The Contribution of Small Phytoplankton Communities to the Total Dissolved Inorganic Nitrogen Assimilation Rates in the East/Japan Sea: An Experimental Evaluation
Abstract
:1. Introduction
2. Materials and Methods
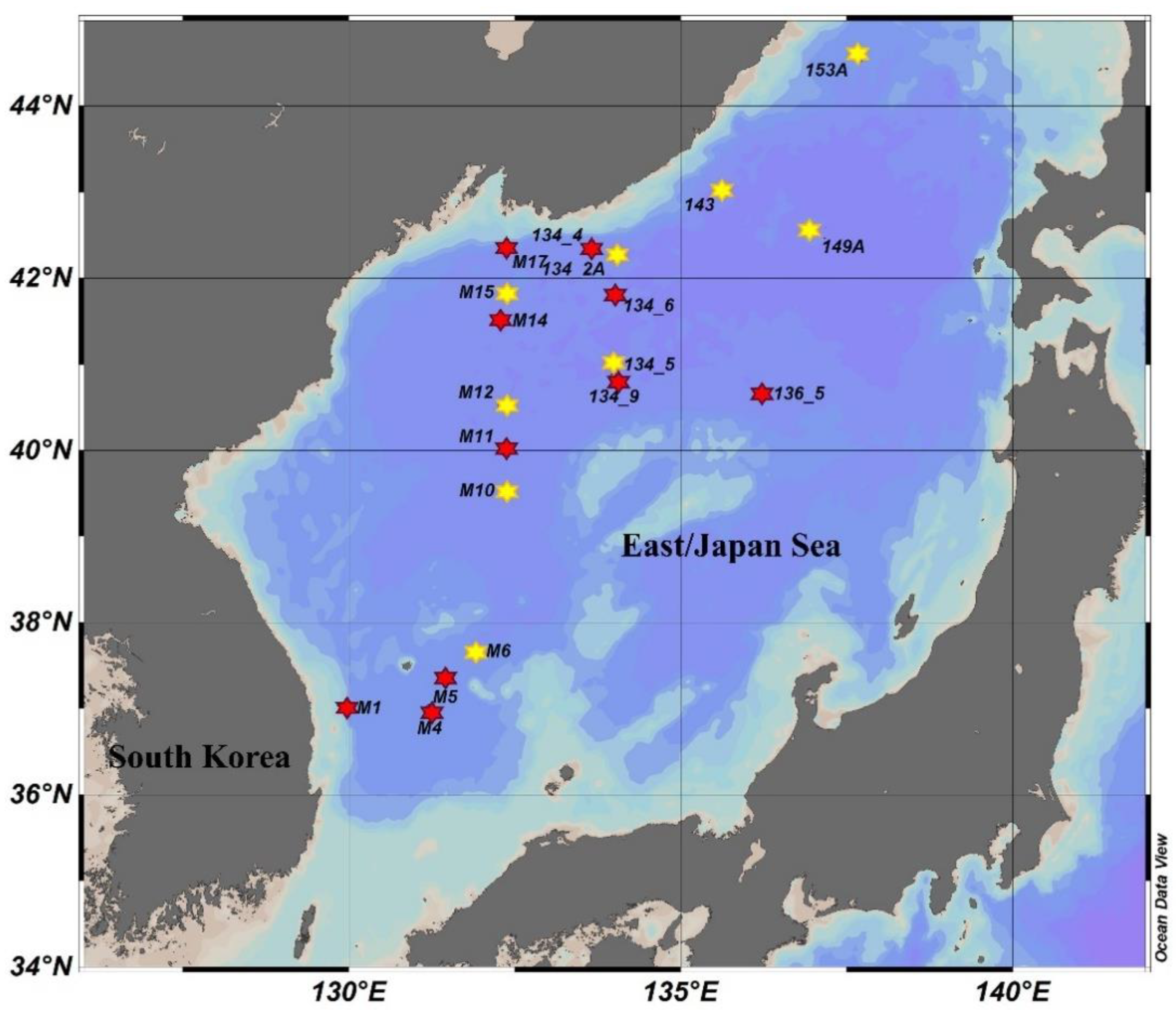
2.1. Study Area and Sampling
2.2. Nutrient Measurements
2.3. DIN Uptake Rate Measurements
3. Results and Discussion
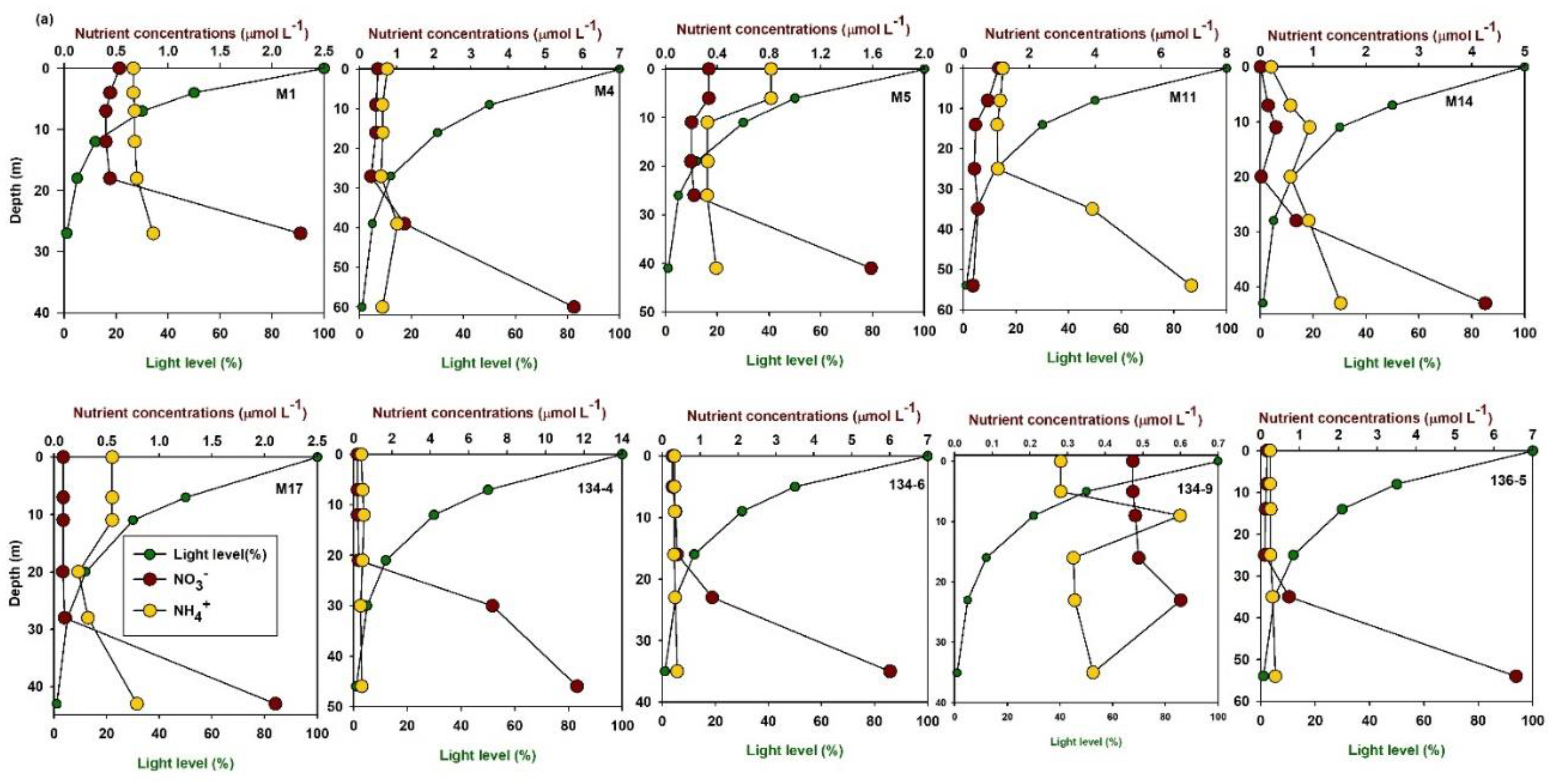
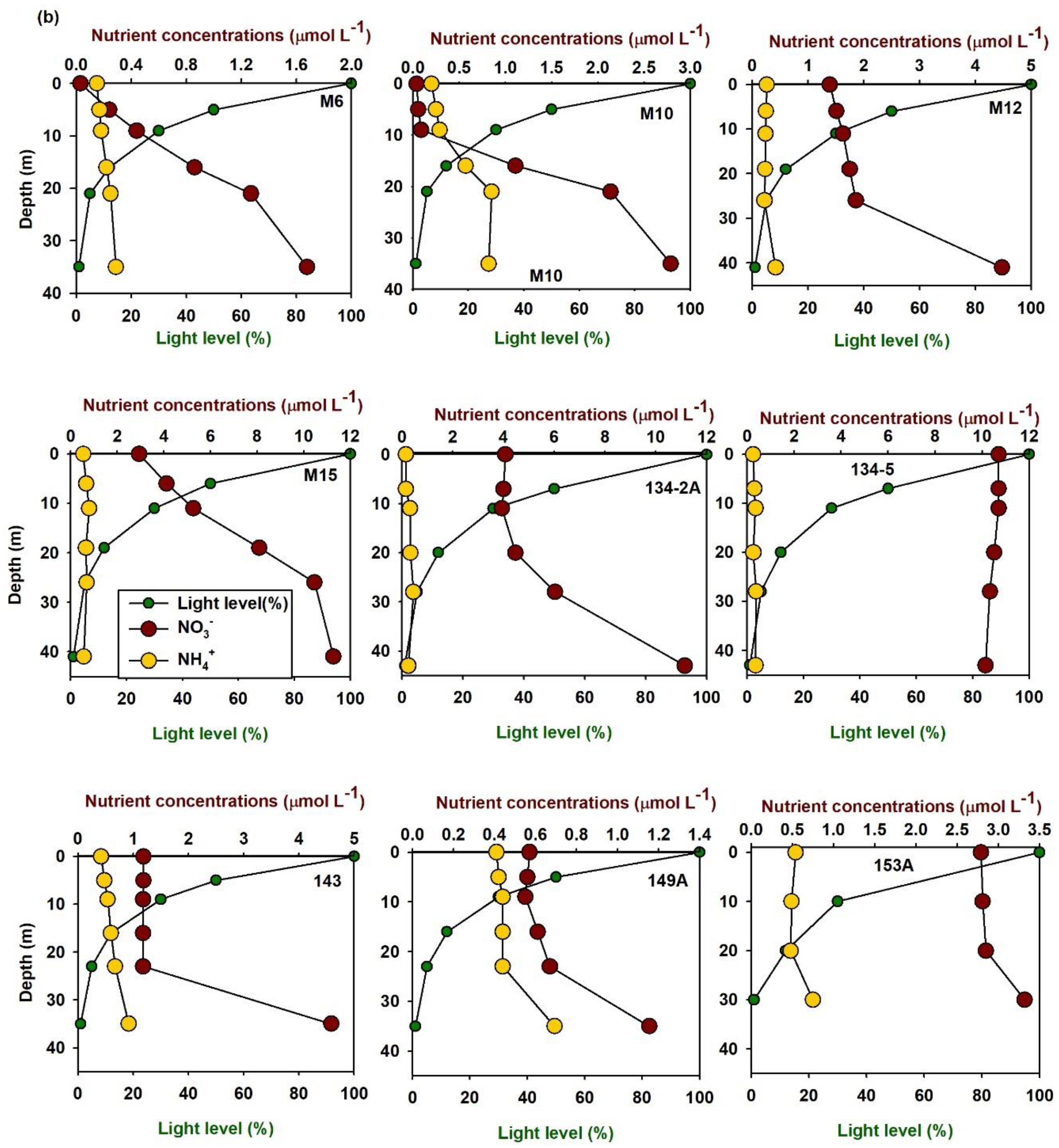
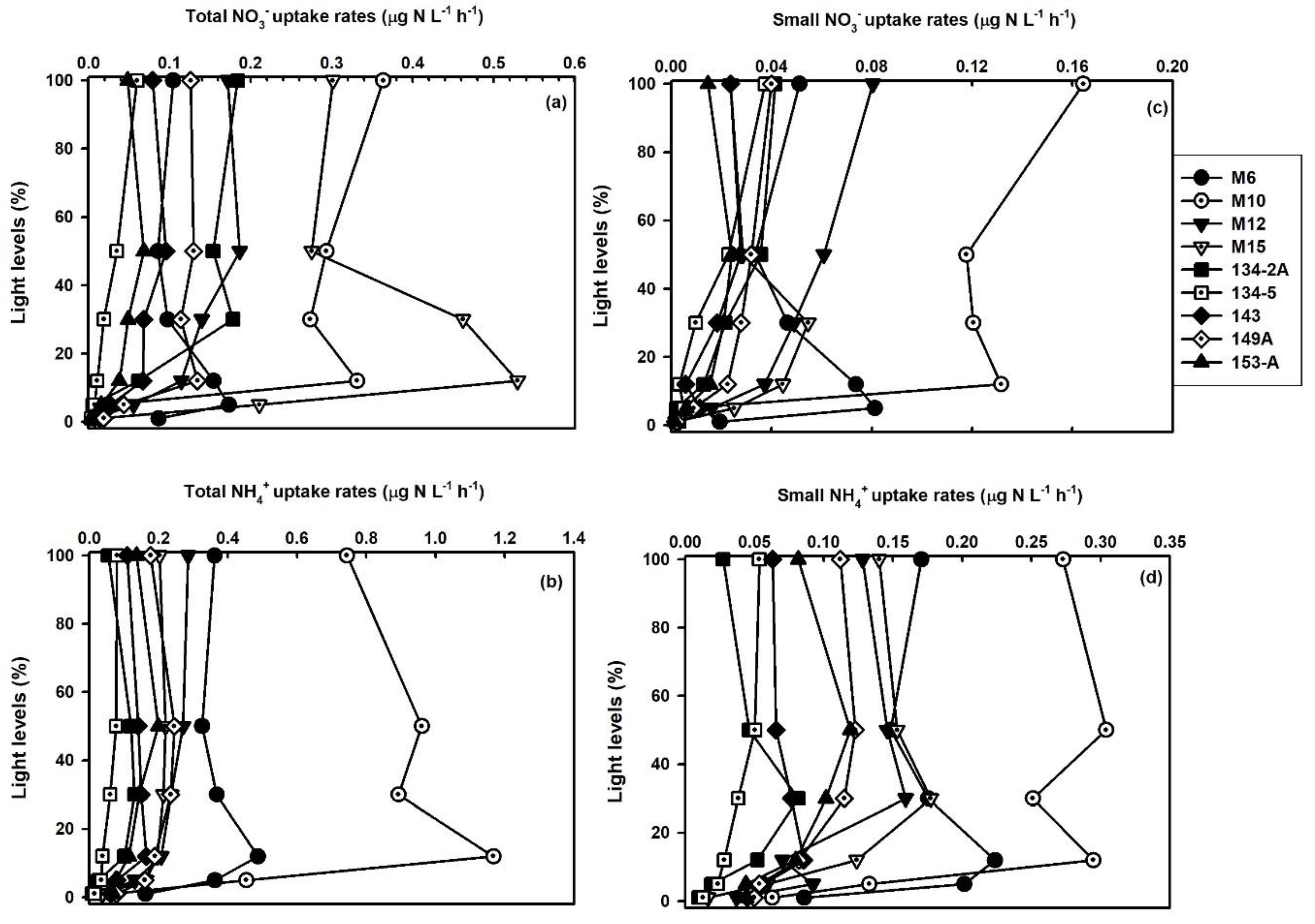
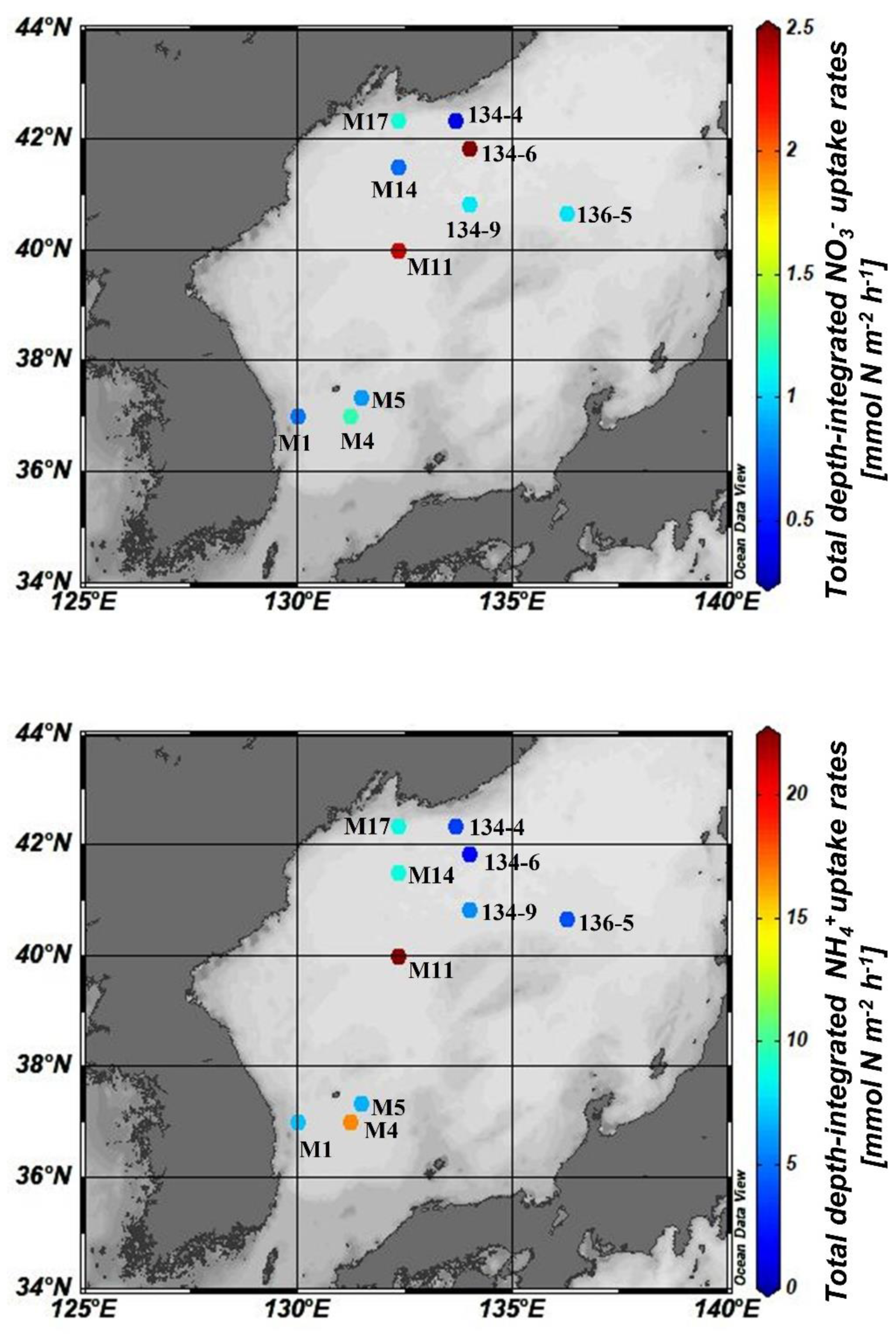
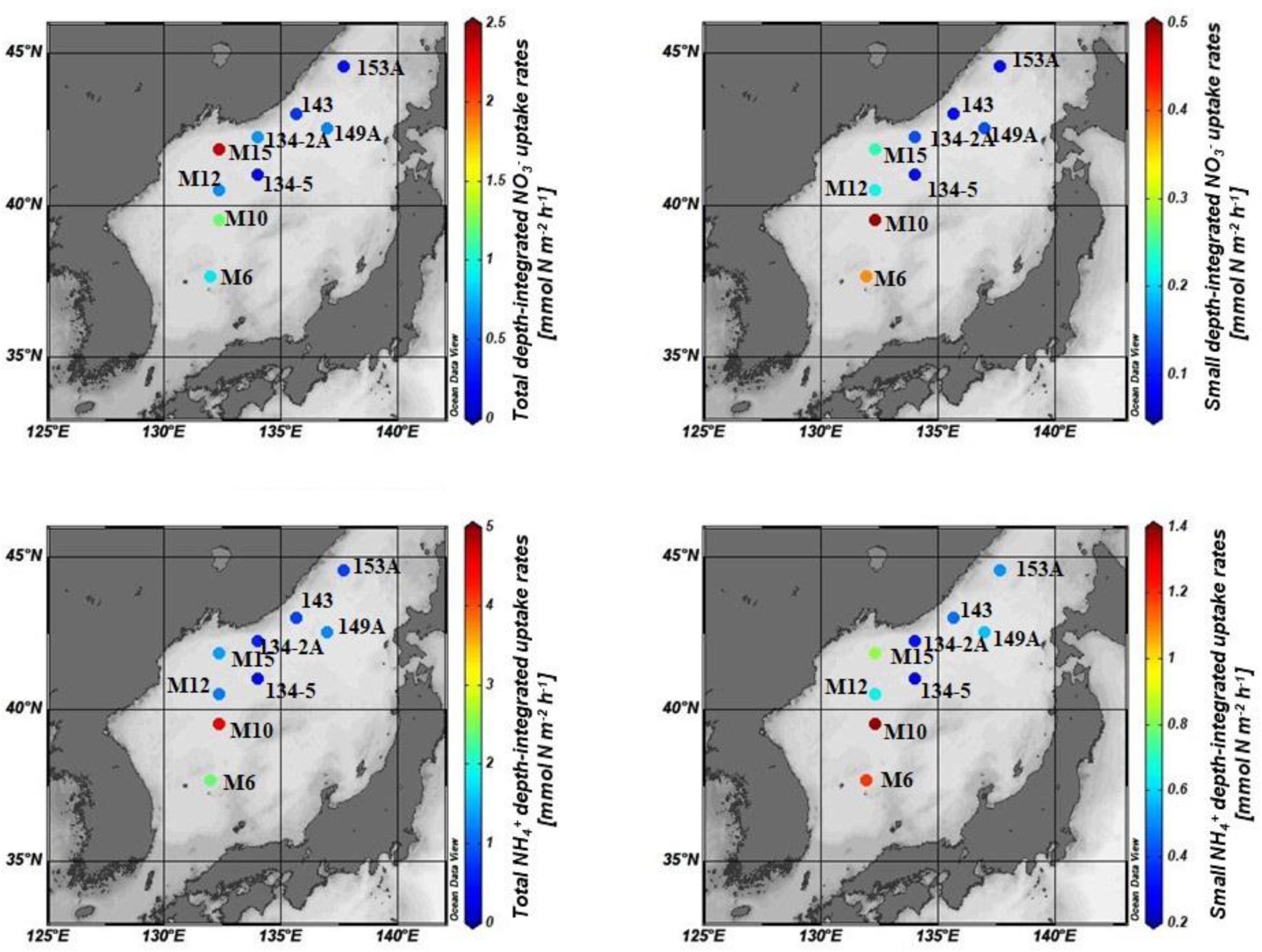
3.1. Spatiotemporal Distribution of DIN Uptake Rates
3.2. Factors Influencing DIN Uptake Rates in the East/Japan Sea
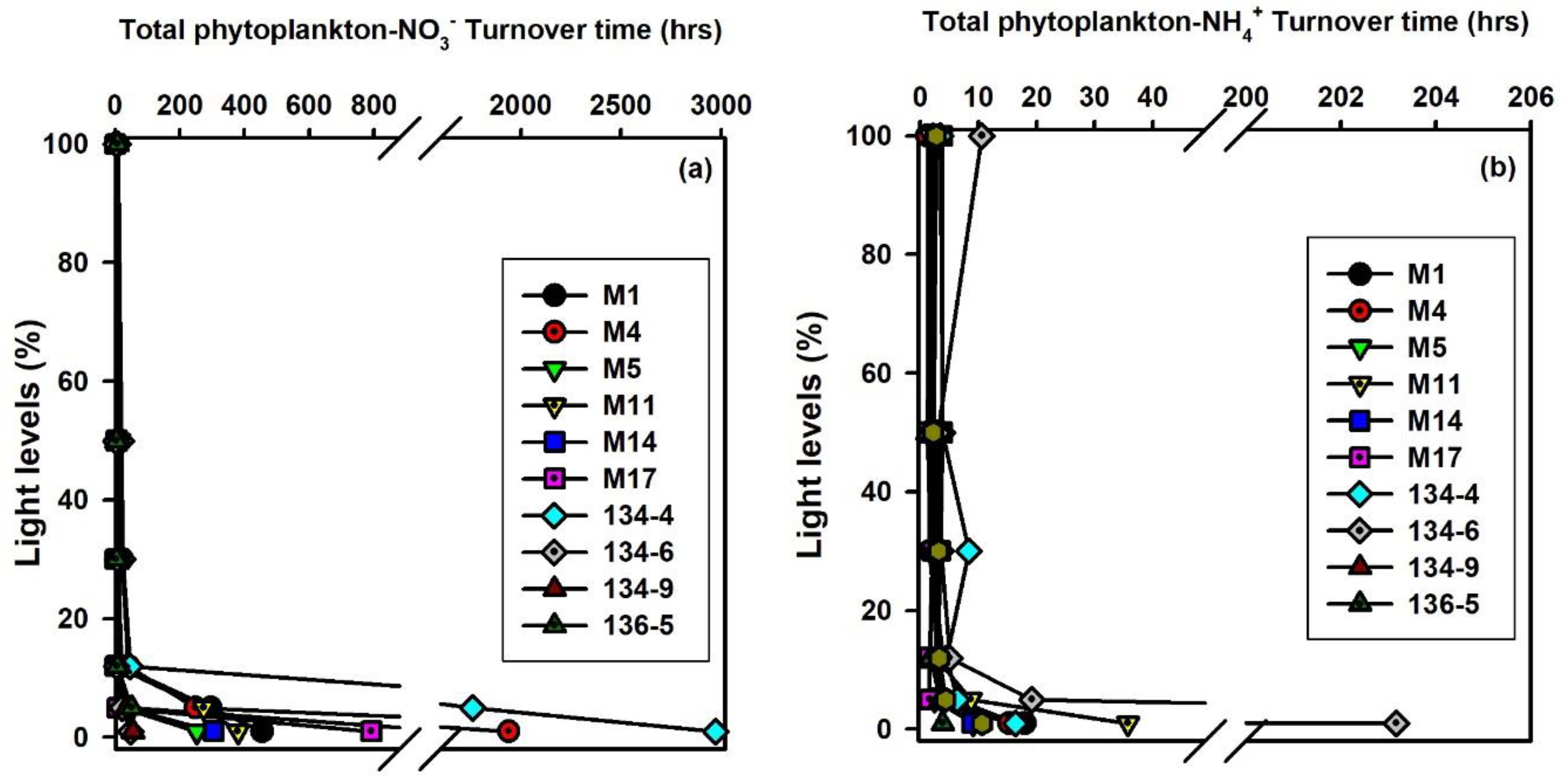
3.3. Turnover Times of Nutrients
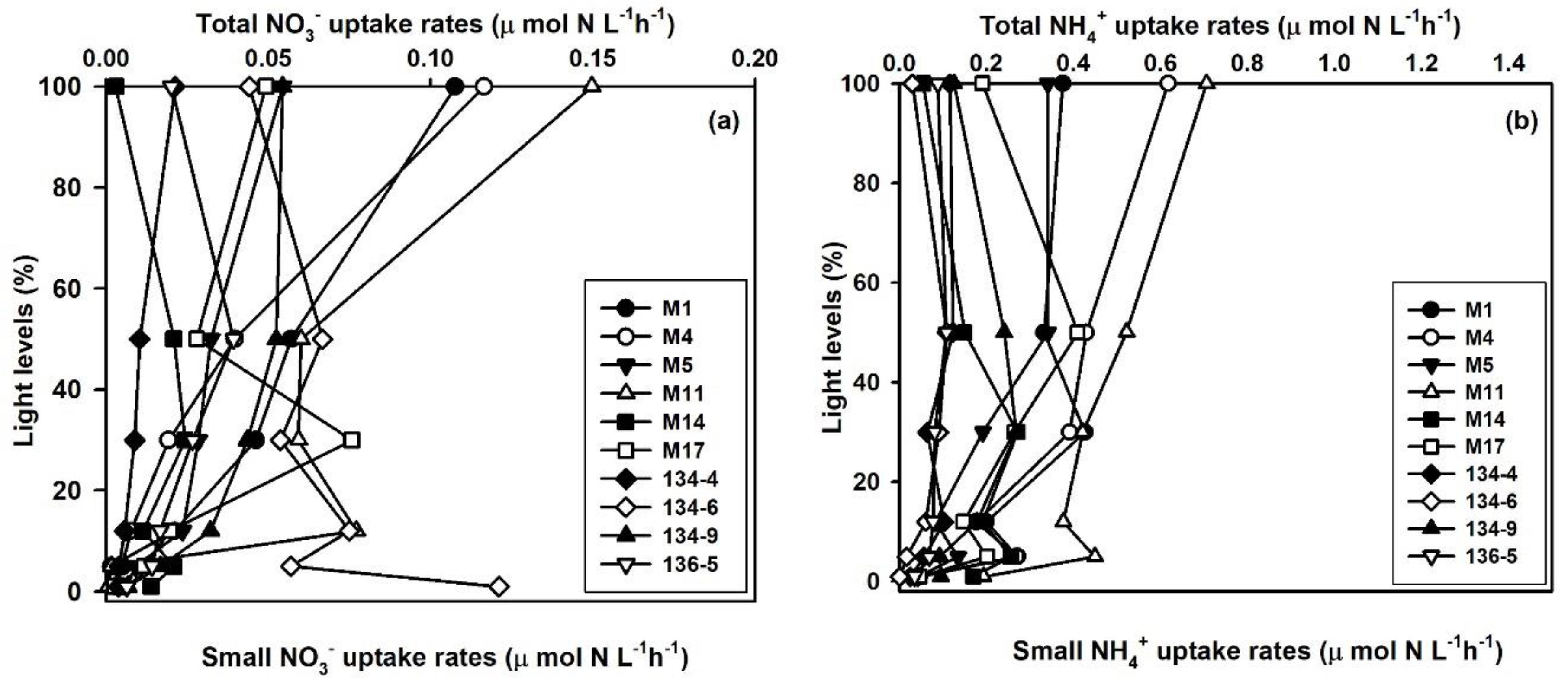
3.4. Contribution of Small Phytoplankton to Total Nitrogen Uptake
3.5. Impact of Small Phytoplankton on the Total DIN Assimilation Rates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawai, H. Transition of current images in the Japan Sea. In Tsushima Warm Current—Ocean Structures and Fishery; The Japanese Society of Fisheries Science: Tokyo, Japan, 1974; pp. 7–26. [Google Scholar]
- Sorokin, Y.I. The heterotrophic phase of plankton succession in the Japan Sea. Mar. Biol. 1977, 41, 107–117. [Google Scholar] [CrossRef]
- Park, K.-A.; Ullman, D.S.; Kim, K.; Chung, J.Y.; Kim, K.-R. Spatial and temporal variability of satellite-observed Subpolar Front in the East/Japan Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 453–470. [Google Scholar] [CrossRef]
- Kang, Y.S.; Kim, J.Y.; Kim, H.G.; Park, J.H. Long term changes in zooplankton and its relationship with squid, Todarodes pacificus, catch in the East/Japan Sea. Fish. Oceanogr. 2002, 11, 337–346. [Google Scholar] [CrossRef]
- Kim, D.; Yang, E.J.; Kim, K.H.; Shin, C.-W.; Park, J.; Yoo, S.; Hyun, J.-H. Impact of an anticyclonic eddy on the summer nutrient and chlorophyll a distribution in the Ulleung Basin, East Sea (Japan Sea). ICES J. Mar. Sci. 2011, 69, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-H.; Son, S.; Park, J.-W.; Kwak, J.H.; Kang, C.-K.; Son, Y.B.; Kwon, J.-N.; Lee, S.H. Enhanced biological activity by an anticyclonic warm eddy during early spring in the East Sea (Japan Sea) detected by the geostationary ocean color satellite. Ocean Sci. J. 2012, 47, 377–385. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Kim, D.; Shin, C.-W.; Noh, J.-H.; Yang, E.-J.; Mok, J.-S.; Kim, S.-H.; Kim, H.-C.; Yoo, S. Enhanced phytoplankton and bacterioplankton production coupled to coastal upwelling and an anticyclonic eddy in the Ulleung basin, East Sea. Aquat. Microb. Ecol. 2008, 54, 45–54. [Google Scholar] [CrossRef]
- Yoo, S.; Park, J. Why is the southwest the most productive region of the East Sea/Sea of Japan? J. Mar. Syst. 2009, 78, 301–315. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Park, H.J.; Choy, E.J.; Jeong, H.D.; Kim, K.R.; Kang, C.-K. Monthly measured primary and new productivities in the Ulleung Basin as a biological “hot spot” in the East/Japan Sea. Biogeosciences 2013, 10, 4405–4417. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.; Park, J.W.; Son, S.; Noh, J.-H.; Jeong, J.-Y.; Kwak, J.H.; Saux-Picart, S.; Choi, J.H.; Kang, C.-K.; Lee, S.H. Long-term annual primary production in the Ulleung Basin as a biological hot spot in the East/Japan Sea. J. Geophys. Res. Ocean. 2014, 119, 3002–3011. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.K.; Kremer, C.T.; Klausmeier, C.A.; Litchman, E. A Global Pattern of Thermal Adaptation in Marine Phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Bhavya, P.S.; Lee, J.H.; Lee, H.W.; Kang, J.J.; Lee, J.H.; Lee, D.; An, S.H.; Stockwell, D.A.; Whitledge, T.E.; Lee, S.H. First in situ estimations of small phytoplankton carbon and nitrogen uptake rates in the Kara, Laptev, and East Siberian seas. Biogeosciences 2018, 15, 5503–5517. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Saino, T. Variation in mesozooplankton community structure in the East/Japan Sea (1991–1999) with possible influence of the ENSO scale climatic variability. Prog. Oceanogr. 2003, 57, 317–339. [Google Scholar] [CrossRef]
- Zhang, C.I.; Lee, J.B.; Young, I.S.; Yoon, S.C.; Kim, S. Variations in the abundance of fisheries resources and ecosystem structure in the East/Japan Sea. Prog. Oceanogr. 2004, 61, 245–265. [Google Scholar] [CrossRef]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on phytoplankton phenology and community structure in the western North Pacific. Geophys. Res. Lett. 2012, 39, L15603. [Google Scholar] [CrossRef]
- Li, W.K.W.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. The smallest algae thrive as the Arctic Ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.S.; Whitledge, T.E.; Stockwell, D.; Son, S.H.; Lee, J.H.; Park, J.W.; Lee, D.B.; Park, J.; Lee, S.H. Primary production in the Chukchi Sea with potential effects of freshwater content. Biogeosciences 2016, 13, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-R.; Kim, K.; Kang, D.-J.; Park, S.Y.; Park, M.-K.; Kim, Y.-G.; Min, H.S.; Min, D. The East Sea (Japan Sea) in Change: A Story of Dissolved Oxygen. Mar. Technol. Soc. J. 1999, 33, 15–22. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.-R.; Min, D.-H.; Volkov, Y.; Yoon, J.-H.; Takematsu, M. Warming and structural changes in the East (Japan) Sea: A clue to future changes in global oceans? Geophys. Res. Lett. 2001, 28, 3293–3296. [Google Scholar] [CrossRef]
- Kang, D.J.; Park, S.; Kim, Y.G.; Kim, K.; Kim, K.-R. A moving-boundary box model (MBBM) for oceans in change: An application to the East/Japan Sea. Geophys. Res. Lett. 2003, 30, 1299. [Google Scholar] [CrossRef]
- Chiba, S.; Aita, M.N.; Tadokoro, K.; Saino, T.; Sugisaki, H.; Nakata, K. From climate regime shifts to lower-trophic level phenology: Synthesis of recent progress in retrospective studies of the western North Pacific. Prog. Oceanogr. 2008, 77, 112–126. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, S.; Dahms, H.-U.; Park, J.W.; Lim, J.-H.; Noh, J.-H.; Kwon, J.-I.; Joo, H.T.; Jeong, J.Y.; Kang, C.-K. Decadal changes of phytoplankton chlorophyll-a in the East Sea/Sea of Japan. Oceanology 2014, 54, 771–779. [Google Scholar] [CrossRef]
- Joo, H.T.; Sun, S.; Park, J.W.; Kang, J.J.; Jeong, J.-Y.; Lee, C.I.; Kang, C.K.; Lee, S.H. Long-term pattern of primary productivity in the East/Japan Sea based on ocean color data derived from MODIS-aqua. Remote Sens. 2016, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Joo, H.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.-K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- French, R.H.; Cooper, J.J.; Vigg, S. Secchi Disc Relationships. J. Am. Water Resour. Assoc. 1982, 18, 121–123. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E. Primary and new production in the deep Canada Basin during summer. Polar Biol. 2002, 28, 190–197. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E.; Kang, S.H. Recent carbon and nitrogen uptake rates of phytoplankton in Bering Strait and the Chukchi Sea. Cont. Shelf Res. 2007, 27, 2231–2249. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: New York, NY, USA, 1984. [Google Scholar]
- Garneau, M.E.; Gosselin, M.; Klein, B.; Tremblay, J.E.; Fouilland, E. New and regenerated production during a late summer bloom in an Arctic polynya. Mar. Ecol. Prog. Ser. 2007, 345, 13–26. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Goering, J.J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol. Oceanogr. 1967, 12, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of phytosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Gandhi, N.; Ramesh, R.; Laskar, A.; Sheshshayee, M.; Shetye, S.S.; Anilkumar, N.; Patil, S.M.; Mohan, R. Zonal variability in primary production and nitrogen uptake rates in the southwestern Indian Ocean and the Southern Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 67, 32–43. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P. The use of 15 N to measure nitrogen uptake in eutrophic oceans; experimental considerations. Limnol. Oceanogr. 1986, 31, 673–689. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.H.; Hwan, J.; Suh, Y.S.; Park, H.J.; Chang, K.I.; Kim, K.R.; Kang, C.K. Summer primary productivity and phytoplankton community composition driven by different hydrographic structures in the East/ Japan Sea and the Western Subarctic Pacific. J. Geophy. Res. Ocean. 2014, 119, 4505–4519. [Google Scholar] [CrossRef]
- Bhavya, P.S.; Kumar, S.; Gupta, G.V.M.; Sudheesh, V.; Sudharma, K.V.; Varrier, D.S.; Dhanya, K.R.; Saravanane, N. Nitrogen Uptake Dynamics in a Tropical Eutrophic Estuary (Cochin, India) and Adjacent Coastal Waters. Chesap. Sci. 2015, 39, 54–67. [Google Scholar] [CrossRef]
- Glibert, P.M.; Biggs, D.C.; McCarthy, J.J. Utilization of ammonium and nitrate during austral summer in the Scotia Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 1982, 29, 837–850. [Google Scholar] [CrossRef]
- Eppley, R.W.; Thomas, W.H. Comparison of half-saturation constants for growth and nitrate uptake of marine phytoplankton. J. Phycol. 1969, 5, 375–379. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Egge, J.K. A theoretical model for nutrient uptake in phytoplankton. Mar. Ecol. Prog. Ser. 1991, 70, 65–72. [Google Scholar] [CrossRef]
- Hein, M.; Folager Pedersen, M.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro and macroalgae. Mar. Ecol. Prog. Ser. 1995, 118, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Shuter, B.G. Size dependence of phophorus and nitrogen subsistence quotas in unicellular microrganisms. Limnol. Oceanogr. 1978, 23, 1248–1255. [Google Scholar] [CrossRef]
- Grover, J.P. Resource competition in a variable environment: Phytoplankton growing according to the variable-internal-stores model. Am. Nat. 1991, 138, 811–835. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Agustí, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Hodal, H.; Kristiansen, S. The importance of small-celled phytoplankton in spring blooms at the marginal ice zone in the northern Barents Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- Malone, T.C. Size-Fractionated Primary Productivity of Marine Phytoplankton. In Primary Productivity in the Sea; Springer: Boston, MA, USA, 1980; pp. 301–319. [Google Scholar]
- Nalewajko, C.; Garside, C. Methodological problems in the simultaneous assessment of photosynthesis and nutrient uptake in phytoplankton as functions of light intensity and cell size. Limnol. Oceanogr. 1983, 28, 591–597. [Google Scholar] [CrossRef]
- Probyn, T.A. Nitrogen uptake by size-fractionated phytoplankton populations in the southern Benguela upwelling system. Mar. Ecol. Prog. Ser. Oldendorf. 1985, 22, 249–258. [Google Scholar] [CrossRef]
- Koike, I.; Holm-Hansen, O.; Biggs, D.C. Inorganic nitrogen metabolism by Antarctic phytoplankton with special reference to ammonium cycling. Mar. Ecol. Prog. Ser. 1986, 30, 105–116. [Google Scholar] [CrossRef]
- Dauchez, S.; Legendre, L.; Fortier, L.; Levasseur, M. Nitrate uptake by size-fractionated phytoplankton on the Scotian Shelf (Northwest Atlantic): Spatial and temporal variability. J. Plank. Res. 1996, 18, 577–595. [Google Scholar] [CrossRef]
- Dauchez, S.; Legendre, L.; Fortier, L.; Levasseur, M. New production and production of large phytoplankton (>5 um) on the Scotian Shelf (NW Atlantic). Mar. Ecol. Prog. Ser. 1996, 135, 215–222. [Google Scholar] [CrossRef]
- Tamigneaux, E.; Vazquez, E.; Mingelbier, M.; Klein, B.; Legendre, L. Environmental control of phytoplankton assemblages in nearshore marine waters, with special emphasis on phototrophic ultraplankton. J. Plankton Res. 1995, 17, 1421–1448. [Google Scholar] [CrossRef]
- Wilkerson, F.; Dugdale, R.; Kudela, R.; Chavez, F.P. Biomass and productivity in Monterey Bay, California: Contribution of the large phytoplankton. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 1003–1022. [Google Scholar] [CrossRef]
- Kim, B.K.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H.; Hahm, D.; Rhee, T.S.; Lee, S.H. Large seasonal variation in phytoplankton production in the Amundsen Sea. Polar Biol. 2015, 38, 319–331. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Joo, H.T.; Kang, S.H.; Kang, C.-K.; Whitledge, T.E. Contribution of small phytoplankton to total primary production in the Chukchi Sea. Cont. Shelf Res. 2013, 68, 43–50. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.-W.; Lee, S.H.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.; Lee, S.H. Seasonal Variations in the Small Phytoplankton Contribution to the Total Primary Production in the Amundsen Sea, Antarctica. J. Geophys. Res. Ocean. 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Kwak, J.H.; Han, E.; Lee, S.H.; Park, H.J.; Kim, K.R.; Kang, C.K. A consistent structure of phytoplankton communities across the warm–cold regions of the water mass on a meridional transect in the East/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 36–44. [Google Scholar] [CrossRef]
- Goldman, J.C. Potential role of large oceanic diatoms in new primary production. Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 159–168. [Google Scholar] [CrossRef]
- Michaels, A.F.; Silver, M.W. Primary production, sinking fluxes, and the microbial food web. Deep Sea Res. Part A Oceanogr. Res. Pap. 1988, 35, 473–490. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Moran, X.A.G.; Lopez-Urrutia, A.; Calvo-Diaz, A.; Li, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Gregg, W.W.; Conkright, M.E.; Ginoux, P.; O’Reilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 15, 1809. [Google Scholar] [CrossRef] [Green Version]


| Year | Light Level (%) | Temperature (°C) | |||||||||
| M1 | M4 | M5 | M11 | M14 | M17 | 134-4 | 134-6 | 134-9 | 136-5 | ||
| 100 | 24.9 | 24.9 | 24.2 | 22.2 | 18.1 | 19.2 | 18.4 | 16.8 | 21.5 | 20.1 | |
| 50 | 24.9 | 24.2 | 17.4 | 19.4 | 16.3 | 20.8 | 20.1 | ||||
| 2012 | 30 | 23.3 | 25.2 | 22.9 | 21.5 | 17.5 | 18.7 | 18.7 | 16.1 | 20.6 | 19.7 |
| 12 | 23.2 | 23.4 | 17.4 | 18.3 | 16.2 | 21.1 | 19.9 | ||||
| 5 | 23.3 | 23.5 | 17.1 | 18.1 | 16.5 | 20.8 | 19.9 | ||||
| 1 | 23.1 | 24.7 | 23.8 | 21.4 | 17.3 | 18.6 | 18.1 | 16.5 | 21.1 | 19.8 | |
| Light Level (%) | Temperature (°C) | ||||||||||
| M6 | M10 | M12 | M15 | 134-2A | 134-5 | 143 | 149A | 153-A | |||
| 100 | 2.58 | 3.59 | 3.18 | 4.33 | 5.97 | 5.07 | 7.77 | 12.1 | 13.6 | ||
| 50 | 2.53 | 3.54 | 3.17 | 4.34 | 6.02 | 5.07 | 7.69 | 11.7 | 13.6 | ||
| 2015 | 30 | 2.55 | 3.54 | 3.03 | 4.34 | 5.84 | 5.07 | 7.44 | 11.0 | 13.0 | |
| 12 | 2.48 | 3.47 | 2.92 | 4.34 | 5.73 | 5.06 | 7.00 | 10.5 | 12.1 | ||
| 5 | 2.29 | 3.14 | 2.45 | 4.30 | 5.72 | 5.06 | 6.46 | 9.58 | 11.0 | ||
| 1 | 1.93 | 2.52 | 2.04 | 4.03 | 5.70 | 4.92 | 4.57 | 9.28 | 10.1 | ||
| Year | Stn. Name | Latitude (°E) | Longitude (°N) | Euphotic | Total NO3− | Small NO3− | Contribution | Total NH4+ | Small NH4+ | Contribution |
|---|---|---|---|---|---|---|---|---|---|---|
| Depth | Uptake Rate | Uptake Rate | (%) | Uptake Rate | Uptake Rate | (%) | ||||
| 2012 | M1 | 37.002 | 129.999 | 27 | 0.750 | - | - | 6.978 | - | - |
| M4 | 36.990 | 131.232 | 60 | 1.214 | - | - | 16.66 | - | - | |
| M5 | 37.349 | 131.459 | 41 | 0.850 | - | - | 6.587 | - | - | |
| M11 | 40.001 | 132.333 | 54 | 2.365 | - | - | 22.38 | - | - | |
| M14 | 41.501 | 132.337 | 43 | 0.726 | - | - | 8.645 | - | - | |
| M17 | 42.340 | 132.335 | 43 | 1.142 | - | - | 8.578 | - | - | |
| 134-4 | 42.335 | 133.667 | 46 | 0.333 | - | - | 3.457 | - | - | |
| 134-6 | 41.831 | 134.003 | 35 | 2.498 | - | - | 1.676 | - | - | |
| 134-9 | 40.838 | 133.997 | 35 | 1.036 | - | - | 5.586 | - | - | |
| 136-5 | 40.653 | 136.264 | 54 | 1.023 | - | - | 3.957 | - | - | |
| 2015 | M6 | 37.661 | 131.944 | 35 | 0.873 | 0.377 | 43.18 | 2.381 | 1.184 | 49.72 |
| M10 | 39.524 | 132.339 | 35 | 1.197 | 0.495 | 41.32 | 4.570 | 1.381 | 30.21 | |
| M12 | 40.513 | 132.331 | 33 | 0.633 | 0.220 | 34.71 | 1.146 | 0.641 | 55.93 | |
| M15 | 41.832 | 132.334 | 41 | 2.363 | 0.242 | 10.24 | 1.351 | 0.823 | 60.94 | |
| 134-2A | 42.255 | 134.011 | 43 | 0.653 | 0.127 | 19.39 | 0.586 | 0.323 | 55.20 | |
| 134-5 | 40.990 | 134.005 | 43 | 0.145 | 0.086 | 59.36 | 0.385 | 0.264 | 68.55 | |
| 143 | 43.000 | 135.657 | 35 | 0.362 | 0.089 | 24.61 | 0.803 | 0.456 | 56.82 | |
| 149A | 42.518 | 136.989 | 35 | 0.599 | 0.131 | 21.86 | 1.232 | 0.570 | 46.25 | |
| 153A | 44.567 | 137.673 | 30 | 0.227 | 0.087 | 38.49 | 0.792 | 0.508 | 64.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhavya, P.S.; Kang, J.J.; Jang, H.K.; Joo, H.; Lee, J.H.; Lee, J.H.; Park, J.W.; Kim, K.; Kim, H.C.; Lee, S.H. The Contribution of Small Phytoplankton Communities to the Total Dissolved Inorganic Nitrogen Assimilation Rates in the East/Japan Sea: An Experimental Evaluation. J. Mar. Sci. Eng. 2020, 8, 854. https://doi.org/10.3390/jmse8110854
Bhavya PS, Kang JJ, Jang HK, Joo H, Lee JH, Lee JH, Park JW, Kim K, Kim HC, Lee SH. The Contribution of Small Phytoplankton Communities to the Total Dissolved Inorganic Nitrogen Assimilation Rates in the East/Japan Sea: An Experimental Evaluation. Journal of Marine Science and Engineering. 2020; 8(11):854. https://doi.org/10.3390/jmse8110854
Chicago/Turabian StyleBhavya, Panthalil S., Jae Joong Kang, Hyo Keun Jang, HuiTae Joo, Jae Hyung Lee, Jang Han Lee, Jung Woo Park, Kwanwoo Kim, Hyung Chul Kim, and Sang Heon Lee. 2020. "The Contribution of Small Phytoplankton Communities to the Total Dissolved Inorganic Nitrogen Assimilation Rates in the East/Japan Sea: An Experimental Evaluation" Journal of Marine Science and Engineering 8, no. 11: 854. https://doi.org/10.3390/jmse8110854
APA StyleBhavya, P. S., Kang, J. J., Jang, H. K., Joo, H., Lee, J. H., Lee, J. H., Park, J. W., Kim, K., Kim, H. C., & Lee, S. H. (2020). The Contribution of Small Phytoplankton Communities to the Total Dissolved Inorganic Nitrogen Assimilation Rates in the East/Japan Sea: An Experimental Evaluation. Journal of Marine Science and Engineering, 8(11), 854. https://doi.org/10.3390/jmse8110854







