Laboratory Culture-Based Characterization of the Resting Stage Cells of the Brown-Tide-Causing Pelagophyte, Aureococcus anophagefferens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Information and RSC Formation
2.2. Basic Morphological Observation
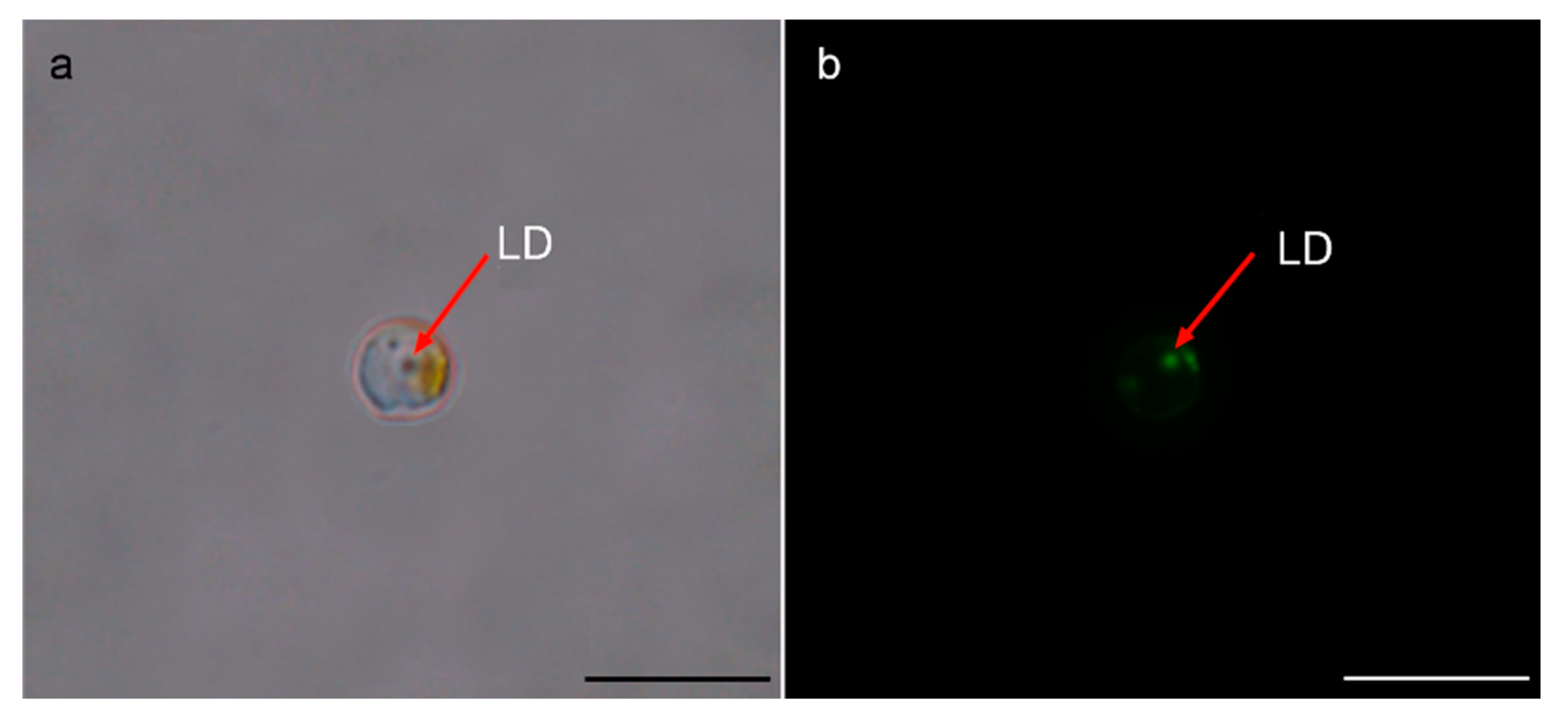
2.3. BODIPY 505/515 Fluorescence Staining
2.4. Adenosine Triphosphate (ATP) Assays
2.5. Germination Experiments
2.6. Statistical Analyses
3. Results
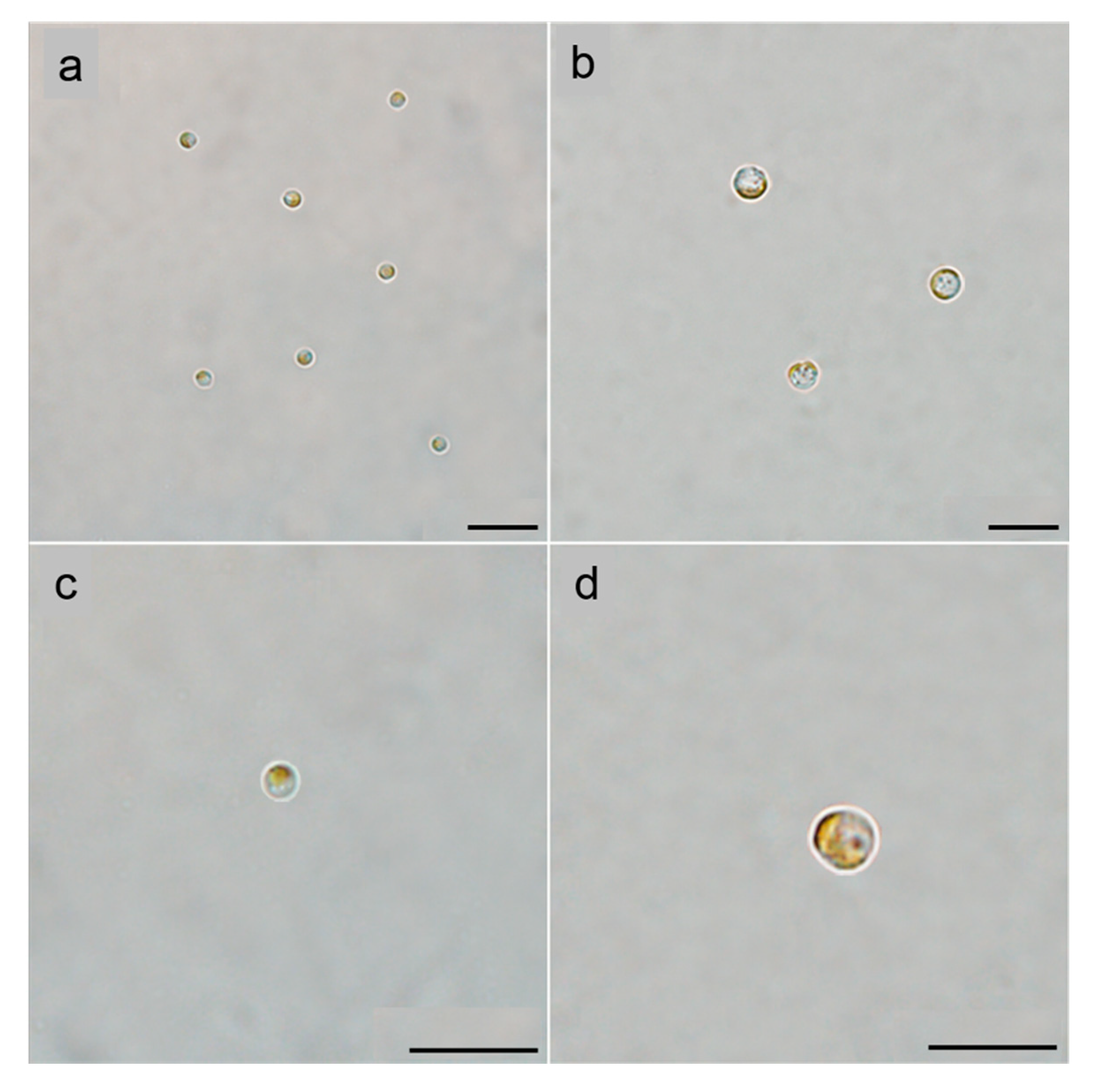
3.1. Morphological Characteristics of Resting Cells
3.2. Metabolic Activity of RSCs as Indicated in Cellular ATP Content
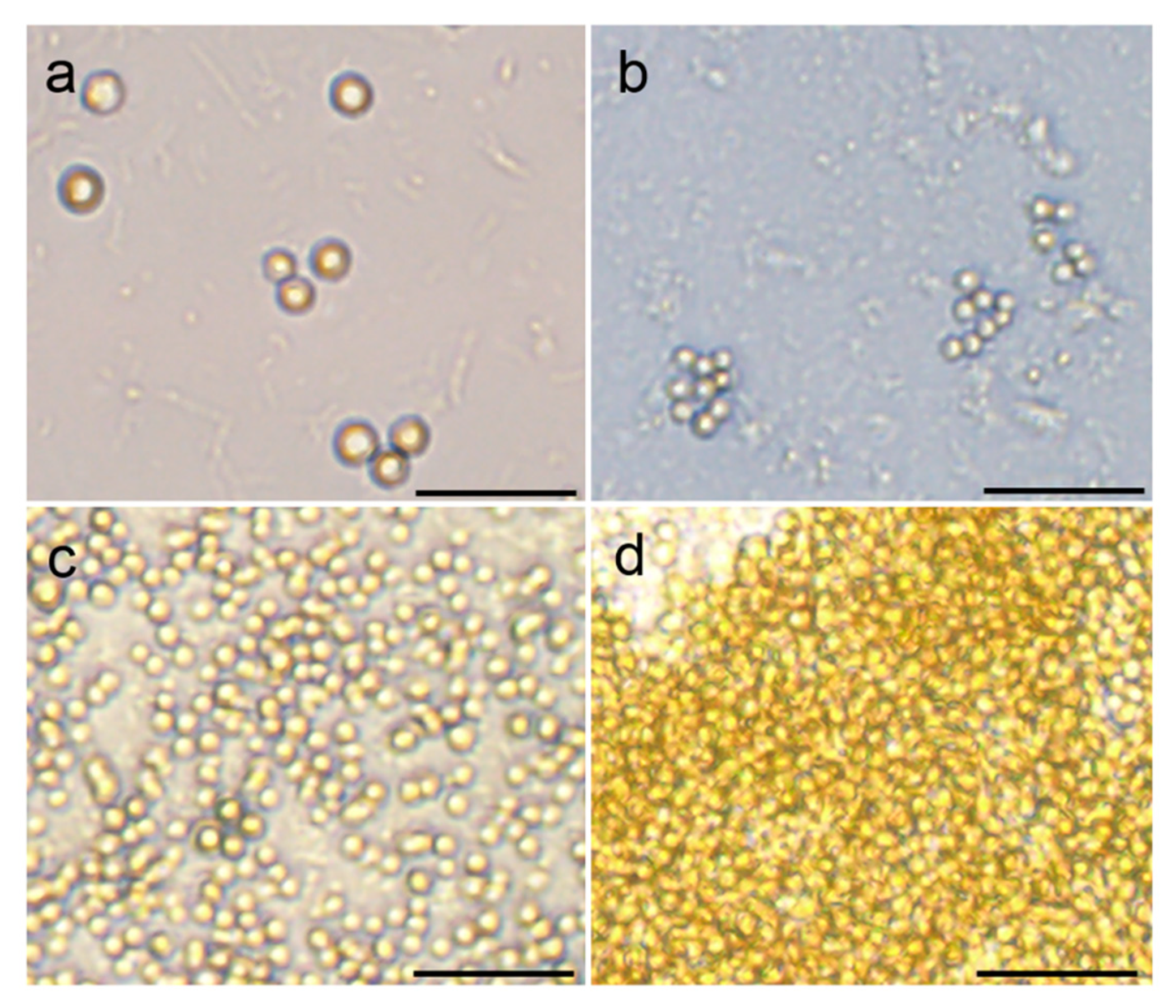
3.3. Resumption of Vegetative Growth from Resting State
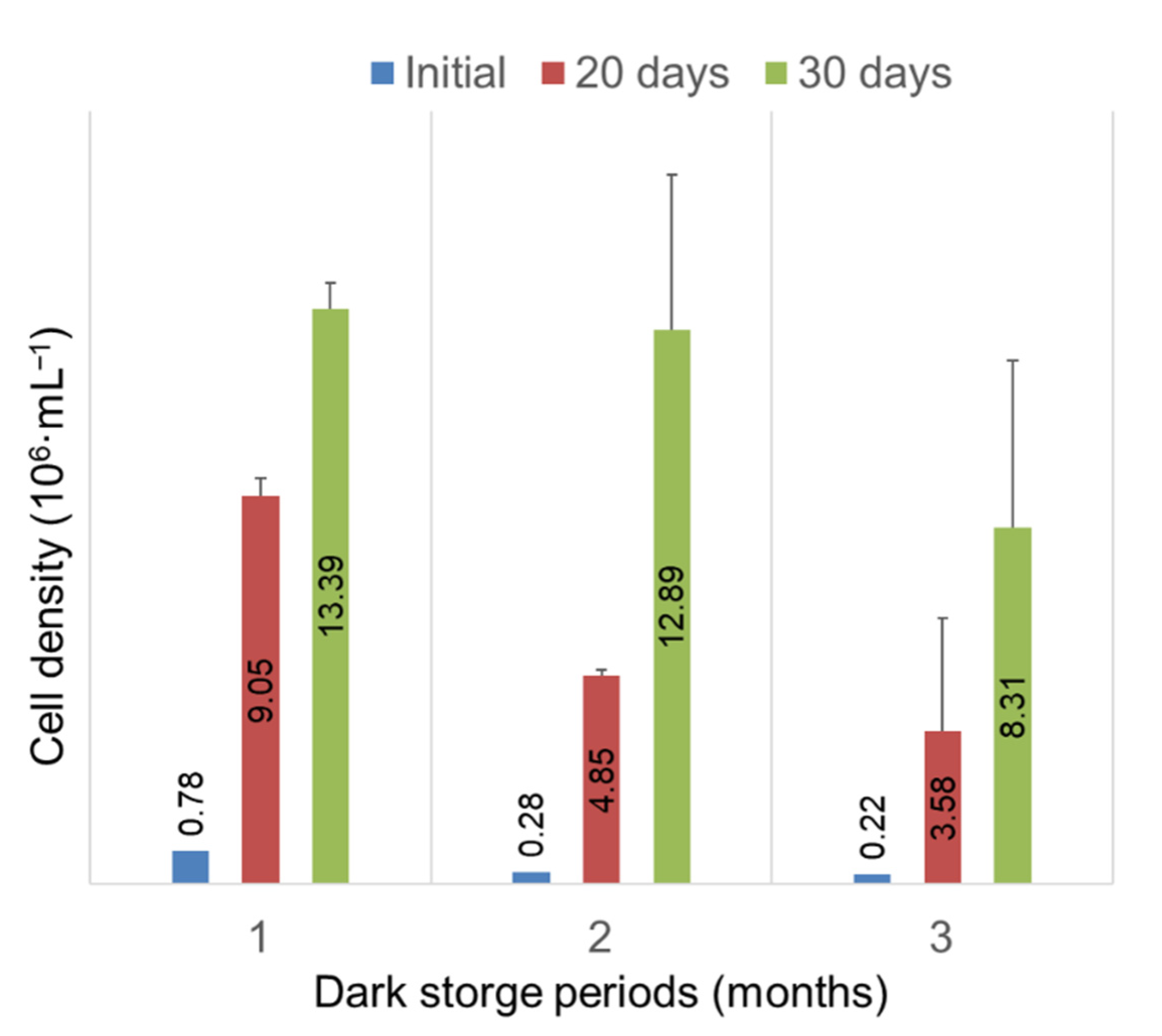
3.4. The Ability of RSCs to Survive Prolonged Darkness and Coldness
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gobler, C.J.; Sunda, W.G. Ecosystem disruptive algal blooms of the brown tide species, Aureococcus anophagefferens and Aureoumbra lagunensis. Harmful Algae 2012, 14, 36–45. [Google Scholar] [CrossRef]
- Sieburth, J.M.; Johnson, P.W.; Hargraves, P.E. Ultrastructure and ecology of Aureococcus anophageferens gen. et sp. nov. (Chrysophyceae): The dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, summer 1985. J. Phycol. 1988, 24, 416–425. [Google Scholar] [CrossRef]
- Cosper, E.M.; Dennison, W.; Milligan, A.; Carpenter, E.J.; Lee, C.; Holzapfel, J.; Milanese, L. An examination of environmental factors important to initiating and sustaining “Brown Tide” blooms. In Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tides and Other Unusual Blooms; Cosper, E.M., Bricelj, V.M., Carpenter, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 317–340. [Google Scholar]
- Olsen, P.S. Development and distribution of a brown-water algal bloom in Barnegat Bay, New Jersey with perspective on resources and other red tides in the region. In Novel Phytoplankton Blooms: Causes and Impacts of Recurrent Brown Tides and Other Unusual Blooms; Cosper, E.M., Bricelj, V.M., Carpenter, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 189–212. [Google Scholar]
- Popels, L.C.; Cary, S.C.; Hutchins, D.A.; Forbes, R.; Pustizzi, F.; Gobler, C.J.; Coyne, K.J. The use of quantitative polymerase chain reaction for the detection and enumeration of the harmful alga Aureococcus anophagefferens in environmental samples along the United States East Coast. Limnol. Oceanogr. Methods 2003, 1, 92–102. [Google Scholar] [CrossRef]
- Probyn, T.; Pitcher, G.; Pienaar, R.; Nuzzi, R. Brown tides and mariculture in Saldanha Bay, South Africa. Mar. Pollut. Bull. 2001, 42, 405–408. [Google Scholar] [CrossRef]
- Probyn, T.A.; Bernard, S.; Pitcher, G.C.; Pienaar, R.N. Ecophysiological studies on Aureococcus anophagefferens blooms in Saldanha Bay, South Africa. Harmful Algae 2010, 9, 123–133. [Google Scholar] [CrossRef]
- Kong, F.; Yu, R.; Zhang, Q.; Yan, T.; Zhou, M. Pigment characterization for the 2011 bloom in Qinhuangdao implicated “brown tide” events in China. Chin. J. Oceanol. Limnol. 2012, 30, 361–370. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Qiu, L.-M.; Yu, R.-C.; Kong, F.-Z.; Wang, Y.-F.; Yan, T.; Gobler, C.J.; Zhou, M.-J. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Smayda, T.J. Reflections on the ballast water dispersal—Harmful algal bloom paradigm. Harmful Algae 2007, 6, 601–622. [Google Scholar] [CrossRef]
- Doblin, M.A.; Popels, L.C.; Coyne, K.J.; Hutchins, D.A.; Cary, S.C.; Dobbs, F.C. Transport of the harmful bloom alga Aureococcus anophagefferens by oceangoing ships and coastal boats. Appl. Environ. Microbiol. 2004, 70, 6495–6500. [Google Scholar] [CrossRef] [Green Version]
- Popels, L.C.; Hutchins, D.A. Factors affecting dark survival of the brown tide alga Aureococcus anophagefferens (Pelagophyceae). J. Phycol. 2002, 38, 738–744. [Google Scholar] [CrossRef]
- Popels, L.C.; MacIntyre, H.L.; Warner, M.E.; Zhang, Y.; Hutchins, D.A. Physiological responses during dark survival and recovery in Aureococcus anophagefferens (Pelagophyceae). J. Phycol. 2007, 43, 32–42. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; de Salas, M.F. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae 2007, 6, 465–485. [Google Scholar] [CrossRef]
- Anderson, D.M.; Coats, D.W.; Tyler, M.A. Encystment of the dinoflagellate Gyrodinium uncatenum: Temperature and nutrient effects. J. Phycol. 1985, 21, 200–206. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y. Technical Guide for Modern Dinoflagellate Cyst Study; WESTPAC-HAB/WEATPAC/IOC; Japanese Society for the Promotion of Science: Tokyo, Japan, 2000; pp. 1–97. [Google Scholar]
- Bravo, I.; Figueroa, R.I. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms 2014, 2, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Smayda, T.J.; Mitchell-Innes, B. Dark survival of autotrophic, planktonic marine diatoms. Mar. Biol. 1974, 25, 195–202. [Google Scholar] [CrossRef]
- Dehning, I.; Tilzer, M.M. Survival of Scenedesmus acuminatus (Chlorophyceae) in darkness. J. Phycol. 1989, 25, 509–514. [Google Scholar] [CrossRef]
- Deventer, B.; Heckman, C.W. Effects of prolonged darkness on the relative pigment content of cultured diatoms and green algae. Aquat. Sci. 1996, 58, 241–252. [Google Scholar] [CrossRef]
- Wilken, S.; Huisman, J.; Naus-Wiezer, S.; Van Donk, E. Mixotrophic organisms become more heterotrophic with rising temperature. Ecol. Lett. 2013, 16, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, M.; Ribeiro, S. The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’. Biol. Rev. 2018, 93, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, R.I.; Estrada, M.; Garcés, E. Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 2018, 73, 44–57. [Google Scholar] [CrossRef] [PubMed]
- von Dassow, P.; Montresor, M. Unveiling the mysteries of phytoplankton life cycles: Patterns and opportunities behind complexity. J. Plankton Res. 2011, 33, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.V.; Dodge, J.D.; Heaney, S.I. Cyst formation in the freshwater dinoflagellate Ceratium hirundinella (Dinophyceae). J. Phycol. 1982, 18, 121–129. [Google Scholar] [CrossRef]
- Anderson, O.R. The ultrastructure and cytochemistry of resting cell formation in Amphora coffaeformis (Bacillariophyceae). J. Phycol. 1975, 11, 272–281. [Google Scholar] [CrossRef]
- Sicko-Goad, L.; Stoermer, E.F.; Kociolek, J.P. Diatom resting cell rejuvenation and formation: Time course, species records and distribution. J. Plankton Res. 1989, 11, 375–389. [Google Scholar] [CrossRef]
- Kang, Y.; Tang, Y.-Z.; Taylor, G.T.; Gobler, C.J. Discovery of a resting stage in the harmful, brown-tide-causing pelagophyte, Aureoumbra lagunensis: A mechanism potentially facilitating recurrent blooms and geographic expansion. J. Phycol. 2017, 53, 118–130. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Ma, Z.; Hu, Z.; Deng, Y.; Yang, A.; Lin, S.; Yi, L.; Chai, Z.; Gobler, C.J. 3000 km and 1500-year presence of Aureococcus anophagefferens reveals indigenous origin of brown tides in China. Mol. Ecol. 2019, 28, 4065–4076. [Google Scholar] [CrossRef]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals, Proceedings of the 1st Conference on Culture of Marine Invertebrate Animals Greenport, New York, October, 1972; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Wu, S.; Zhang, B.; Huang, A.; Huan, L.; He, L.; Lin, A.; Niu, J.; Wang, G. Detection of intracellular neutral lipid content in the marine microalgae Prorocentrum micans and Phaeodactylum tricornutum using Nile red and BODIPY 505/515. J. Appl. Phycol. 2014, 26, 1659–1668. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 2012, 20, 71–80. [Google Scholar] [CrossRef]
- Sandgren, C.D. Characteristics of sexual and asexual resting cyst (statospore) formation in Dinobryon cylindricum imhof (Chrysophyta). J. Phycol. 1981, 17, 199–210. [Google Scholar] [CrossRef]
- Sandgren, C.D. Chrysophyte reproduction and resting cysts: A paleolimnologist’s primer. J. Paleolimnol. 1991, 5, 1–9. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, B.S.; Wang, P.; Kim, J.H.; Youn, S.H.; Han, M.-S. Cyst morphology and germination in Heterosigma akashiwo (Raphidophyceae). Phycologia 2015, 54, 435–439. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea: A potential mechanism contributing to the ubiquitous distribution of a harmful alga. J. Phycol. 2015, 51, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.Y.; Hu, Z.; Liu, Y.Y.; Tang, Y.Z. Proof of homothally of Pheopolykrikos hartmannii and details of cyst germination process. J. Oceanol. Limnol. 2020, 38, 114–123. [Google Scholar] [CrossRef]
- Lomas, M.W.; Gobler, C.J. Aureococcus anophagefferens research: 20 years and counting. Harmful Algae 2004, 3, 273–277. [Google Scholar] [CrossRef]
- Wazniak, C.E.; Glibert, P.M. Potential impacts of brown tide, Aureococcus anophagefferens, on juvenile hard clams, Mercenaria mercenaria, in the Coastal Bays of Maryland, USA. Harmful Algae 2004, 3, 321–329. [Google Scholar] [CrossRef]
- Gobler, C.J. Harmful Algal Species Fact Sheet: Aureococcus anophagefferens Hargraves et Sieburth & Aureoumbra lagunensis De Yoe et Stockwell—Brown Tides. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 583–584. [Google Scholar]
- Huang, B.; Liang, Y.; Pan, H.; Xie, L.; Jiang, T.; Jiang, T. Hemolytic and cytotoxic activity from cultures of Aureococcus anophagefferens—A causative species of brown tides in the north-western Bohai Sea, China. Chemosphere 2020, 247, 125819. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Bravo, I.; Garcés, E. Effects of nutritional factors and different parental crosses on the encystment and excystment of Alexandrium catenella (Dinophyceae) in culture. Phycologia 2005, 44, 658–670. [Google Scholar] [CrossRef]
- Anderson, D.M.; Taylor, C.D.; Armbrust, E.V. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 1987, 32, 340–351. [Google Scholar] [CrossRef]
- Itakura, S.; Nagasaki, K.; Yamaguchi, M.; Imai, I. Cyst formation in the red tide flagellate Heterosigma akashiwo (Raphidophyceae). J. Plankton Res. 1996, 18, 1975–1979. [Google Scholar] [CrossRef]
- Anderson, D.M. Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae). J. Phycol. 1980, 16, 166–172. [Google Scholar] [CrossRef]
- Mertens, K.N.; Gu, H.; Gurdebeke, P.R.; Takano, Y.; Clarke, D.; Aydin, H.; Li, Z.; Pospelova, V.; Shin, H.H.; Li, Z.; et al. A review of rare, poorly known, and morphologically problematic extant marine organic-walled dinoflagellate cyst taxa of the orders Gymnodiniales and Peridiniales from the Northern Hemisphere. Mar. Micropaleontol. 2020, 159, 101773. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Deng, Y.; Tang, Y.Z. Evidence for resting cyst production in the cosmopolitan toxic dinoflagellate Karlodinium veneficum and the cyst distribution in the China seas. Harmful Algae 2020, 93, 101788. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, N.; Head, M.J.; Limoges, A.; Pospelova, V.; Mertens, K.N.; Matthiessen, J.; De Schepper, S.; de Vernal, A.; Eynaud, F.; Londeix, L.; et al. An overview and brief description of common marine organic-walled dinoflagellate cyst taxa occurring in surface sediments of the Northern Hemisphere. Mar. Micropaleontol. 2020, 159, 101814. [Google Scholar] [CrossRef]
- Limoges, A.; Van Nieuwenhove, N.; Head, M.J.; Mertens, K.N.; Pospelova, V.; Rochon, A. A review of rare and less well known extant marine organic-walled dinoflagellate cyst taxa of the orders Gonyaulacales and Suessiales from the Northern Hemisphere. Mar. Micropaleontol. 2020, 159, 101801. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Deng, Y.; Tang, Y.Z. Evidence for production of sexual resting cysts by the toxic dinoflagellate Karenia mikimotoi in clonal cultures and marine sediments. J. Phycol. 2020, 56, 121–134. [Google Scholar] [CrossRef]
- Bibby, B.T.; Dodge, J.D. The encystment of a freshwater dinoflagellate: A light and electron-microscopical study. Br. Phycol. Bull. 1972, 7, 85–100. [Google Scholar] [CrossRef]
- Loeblich, A.R.; Loeblich, L.A. 13—Dinoflagellate Cysts. In Dinoflagellates; Spector, D.L., Ed.; Academic Press: San Diego, CA, USA, 1984; pp. 443–480. [Google Scholar]
- Bloch, K. Sterol molecule: Structure, biosynthesis, and function. Steroids 1992, 57, 378–383. [Google Scholar] [CrossRef]
- Volkman, J. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar] [CrossRef]
- Pichrtová, M.; Arc, E.; Stöggl, W.; Kranner, I.; Hájek, T.; Hackl, H.; Holzinger, A. Formation of lipid bodies and changes in fatty acid composition upon pre-akinete formation in Arctic and Antarctic Zygnema (Zygnematophyceae, Streptophyta) strains. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [Green Version]
- Barati, B.; Gan, S.Y.; Lim, P.E.; Beardall, J.; Phang, S.M. Green algal molecular responses to temperature stress. Acta Physiol. Plant. 2019, 41, 26. [Google Scholar] [CrossRef]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.E.; Morris, I. Synthesis of lipid during photosynthesis by phytoplankton of the Southern Ocean. Science 1980, 207, 197–199. [Google Scholar] [CrossRef]

| Cell Type | ATP Content (nmol/107 Cells) |
|---|---|
| Resting stage cells | 2.9 ± 0.5 |
| Vegetative cells | 31.6 ± 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Hu, Z.; Deng, Y.; Shang, L.; Gobler, C.J.; Tang, Y.Z. Laboratory Culture-Based Characterization of the Resting Stage Cells of the Brown-Tide-Causing Pelagophyte, Aureococcus anophagefferens. J. Mar. Sci. Eng. 2020, 8, 1027. https://doi.org/10.3390/jmse8121027
Ma Z, Hu Z, Deng Y, Shang L, Gobler CJ, Tang YZ. Laboratory Culture-Based Characterization of the Resting Stage Cells of the Brown-Tide-Causing Pelagophyte, Aureococcus anophagefferens. Journal of Marine Science and Engineering. 2020; 8(12):1027. https://doi.org/10.3390/jmse8121027
Chicago/Turabian StyleMa, Zhaopeng, Zhangxi Hu, Yunyan Deng, Lixia Shang, Christophere J. Gobler, and Ying Zhong Tang. 2020. "Laboratory Culture-Based Characterization of the Resting Stage Cells of the Brown-Tide-Causing Pelagophyte, Aureococcus anophagefferens" Journal of Marine Science and Engineering 8, no. 12: 1027. https://doi.org/10.3390/jmse8121027
APA StyleMa, Z., Hu, Z., Deng, Y., Shang, L., Gobler, C. J., & Tang, Y. Z. (2020). Laboratory Culture-Based Characterization of the Resting Stage Cells of the Brown-Tide-Causing Pelagophyte, Aureococcus anophagefferens. Journal of Marine Science and Engineering, 8(12), 1027. https://doi.org/10.3390/jmse8121027






