, N.; Karakassis, I. Metabolic imbalance in coastal vegetated (Posidonia oceanica) and unvegetated benthic ecosystems. Ecosystems 2010, 13, 459–471. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Duarte, C.M.; Gattuso, J.-P. Respiration in coastal benthic communities. In Respiration in Aquatic Ecosystems; del Giorgio, P.A., le Williams, P.J., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 202–224. [Google Scholar]
- Jokiel, P.L.; Morrissey, J.I. Influence of size on primary production in the reef coral Pocillopora damicornis and the macroalga Acanthophora spicifera. Mar. Biol. 1986, 91, 15–26. [Google Scholar] [CrossRef]
- Dalsgaard, T. Benthic primary production and nutrient cycling in sediments with benthic microalgae and transient accumulation of macroalgae. Limnol. Oceangr. 2003, 48, 2138–2150. [Google Scholar] [CrossRef]
- Sundbäck, K.; Miles, A.; Hulth, S.; Pihl, L.; Engström, P.; Selander, E.; Svenson, A. Importance of benthic nutrient regeneration during initiation of macroalgal blooms in shallow bays. Mar. Ecol. Prog. Ser. 2003, 246, 115–126. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; McGlathery, K.; Rysgaard, S.; Christensen, P.B. Production within dense mats of the filamentous macroalga Chaetomorpha linum in relation to light and nutrient availability. Mar. Ecol. Prog. Ser. 1996, 134, 207–216. [Google Scholar] [CrossRef]
- Littler, M.M.; Taylor, P.R.; Littler, D.S.; Sims, R.H.; Norris, J.N. The Distribution, Abundance and Primary Productivity of Submerged Macrophytes in a Belize Barrier Reef Mangrove System. Atoll Res. Bull. 1985, 289, 1–22. [Google Scholar] [CrossRef]
- Jackson, G.A. Nutrients and production of giant kelp, Macrocystis pyrifera, off southern California. Limnol. Oceangr. 1977, 22, 979–995. [Google Scholar] [CrossRef]
- Jackson, G.A. Modelling the growth and harvest yield of the giant kelp Macrocystis pyrifera. Mar. Biol. 1987, 95, 611–624. [Google Scholar] [CrossRef]
- Mann, K.H. Ecological energetics of the sea-weed zone in a marine bay in the Atlantic coast of Canada. II. Productivity of the seaweeds. Mar. Biol. 1972, 14, 199–209. [Google Scholar]
- Pedersen, M.F.; Nejrup, L.B.; Fredriksen, S.; Christie, H.; Norderhaug, K.M. Effects of wave exposure on population structure, demography, biomass and productivity of the kelp Laminaria hyperborea. Mar. Ecol. Prog. Ser. 2012, 451, 45–60. [Google Scholar] [CrossRef]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Doughty, C.L.; Langley, J.A.; Walker, W.S.; Feller, I.C.; Schaub, R.; Chapman, S.K. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuaries Coasts 2016, 39, 385–396. [Google Scholar] [CrossRef]
- Vaughn, D.R.; Bianchi, T.S.; Shields, M.R.; Kenney, W.F.; Osborne, T.Z. Increased Organic Carbon Burial in Northern Florida Mangrove-Salt Marsh Transition Zones. Glob. Biogeochem. Cycles 2020, 34, e2019GB006334. [Google Scholar] [CrossRef]
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- FitzGerald, D.M.; Hughes, Z. Marsh processes and their response to climate change and sea-level rise. Annu. Rev. Earth Planet. Sci. 2019, 47, 481–517. [Google Scholar] [CrossRef]
- Alongi, D.M. The impact of climate change on mangrove forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]



| Component | Mangrove Forests | Salt Marshes |
|---|---|---|
| Aboveground biomass (AGB) | 109.3 ± 5.0 (94.1) | 4.3 ± 0.10 (2.4) |
| Belowground biomass (BGB) | 80.9 ± 9.5 (34.2) | 12.9 ± 1.2 (9.6) |
| Soil (0–1 m depth) | 565.4 ± 25.7 (500.5) | 317.2 ± 19.1 (282.2) |
| Total C stock (Mg Corg ha−1) | 738.9 ± 27.9 (702.5) | 334.4 ± 3.5 (294.2) |
| Global area (km2) | 86,495 | 54,951 |
| Global C stock (Pg Corg) | 6.17 | 1.84 |
| Component | Mangrove Forests | Salt Marshes |
|---|---|---|
| GPP | 35.3 ± 6.0 (26.6) | 17.7 ± 1.9 (16.6) |
| Aboveground NPP | 13.2 ± 1.7 (9.0) | 5.0 ± 0.3 (3.7) |
| Belowground NPP | 5.2 ± 4.4 (5.1) | 12.6 ± 1.1 (9.4) |
| RC | 22.3 ± 4.9 (15.7) | 8.4 ± 1.2 (6.4) |
| Microalgal GPP | 4.4 ± 2.2 (3.3) | 1.8 ± 0.8 (1.3) |
| Microalgal NPP | 2.1 ± 0.7 (1.8) | 1.5 ± 0.2 (1.1) |
| PGPP/RC | 1.6 ± 0.1 (1.5) | 1.0 ± 1.3 (1.0) |
| Component | Mangrove Forests | Salt Marshes |
|---|---|---|
| Soil respiration | 6.13 ± 0.62 (DIC, CO2); 3.88 ± 0.29 (DO, O2) | 5.64 ± 0.63 (3.66) |
| Soil DIC production | 18.27 ± 2.30 (15.5) | 6.92 ± 1.61 (3.8) |
| Soil CH4 release | 0.015 ± 0.006 (0.004) | 0.142 ± 0.02 (0.07) |
| Corg burial | 1.62 ± 0.67 (1.33) | 3.82 ± 0.58 (1.84) |
| Component | Mangrove Forests | Salt Marshes |
|---|---|---|
| Water-air CO2 release | 3.35 ± 0.35 (2.19) | 2.981 ± 0.33 (1.55) |
| Water-air CH4 release | 0.0116 ± 0.003 (0.01) | 0.104 ± 0.047 (0.006) |
| Export Component | Mangrove Forests | Salt Marshes |
|---|---|---|
| POC | 1.73 ± 0.23 (1.76) | 0.60 ± 0.11 (0.31) |
| DOC | 5.90 ± 1.95 (1.43) | 2.55 ± 0.55 (1.33) |
| DIC | 14.34 ± 2.26 (10.82) | 5.28 ± 1.212 (3.995) |
| CH4 | 0.0277 ± 0.0135 (0.026) | 0.109 ± 0.0786 (0.0081) |
| Ecosystem | Area | RE | Global RE | GPP | Global GPP | Mean PGPP/RE | NEP | Global NEP |
|---|---|---|---|---|---|---|---|---|
| Salt marsh | 5.5 | 1727 | 95 | 2109 | 116 | 1.22 | 382 | 21 |
| Mangrove | 8.6 | 3558 | 306 | 4186 | 360 | 1.18 | 628 | 54 |
| Seagrass | 16.0 | 2133 | 342 | 2752 | 441 | 1.29 | 619 | 99 |
| Macroalgae | 354.0 | 1572 | 5565 | 2159 | 7643 | 1.37 | 587 | 2078 |
| Coral reef | 60.0 | 1572 | 943 | 1720 | 1032 | 1.09 | 148 | 84 |
| Global coastal ocean | 2750 | 1034 | 28,435 | 1028 | 28,270 | 0.98 | −6 | −165 |
| Salt marsh contribution | 0.20% | - | 0.33% | - | 0.47% | - | - | 1.0% |
| Mangrove contribution | 0.31% | - | 1.08% | - | 1.27% | - | - | 2.4% |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, D.M. Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. J. Mar. Sci. Eng. 2020, 8, 767. https://doi.org/10.3390/jmse8100767
Alongi DM. Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. Journal of Marine Science and Engineering. 2020; 8(10):767. https://doi.org/10.3390/jmse8100767
Chicago/Turabian StyleAlongi, Daniel M. 2020. "Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis" Journal of Marine Science and Engineering 8, no. 10: 767. https://doi.org/10.3390/jmse8100767
APA StyleAlongi, D. M. (2020). Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. Journal of Marine Science and Engineering, 8(10), 767. https://doi.org/10.3390/jmse8100767





