Lagoon Biogeochemical Processing is Reflected in Spatial Patterns of Sediment Stable Isotopic Ratios
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mancinelli, G.; Vizzini, S. Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: State of the art, knowledge gaps, and community-scale perspectives. Estuar. Coast. Shelf Sci. 2015, 156, 195–204. [Google Scholar] [CrossRef]
- Oczkowski, A.; Kreakie, B.; McKinney, R.A.; Prezioso, J. Patterns in stable isotope values of N and carbon in particulate matter from the Northwest Atlantic continental shelf, from the Gulf of Maine to Cape Hatteras. Front. Mar. Sci. 2016, 3, 252. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Hollander, D.J.; Peebles, E.B. Seasonal δ13C and δ15N isoscapes of fish populations along a continental shelf trophic gradient. Cont. Shelf Res. 2013, 68, 112–122. [Google Scholar] [CrossRef]
- Vokhshoori, N.L.; McCarthy, M.D. Compound-specific δ15N amino acid measurements in littoral mussels in the California upwelling ecosystem: A new approach to generating baseline δ15N isoscapes for coastal ecosystems. PLoS ONE 2014, 9, e98087. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Middelburg, J.J.; McClelland, J.W.; Jake Vander Zanden, M. Stable isotope tracers: Enriching our perspectives and questions on sources, fates, rates, and pathways of major elements in aquatic systems. Limnol. Oceanogr. 2019, 64, 950–981. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.S.; Koch, P.L.; Newsome, S.D.; McMahon, K.W.; Aurioles, D. Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In Isoscapes; West, J.B., Bowen, G.J., Dawson, T.E., Tu, K.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 299–318. [Google Scholar]
- Costanzo, S.D.; Udy, J.; Longstaff, B.; Jones, A. Using N stable isotope ratios (δ15N) of macroalgae to determine the effectiveness of sewage upgrades: Changes in the extent of sewage plumes over four years in Moreton Bay, Australia. Mar. Pollut. Bull. 2005, 51, 212–217. [Google Scholar] [CrossRef]
- Carriquiry, J.D.; Jorgensen, P.; Villaescusa, J.A.; Ibarra-Obando, S.E. Isotopic and elemental composition of marine macrophytes as biotracers of nutrient recycling within a coastal lagoon in Baja California, Mexico. Estuar. Coast 2016, 39, 451–461. [Google Scholar] [CrossRef]
- Pruell, R.J.; Taplin, B.K. Carbon and N isotope ratios of juvenile winter flounder as indicators of inputs to estuarine systems. Mar. Pollut. Bull. 2015, 101, 624–631. [Google Scholar] [CrossRef]
- Cornwell, J.C.; Kemp, W.M.; Kana, T.M. Denitrification in coastal ecosystems: Methods, environmental controls, and ecosystem level controls, a review. Aquat. Ecol. 1999, 33, 41–54. [Google Scholar] [CrossRef]
- Giblin, A.E.; Tobias, C.R.; Song, B.; Weston, N.; Banta, G.T.; Rivera-Monroy, V.H. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the N cycle of coastal ecosystems. Oceanography 2013, 26, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Marchant, H.K.; Lavik, G.; Holtappels, M.; Kuypers, M.M. The fate of nitrate in intertidal permeable sediments. PLoS ONE 2014, 9, e104517. [Google Scholar] [CrossRef] [PubMed]
- Piehler, M.F.; Smyth, A.R. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Codispoti, L.A.; Brandes, J.A.; Christensen, J.P.; Devol, A.H.; Naqvi, S.W.A.; Paerl, H.W.; Yoshinari, T. The oceanic fixed N and nitrous oxide budgets: Moving targets as we enter the anthropocene? Sci. Mar. 2001, 65, 85–105. [Google Scholar] [CrossRef]
- An, S.; Gardner, W.S.; Kana, T. Simultaneous measurement of denitrification and N fixation using isotope pairing with membrane inlet mass spectrometry analysis. Appl. Environ. Microbiol. 2001, 67, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Newell, R.I.; Cornwell, J.C.; Owens, M.S. Influence of simulated bivalve biodeposition and microphytobenthos on sediment N dynamics: A laboratory study. Limnol. Oceanogr. 2002, 47, 1367–1379. [Google Scholar] [CrossRef]
- Howarth, R.W. Nutrient limitation of net primary production in marine ecosystems. Annu. Rev. Ecol. Syst. 1988, 19, 89–110. [Google Scholar] [CrossRef]
- Fulweiler, R.W.; Brown, S.M.; Nixon, S.W.; Jenkins, B.D. Evidence and a conceptual model for the cooccurrence of N fixation and denitrification in heterotrophic marine sediments. Mar. Ecol. Prog. Ser. 2013, 482, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Bentzon-Tilia, M.; Traving, S.J.; Mantikci, M.; Knudsen-Leerbeck, H.; Hansen, J.L.; Markager, S.; Riemann, L. Significant N 2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J. 2015, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Oczkowski, A.J.; Santos, E.A.; Martin, R.M.; Gray, A.B.; Hanson, A.R.; Watson, E.B.; Huertas, E.; Wigand, C. Unexpected nitrogen sources in a tropical urban estuary. J. Geophys. Res. Biogeosci. 2020, 125. [Google Scholar] [CrossRef]
- Fulweiler, R.W.; Nixon, S.W.; Buckley, B.A.; Granger, S.L. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 2007, 448, 180–182. [Google Scholar] [CrossRef]
- Moisander, P.H.; Beinart, R.A.; Hewson, I.; White, A.E.; Johnson, K.S.; Carlson, C.A.; Montoya, J.P.; Zehr, J.P. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science 2010, 327, 1512–1514. [Google Scholar] [CrossRef]
- Russell, D.G.; Warry, F.Y.; Cook, P.L. The balance between N fixation and denitrification on vegetated and non-vegetated intertidal sediments. Limnol. Oceanogr. 2016, 61, 2058–2075. [Google Scholar] [CrossRef]
- Jørgensen, B.B. Mineralization of organic matter in the sea bed—The role of sulphate reduction. Nature 1982, 296, 643–645. [Google Scholar] [CrossRef]
- Canfield, D.E.; Jørgensen, B.B.; Fossing, H.; Glud, R.; Gundersen, J.; Ramsing, N.B.; Thamdrup, B.; Hansen, J.W.; Nielsen, L.P.; Hall, P.O.J. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 1993, 113, 27–40. [Google Scholar] [CrossRef]
- Böttcher, M.; Brumsack, H.-J.; Dürselen, C.-D. The isotopic composition of modern seawater sulfate: I. Coastal waters with special regard to the North Sea. J. Mar. Syst. 2007, 67, 73–82. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. The production of 34s-depleted sulfide during bacterial disproportionation of elemental sulfur. Science 1994, 266, 1973–1975. [Google Scholar] [CrossRef]
- Winner, W.E.; Smith, C.L.; Koch, G.W.; Mooney, H.A.; Bewley, J.D.; Krouse, H.R. Rates of emission of H2S from plants and patterns of stable S isotope fractionation. Nature 1981, 289, 672–673. [Google Scholar] [CrossRef]
- Hofmann, M.; Wolf-Gladrow, D.A.; Takahashi, T.; Sutherland, S.C.; Six, K.D.; Maier Reimer, E. Stable carbon isotope distribution of particulate organic matter in the ocean: A model study. Mar. Chem. 2000, 72, 131–150. [Google Scholar] [CrossRef]
- Hu, X.; Burdige, D.J.; Zimmerman, R.C. δ13C is a signature of light availability and photosynthesis in seagrass. Limnol. Oceanogr. 2012, 57, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Simenstad, C.A.; Wissmar, R.C. d13C evidence of the origins and fates of organic carbon in estuarine and nearshore food webs. Mar. Ecol. Prog. Ser. 1985, 22, 141–152. [Google Scholar] [CrossRef]
- Marbà, N.; Holmer, M.; Gacia, E.; Barron, C. Seagrass Beds and Coastal Biogeochemistry. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 135–157. [Google Scholar]
- Holmer, M. Productivity and biogeochemical cycling in seagrass ecosystems. In Coastal Wetlands, 2nd ed.; Perillo, M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–477. [Google Scholar]
- Beer, S.; Rehnberg, J. The acquisition of inorganic carbon by the seagrass Zostera marina. Aquat. Bot. 1997, 56, 277–283. [Google Scholar] [CrossRef]
- Frederiksen, M.S.; Holmer, M.; Borum, J.; Kennedy, H. Temporal and spatial variation of sulfide invasion in eelgrass (Zostera marina) as reflected by its S isotopic composition. Limnol. Oceanogr. 2006, 51, 2308–2318. [Google Scholar] [CrossRef]
- Kharlamenko, V.I.; Kiyashko, S.I.; Imbs, A.B.; Vyshkvartzev, D.I. Identification of food sources of invertebrates from the seagrass Zostera marina community using carbon and S stable isotope ratio and fatty acid analyses. Mar. Ecol. Prog. Ser. 2001, 220, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Schubert, P.R.; Karez, R.; Reusch, T.B.; Dierking, J. Isotopic signatures of eelgrass (Zostera marina L.) as bioindicator of anthropogenic nutrient input in the western Baltic Sea. Mar. Pollut. Bull. 2013, 72, 64–70. [Google Scholar] [CrossRef]
- Holmer, M.; Hasler-Sheetal, H. Sulfide intrusion in seagrasses assessed by stable S isotopes—A synthesis of current results. Front. Mar. Sci. 2014, 1, 64. [Google Scholar] [CrossRef] [Green Version]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Tallis, H.M.; Ruesink, J.L.; Dumbauld, B.; Hacker, S.; Wisehart, L.M. Oysters and aquaculture practices affect eelgrass density and productivity in a Pacific Northwest estuary. J. Shellfish Res. 2009, 28, 251–261. [Google Scholar] [CrossRef]
- Simenstad, C.A.; Fresh, K.L. Influence of intertidal aquaculture on benthic communities in Pacific Northwest estuaries: Scales of disturbance. Estuaries 1995, 18, 43–70. [Google Scholar] [CrossRef]
- Kellogg, M.L.; Smyth, A.R.; Luckenbach, M.W.; Carmichael, R.H.; Brown, B.L.; Cornwell, J.C.; Piehler, M.F.; Owens, M.S.; Dalrymple, D.J.; Higgins, C.B. Use of oysters to mitigate eutrophication in coastal waters. Estuar. Coast. Shelf Sci. 2014, 151, 156–168. [Google Scholar] [CrossRef]
- Nelson, K.A.; Leonard, L.A.; Posey, M.H.; Alphin, T.D.; Mallin, M.A. Using transplanted oyster (Crassostrea virginica) beds to improve water quality in small tidal creeks: A pilot study. J. Exp. Mar. Biol. Ecol. 2004, 298, 347–368. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Humphries, A.T.; Casas, S.M.; La Peyre, J.F. Temporal variation in development of ecosystem services from oyster reef restoration. Ecol. Eng. 2014, 63, 34–44. [Google Scholar] [CrossRef]
- Crawford, C.M.; Macleod, C.K.; Mitchell, I.M. Effects of shellfish farming on the benthic environment. Aquaculture 2003, 224, 117–140. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Muñoz, A.; Buddemeier, R.W.; Camacho-Ibar, V.; Carriquiry, J.D.; Ibarra-Obando, S.E.; Massey, B.W.; Smith, S.V.; Wulff, F. Sustainability of coastal resource use in San Quintin, Mexico. AMBIO 2001, 30, 142–150. [Google Scholar] [CrossRef]
- Sigman, D.M.; Robinson, R.; Knapp, A.N.; Van Geen, A.; McCorkle, D.C.; Brandes, J.A.; Thunell, R.C. Distinguishing between water column and sedimentary denitrification in the Santa Barbara Basin using the stable isotopes of nitrate. Geochem. Geophys. Geosyst. 2003, 4, 1040. [Google Scholar] [CrossRef] [Green Version]
- Kessler, A.J.; Bristow, L.A.; Cardenas, M.B.; Glud, R.N.; Thamdrup, B.; Cook, P.L. The isotope effect of denitrification in permeable sediments. Geochim. Cosmochim. Acta 2014, 133, 156–167. [Google Scholar] [CrossRef]
- Camacho-Ibar, V.F.; Carriquiry, J.D.; Smith, S.V. Non-conservative P and N fluxes and net ecosystem production in San Quintin Bay, Mexico. Estuaries 2003, 26, 1220–1237. [Google Scholar] [CrossRef]
- Ibarra-Obando, S.E.; Smith, S.V.; Poumian-Tapia, M.; Camacho-Ibar, V.; Carriquiry, J.D.; Montes-Hugo, M. Benthic metabolism in San Quintin Bay, Baja California, Mexico. Mar. Ecol. Prog. Ser. 2004, 283, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Medina, F.; Suarez, F.; Espindola, J.M. Historic and Holocene volcanic centers in NW Mexico. Bull. Volcanol. 1989, 51, 91–93. [Google Scholar] [CrossRef]
- Luhr, J.F.; Aranda-Gómez, J.J.; Housh, T.B. San Quintin volcanic field, Baja California Norte, Mexico: Geology, petrology, and geochemistry. J. Geophys. Res. Solid Earth 1995, 100, 10353–10380. [Google Scholar] [CrossRef]
- Navarro, E.; Daesslé, L.W.; Camacho-Ibar, V.F.; Ortiz-Hernández, M.C.; Gutiérrez-Galindo, E.A. La geoquímica de Fe, Ti y Al como indicadora de la sedimentación volcanoclástica en la laguna costera de San Quintín, Baja California, México. Cienc. Mar. 2006, 32, 205–217. [Google Scholar] [CrossRef]
- Gorsline, D.S.; Stewart, R.A. Benthic marine exploration of Bahia de San Quintin, Baja, California, 1960–1961: Marine and Quaternary geology. Pac. Nat. 1962, 3, 282–319. [Google Scholar]
- SMN (Servicio Meterorlógico Nacional) 2019. Normales Climatologías. ESTACION: 00002032 LAS ESCOBAS. 17 September 2019. Available online: https://smn.conagua.gob.mx/tools/RESOURCES/Normales5110/NORMAL02032.TXT (accessed on 18 September 2020).
- Barnard, J.L. Benthic marine exploration of Bahia de San Quintin, Baja California, 1960–1961. Pac. Nat. 1962, 3, 250–274. [Google Scholar]
- García-Esquivel, Z.; González-Gómez, M.A.; Ley-Lou, F.; Mejía-Trejo, A. Oyster culture potential in the west arm of San Quintín Bay: Current biomass and preliminary estimate of the carrying capacity. Cienc. Mar. 2004, 30, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.J.; Howarth, R.W. S, carbon, and N isotopes used to trace organic matter flow in the salt-marsh estuaries of Sapelo Island, Georgia 1. Limnol. Oceanogr. 1987, 32, 1195–1213. [Google Scholar] [CrossRef]
- Gray, A.B.; Pasternack, G.B.; Watson, E.B. Hydrogen peroxide treatment effects on the particle size distribution of alluvial and marsh sediments. Holocene 2010, 20, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Blott, S.J.; Pye, K. Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Friedman, G.M.; Sanders, J.E. Principles of Sedimentology; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Angulo-Larios, N. Hidrodinámica de la Bahía de San Quintín, B.C. Master’s Thesis, Facultad de Ciencias Marinas (FCM), Universidad Autónoma de Baja California (UABC), Ensenada, MX, USA, 2006. [Google Scholar]
- Canu, D.M.; Aveytua-Alcázar, L.; Camacho-Ibar, V.F.; Querin, S.; Solidoro, C. Hydrodynamic properties of San Quintin Bay, Baja California: Merging models and observations. Mar. Pollut. Bull. 2016, 108, 203–214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Mevik, B.H.; Wehrens, R.; Liland, K.; Hiemstra, P.; Wehrens, R. PLS: Partial Least Squares and Principal Component Regression, R package version 2.7-2; 2019. Available online: https://cran.r-project.org/web/packages/pls/index.html (accessed on 18 September 2020).
- Liland, H.V.; Mehmood, T.; Sæbø, S. plsVarSel: Variable Selection in Partial Least Squares, R Package Version 0.9.4; 2017. Available online: https://mran.microsoft.com/snapshot/2018-04-07/web/packages/plsVarSel/index.html (accessed on 18 September 2020).
- Harrell, F.E.; Dupont, C. Package Hmisc: Harrell Miscellaneous, Package Version 4.2-0; 2019. Available online: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed on 18 September 2020).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Corrplot: Visualization of a Correlation Matrix, R. Package Version 0.84; 2017. Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 18 September 2020).
- McLeod, A.I. Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test, R Package Version 2.2; 2015. Available online: https://cran.r-project.org/web/packages/pls/kendall.html (accessed on 18 September 2020).
- Tanner, W.F. (Ed.) William F. Tanner on Environmental Clastic Granulometry; Florida Geological Survey: Tallahassee, FL, USA, 1995. [Google Scholar]
- Lario, J.; Spencer, C.; Plater, A.J.; Zazo, C.; Goy, J.L.; Dabrio, C.J. Particle size characterisation of Holocene back-barrier sequences from North Atlantic coasts (SW Spain and SE England). Geomorphology 2002, 42, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Lara-Lara, J.R.; Alvarez-Borrego, S. Ciclo anual de clorofilas y produccion organica primaria en Bahia de San Quintiri, B.C. Cienc. Mar. 1975, 2, 77–97. [Google Scholar] [CrossRef] [Green Version]
- Ibarra-Obando, S.E.; Camacho-Ibar, V.F.; Carriquiry, J.D.; Smith, S.V. Upwelling and lagoonal ecosystems of the dry Pacific coast of Baja California. In Coastal Marine Ecosystems of Latin America; Seelinger, U., Kjerfve, B., Eds.; Springer: Heidelberg, Germany, 2001; pp. 315–330. [Google Scholar]
- Hernández-Ayón, J.M.; Camacho-Ibar, V.F.; Mejía-Trejo, A.; Cabello-Pasini, A. Variabilidad del CO2 total durante eventos de surgencia en Bahía San Quintín, Baja California, México. In Carbono en Ecosistemas Acuáticos de México; Secretaría de Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, Centro de Investigaciones Científicas y de Educación de Ensenada: Ciudad de México, México, 2007; pp. 187–200. [Google Scholar]
- Sandoval-Gil, J.M.; Camacho-Ibar, V.F.; del Carmen Ávila-López, M.; Hernández-López, J.; Zertuche-González, J.A.; Cabello-Pasini, A. Dissolved inorganic nitrogen uptake kinetics and δ15N of Zostera marina L. (eelgrass) in a coastal lagoon with oyster aquaculture and upwelling influence. J. Exp. Mar. Biol. Ecol. 2015, 472, 1–13. [Google Scholar] [CrossRef]
- Hernández-López, J.; Camacho-Ibar, V.F.; Macías-Tapia, A.; McGlathery, K.J.; Daesslé, L.W.; Sandoval-Gil, J.M. Benthic N fixation in Zostera marina meadows in an upwelling-influenced coastal lagoon. Cienc. Mar. 2017, 43, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.P.; Carpenter, E.J.; Capone, D.G. N fixation and N isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol. Oceanogr. 2002, 47, 1617–1628. [Google Scholar] [CrossRef] [Green Version]
- Newell, S.E.; McCarthy, M.J.; Gardner, W.S.; Fulweiler, R.W. Sediment N fixation: A call for re-evaluating coastal N budgets. Estuaries Coast. 2016, 39, 1626–1638. [Google Scholar] [CrossRef]
- Detmers, J.; Brüchert, V.; Habicht, K.S.; Kuever, J. Diversity of S isotope fractionations by sulfate-reducing prokaryotes. Appl. Environ. Microbiol. 2001, 67, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Strauss, H. The isotopic composition of sedimentary S through time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 132, 97–118. [Google Scholar] [CrossRef]
- Invers, O.; Zimmerman, R.C.; Alberte, R.S.; Perez, M.; Romero, J. Inorganic carbon sources for seagrass photosynthesis: An experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Biol. Ecol. 2001, 265, 203–217. [Google Scholar] [CrossRef]
- Pedersen, O.; Binzer, T.; Borum, J. Sulphide intrusion in eelgrass (Zostera marina L.). Plant Cell Environ. 2004, 27, 595–602. [Google Scholar] [CrossRef]
- Kennedy, H.; Beggins, J.; Duarte, C.M.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycles 2010, 24, GB4026. [Google Scholar] [CrossRef] [Green Version]
- Oczkowski, A.; Markham, E.; Hanson, A.; Wigand, C. Carbon stable isotopes as indicators of coastal eutrophication. Ecol. Appl. 2014, 24, 457–466. [Google Scholar] [CrossRef]
- Ribas-Ribas, M.; Hernández-Ayón, J.M.; Camacho-Ibar, V.F.; Cabello-Pasini, A.; Mejia-Trejo, A.; Durazo, R.; Galindo-Bect, S.; Souza, A.J.; Forja, J.M.; Siqueiros-Valencia, A. Effects of upwelling, tides and biological processes on the inorganic carbon system of a coastal lagoon in Baja California. Estuarine. Coast. Shelf Sci. 2011, 95, 367–376. [Google Scholar] [CrossRef]
- Welsh, D.T. N fixation in seagrass meadows: Regulation, plant–bacteria interactions and significance to primary productivity. Ecol. Lett. 2000, 3, 58–71. [Google Scholar] [CrossRef]
- Moriarty, D.J.W.; Iverson, R.L.; Pollard, P.C. Exudation of organic carbon by the seagrass Halodule wrightii Aschers. And its effect on bacterial growth in the sediment. J. Exp. Mar. Biol. Ecol. 1986, 96, 115–126. [Google Scholar] [CrossRef]
- Holmer, M.; Andersen, F.Ø.; Nielsen, S.L.; Boschker, H.T. The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aquat. Bot. 2001, 71, 1–17. [Google Scholar] [CrossRef]
- Hemminga, M.A. The root/rhizome system of seagrasses: An asset and a burden. J. Sea Res. 1998, 39, 183–196. [Google Scholar] [CrossRef]
- Humphries, A.T.; Ayvazian, S.G.; Carey, J.C.; Hancock, B.T.; Grabbert, S.; Cobb, D.; Strobel, C.J.; Fulweiler, R.W. Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove N at comparable high rates. Front. Mar. Sci. 2016, 3, 74. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, J.M.; Hollibaugh, J.T.; Mortazavi, B. Living oysters and their shells as sites of nitrification and denitrification. Mar. Pollut. Bull. 2016, 112, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Arfken, A.; Song, B.; Bowman, J.S.; Piehler, M. Denitrification potential of the eastern oyster microbiome using a 16S rRNA gene based metabolic inference approach. PLoS ONE 2017, 12, e0185071. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.H.; Mok, J.S.; Lee, J.S.; An, S.U.; Lee, W.C.; Jung, R.H. Impacts of long-line aquaculture of Pacific oysters (Crassostrea gigas) on sulfate reduction and diffusive nutrient flux in the coastal sediments of Jinhae–Tongyeong, Korea. Mar. Pollut. Bull. 2013, 74, 187–198. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Ning, X. Reduced inorganic S in sediments of the mariculture region of Sanggou Bay, China. Aquacult. Environ. Interac. 2016, 8, 233–246. [Google Scholar] [CrossRef] [Green Version]
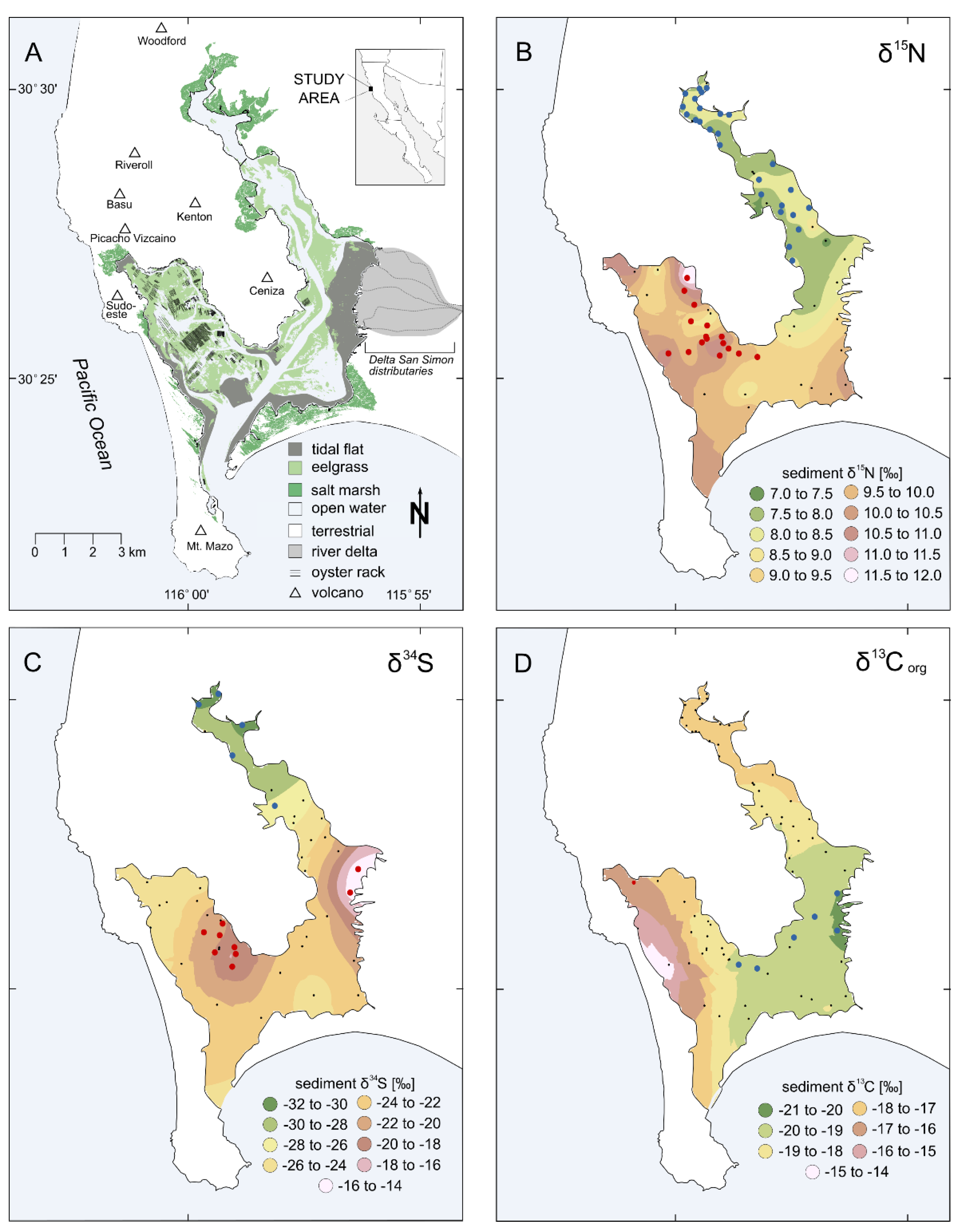
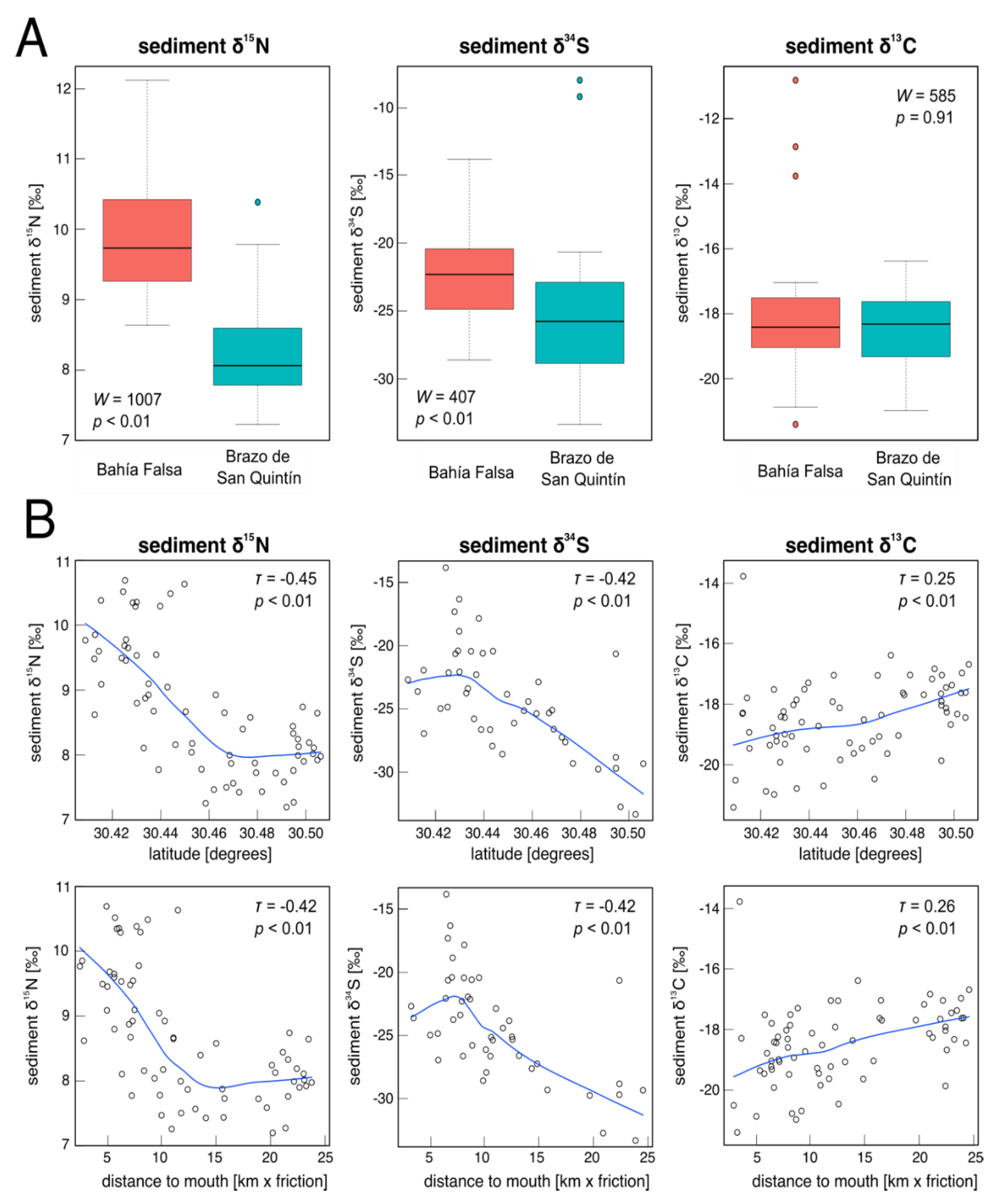
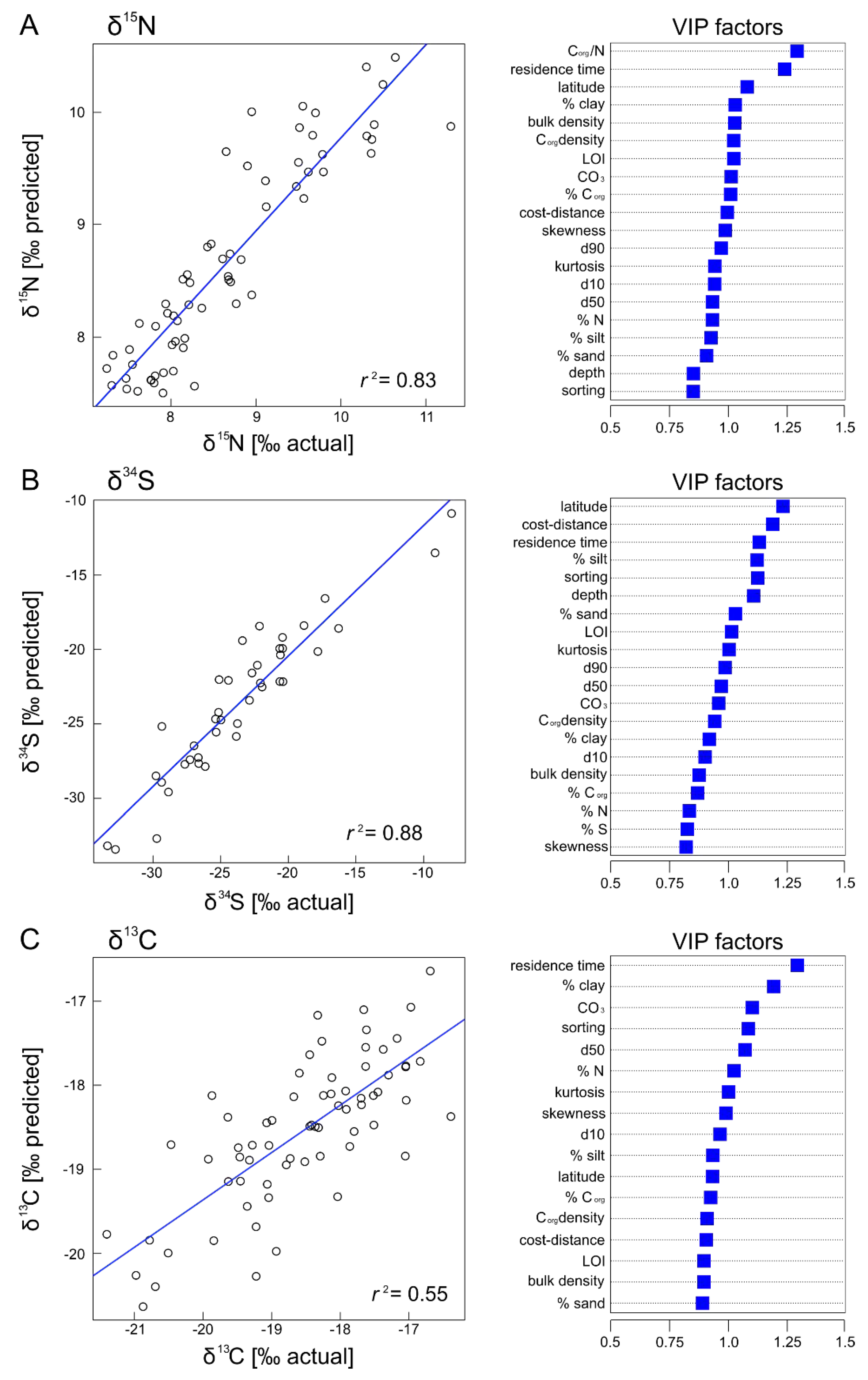
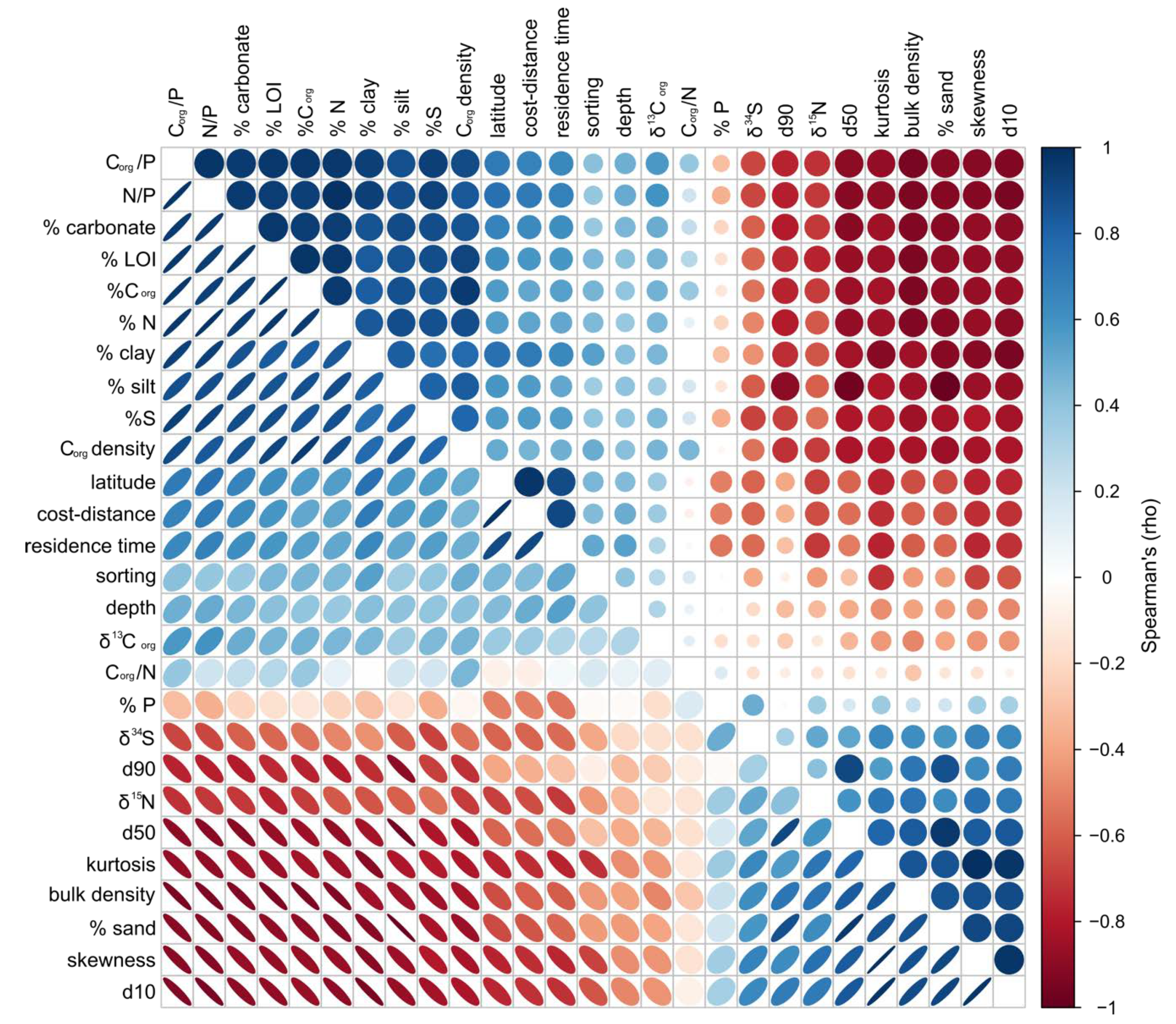
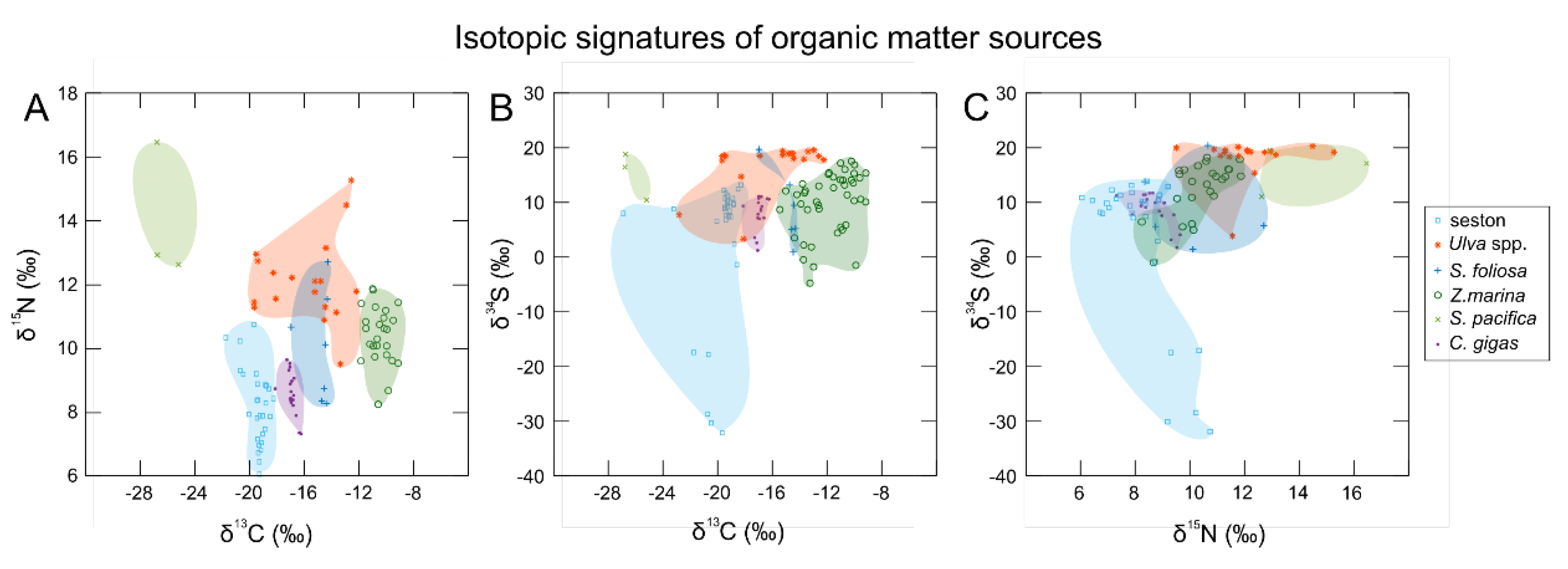
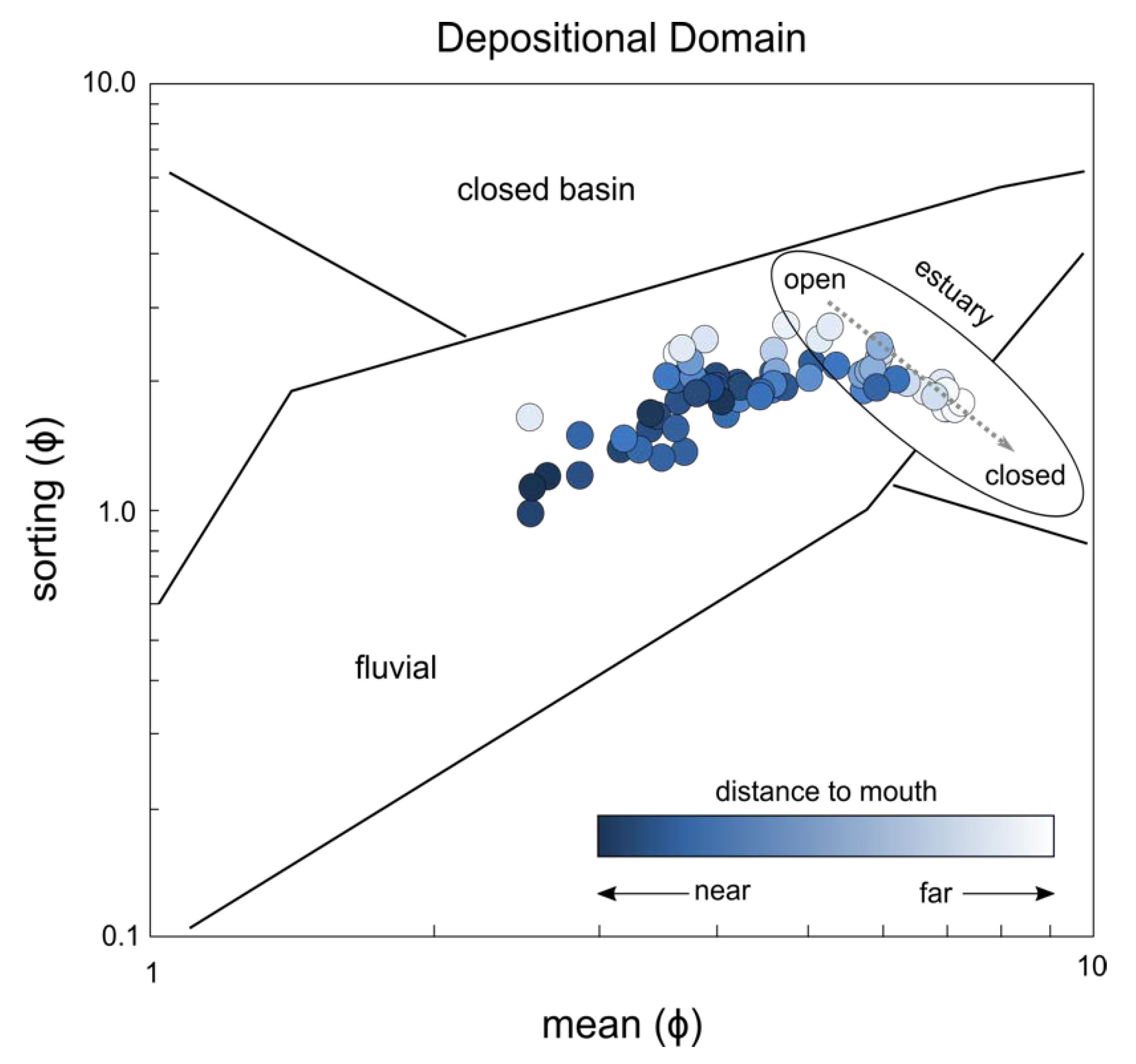

| Material | Collection Date | Number of Samples |
|---|---|---|
| Zostera marina | 9 July 2018 | 18 |
| Zostera marina | 29 November 2016 | 26 |
| Seston | 25 August 2017 | 14 |
| Seston | 10 July 2018 | 10 |
| Ulva spp. | 1 December 2016 | 17 |
| Crassostrea gigas† | 23 August 2017 | 18 |
| Spartina foliosa | 29 November 2016 | 6 |
| Salicornia pacifica | 29 November 2016 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, E.B.; Hinojosa-Corona, A.; Krause, J.R.; Herguera, J.C.; McDonnell, J.; Villegas Manríquez, K.R.; Gannon, M.E.; Gray, A.B. Lagoon Biogeochemical Processing is Reflected in Spatial Patterns of Sediment Stable Isotopic Ratios. J. Mar. Sci. Eng. 2020, 8, 874. https://doi.org/10.3390/jmse8110874
Watson EB, Hinojosa-Corona A, Krause JR, Herguera JC, McDonnell J, Villegas Manríquez KR, Gannon ME, Gray AB. Lagoon Biogeochemical Processing is Reflected in Spatial Patterns of Sediment Stable Isotopic Ratios. Journal of Marine Science and Engineering. 2020; 8(11):874. https://doi.org/10.3390/jmse8110874
Chicago/Turabian StyleWatson, Elizabeth Burke, Alejandro Hinojosa-Corona, Johannes R. Krause, Juan Carlos Herguera, Julianna McDonnell, Karen Raquel Villegas Manríquez, Michelle E. Gannon, and Andrew B. Gray. 2020. "Lagoon Biogeochemical Processing is Reflected in Spatial Patterns of Sediment Stable Isotopic Ratios" Journal of Marine Science and Engineering 8, no. 11: 874. https://doi.org/10.3390/jmse8110874





