Effects of Temperature on the Survival and Larval Development of Deiratonotus Japonicus (Brachyura, Camptandriidae) as a Biological Indicator
Abstract
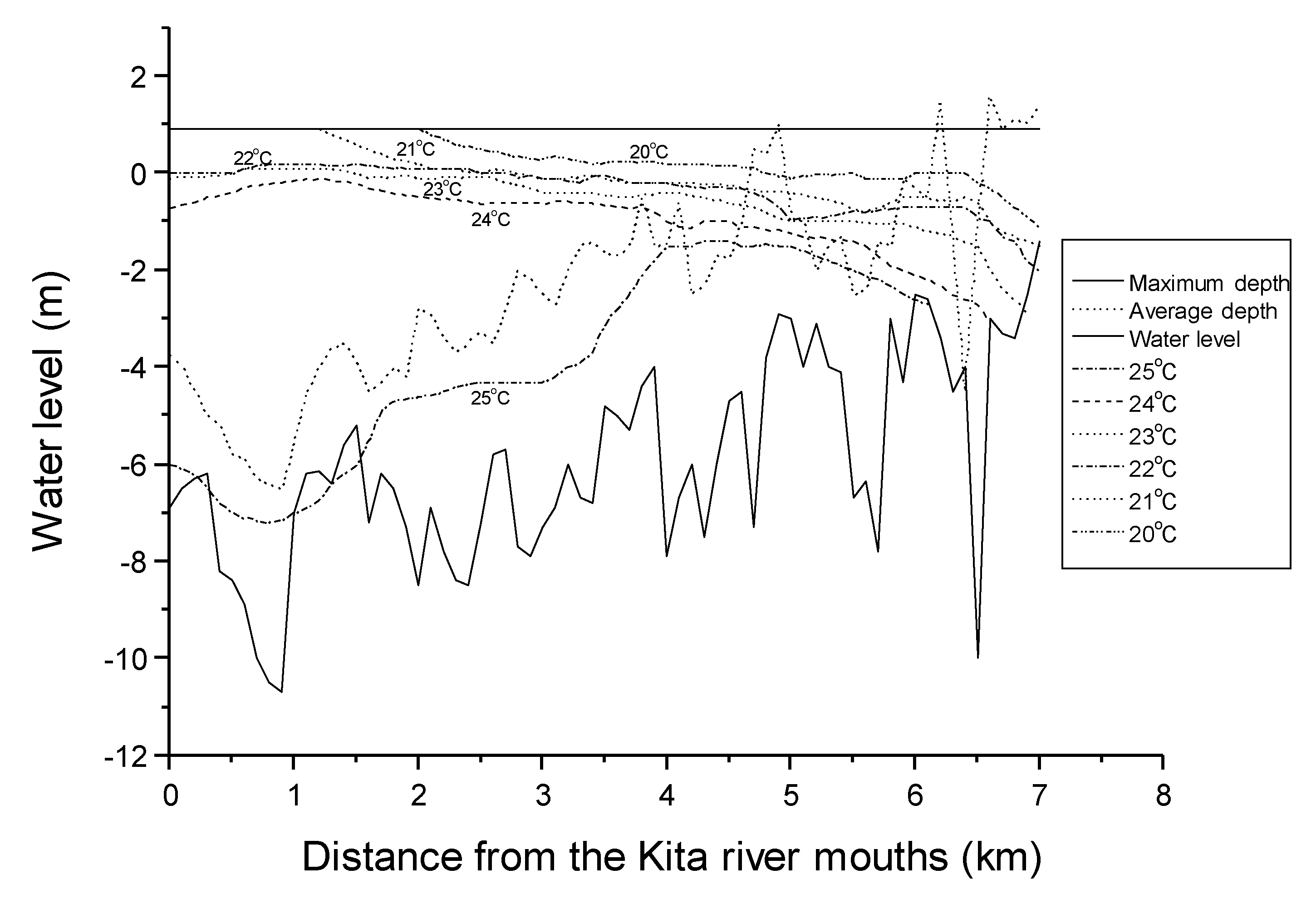
:1. Introduction
2. Materials and Methods
3. Results
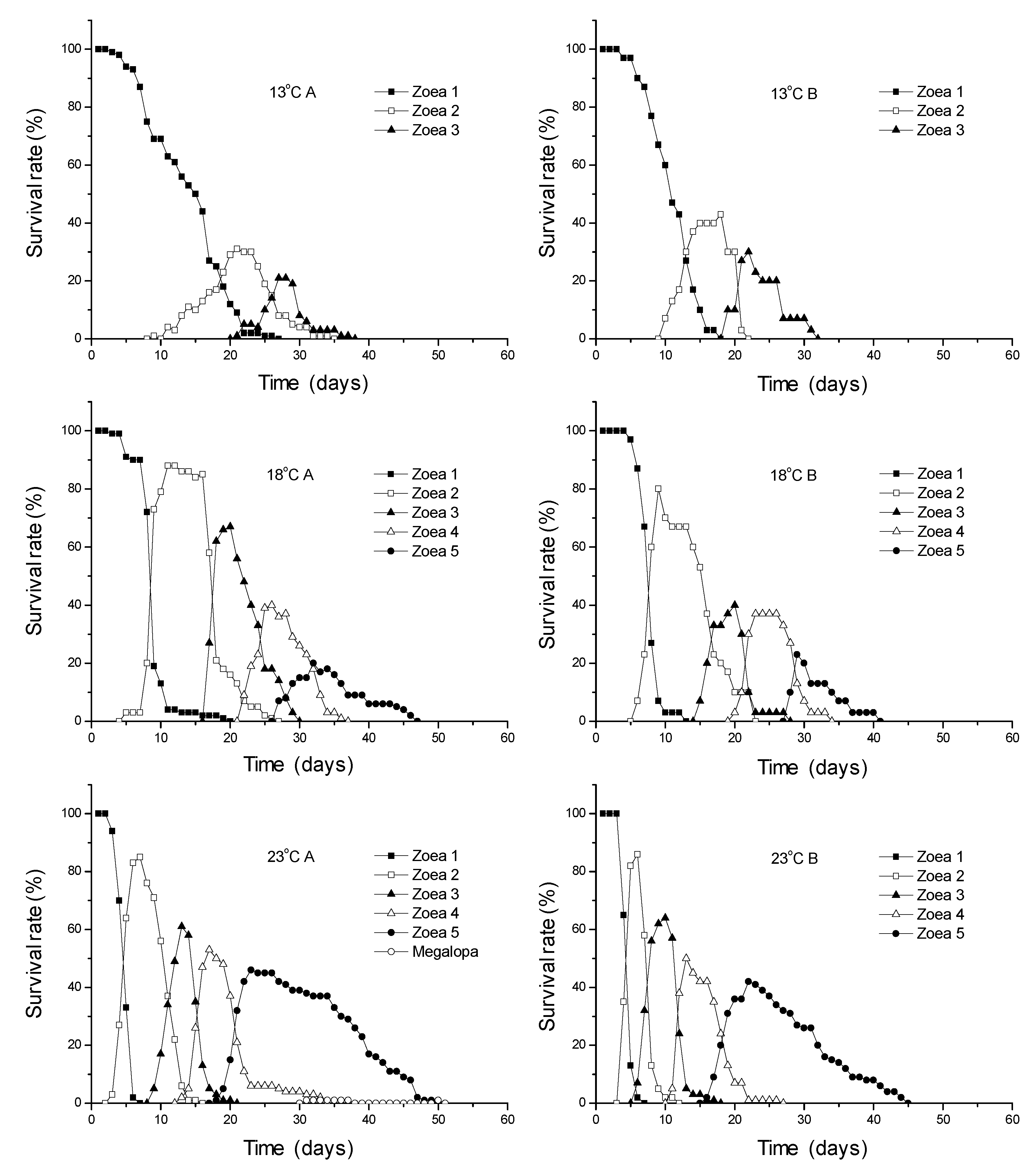
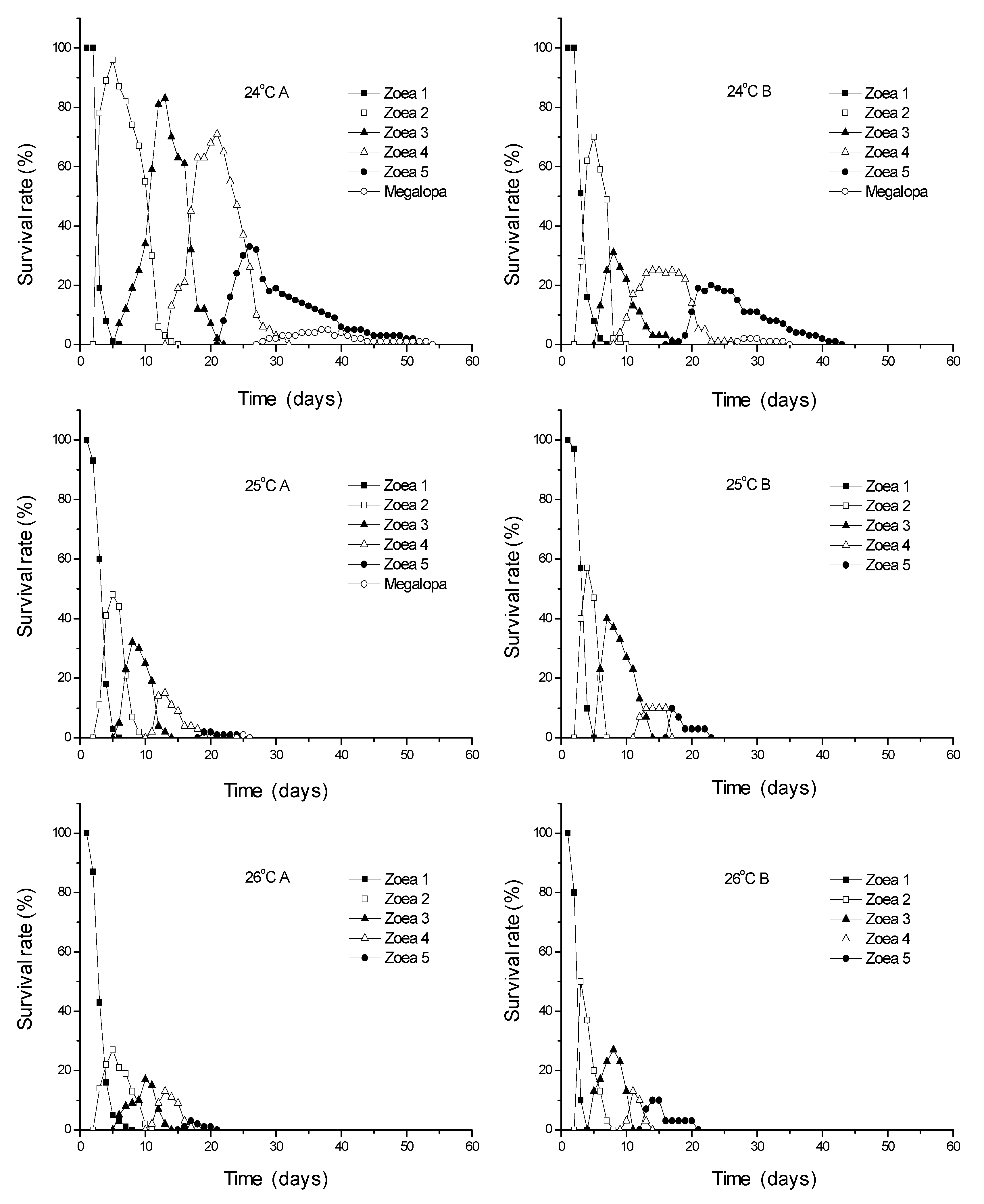
3.1. Survival Rates
3.2. Growth and Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakai, T. Brachyura from the coast of Kyushu, Japan. Sci. Rep. Tokyo Bunrika Daigaku 1934, 1, 321–322. [Google Scholar]
- Available online: https://www.env.go.jp/en/index.html (accessed on 24 January 2019).
- Terada, M. Early zoeal stage of Deiratonotus japonicas (Sakai, 1934) (Ocypodidae, Camptandriinae) obtained under laboratory conditions. Crustacean Res. 1995, 24, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, H.; Kusuda, T.; Lee, S.Y.; Hara, A.; Murakami, K. Study on the influence of water quality and the environmental condition of habitat of Deiratonotus japonicus in the Kita River. Env. Eng. Res. 2000, 37, 173–181. [Google Scholar]
- Nakasone, S.; Irei, M. Ocypodidae. In The flora and Fauna of Inland Waters in the Ryukyu Island; Nishida, M., Shikatani, N., Shokita, S., Eds.; Tokai University Press: Tokyo, Japan, 2003; pp. 266–272. [Google Scholar]
- Kawamoto, M.; Wada, K.; Kawane, M.; Kamada, M. Population subdivision of the Brackish-water crab Deiratonotus cristatus on the Japanese Coast. Zool. Sci. 2012, 29, 21–29. [Google Scholar] [CrossRef]
- Yamanishi, H.; Kusuda, T.; Hirata, M.; Oh, I.K.; Lee, S.Y. Characteristics of crossing distribution and the preference conditions on habitation of Deiratonotus japonicus. Env. Eng. Res. 2001, 38, 1–11. [Google Scholar]
- Kwanae, M.; Wada, K.; Kitaura, J.; Watanabe, K. Taxonomic re-examination of the two camptandriid crab species Deiratonotus japonicus (Sakai, 1934) and D. tondensis Sakai, 1983, and genetic differentiation among their local populations. J. Nat. Hist. 2005, 39, 3903–3918. [Google Scholar] [CrossRef]
- Kwanae, M.; Wada, K.; Watanabe, K. Comparisons of genetic population structures in four intertidal brachyuran species of contrasting habitat characteristics. Mar. Biol. 2008, 156, 193–203. [Google Scholar] [CrossRef]
- Waiho, K.; Fazhan, H.; Quinitio, E.T.; Baylon, J.C.; Fujaya, Y.; Azmie, G.; Wu, Q.; Shi, X.; Ikhwanuddin, M.; Ma, H. Larval rearing of mud crab (Scylla): What lies ahead. Aquaculture 2018, 493, 37–50. [Google Scholar] [CrossRef]
- Gimenez, L.; Anger, K. Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J. Exp. Mar. Bio. Ecol. 2001, 260, 241–257. [Google Scholar] [CrossRef]
- Gimenez, L.; Anger, K. Larval performance in an estuarine crab, Chasmagnathus granulate, is a consequence of both larval and embryonic experience. Mar. Ecol. Prog. Ser. 2003, 249, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, L.; Torres, G. Larval growth in the estuarine crab Chasmagnathus granulate: The importance of salinity experienced during embryonic development, and the initial larval biomass. Mar. Bio. 2002, 141, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.D.; Bert, T.M.; Tweedale, W.A.; Torres, J.J.; Lindberg, W.J. The effects of temperature and salinity on survival and development of early life stage Florida stone crabs Menipp mercenaria (Say). J. Exp. Mar. Biol. Ecol. 1992, 157, 115–136. [Google Scholar] [CrossRef]
- Thomas, C.W.; Clear, B.J.; Hart, P.R. The effect of temperature on survival, growth, feeding and metabolic activity of the southern rock lobster, Jasus edwardsii. Aquculture 2000, 185, 73–84. [Google Scholar] [CrossRef]
- Newman, B.K.; Papadopoulos, I.; Vorsatz, J.; Wooldridge, T.H. Influence of temperature on the larval development of Upogebia Africana and U. capensis (Decapoda: Thalassinidae: Upogebiidae) in the laboratory. Mar. Ecol. Prog. Ser. 2006, 325, 165–180. [Google Scholar] [CrossRef]
- Webb, J.B.; Eckert, G.L.; Shirley, T.C.; Tamone, S.L. Changes in zoeae of the snow crab, Chionoecetes opilio, with variation in incubation temperature. J. Exp. Mar. Biol. Ecol. 2006, 339, 96–103. [Google Scholar] [CrossRef]
- Yamamoto, T.; Jinbo, T.; Hamasaki, K. Intrinsic optimum temperature for the development of decapod crustacean larvae based on a thermodynamic model. J. Crustacean Biol. 2017, 37, 272–277. [Google Scholar] [CrossRef]
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.D.S.; Abol-Munafi, A.B. Effects of climate-induced water temperature changes on the life history of brachyuran crabs. Rev. Aquacult. 2019, 1–6. [Google Scholar] [CrossRef]
- Ohashi, K.; Hamasaki, K.; Dan, S.; Kitada, S. Artificial incubation and hatching of embryos of the coconut crab Birgus latro (Decapoda: Anomura: Coenobitidae). Crustacean Res. 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The principles and practice of statistics in biological research; Freeman: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Anger, K.; Thatje, S.; Lovrich, G.; Calcagno, J. Larval and early juvenile development of Paralomis granulsa reared at different temperatures: Tolerance of cold and food limitation in a lithodid crab from high latitudes. Mar. Ecol. Prog. Ser. 2003, 253, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Dawirs, R.R. Effects of temperature and salinity on larval development of Pagurus bernhardus (Decapoda, Paguridae). Mar. Ecol. Prog. Ser. 1979, 1, 323–329. [Google Scholar] [CrossRef]
- Kogane, T.; Hamasaki, K.; Nogami, K. Effect of temperature on survival and developmental period of larval snow crab Chionoecetes opilio (Brachyura: Majidae) reared in the laboratory. Nippon Suisan Gakkaishi 2005, 71, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Eggleston, D.B.; Armstrong, D.A. Pre- and post-settlement determinant of estuarine Dungeness crab recruitment. Ecol. Monogr. 1995, 65, 193–216. [Google Scholar] [CrossRef]
- Epifanio, C.E.; Little, K.T.; Rowe, P.M. Dispersal and recruitment of fiddler crab larvae in the Delaware River estuary. Mar. Ecol. Prog. Ser. 1988, 43, 181–188. [Google Scholar] [CrossRef]
- Rothlisberg, P.C. Larval transport in coastal crustacean three case histories. In Lecture Notes on Coastal and Estuarine Studies; Jansson, B.O., Ed.; Springer: Berlin, Germany, 1988; Volume 22, pp. 273–290. [Google Scholar]
- DeVries, M.C.; Tankersley, R.A.; Forward, R.B., Jr.; Kirby-Smith, W.W.; Luettich, R.A. Abundance of estuarine crab larvae is associated with tidal hydrologic variables. Mar. Biol. 1994, 118, 403–413. [Google Scholar] [CrossRef]
- Zeng, C.; Naylor, E. Occurrence in coastal waters and endogenous tidal swimming rhythms of late megalopae of the shore crab Carcinus maenas: Implications for onshore recruitment. Mar. Ecol. Prog. Ser. 1996, 136, 69–79. [Google Scholar] [CrossRef]
- Forward, R.B., Jr.; Lohmann, K.J.; Cronin, T.W. Rhythms in larval release by an estuarine crab (Rhithropanopeus harrisii). Biol. Bull. Mar. Biol. Lab. Woods Hole 1982, 163, 287–300. [Google Scholar] [CrossRef]
- De Vries, M.C.; Forward, R.B., Jr. Rhythms in larval release of the sublittoral crab Neopanope sayi and the supralittoral crab Sesarma cinereum (Decapoda: Brachyura). Mar. Biol. 1989, 100, 241–248. [Google Scholar] [CrossRef]
- Forward, R.B., Jr. Larval release rhythms of decapod crustaceans: An overview. Bull. Mar. Sci. 1987, 41, 165–176. [Google Scholar]
- Ziegler, T.A.; Forward, R.B., Jr. Larval release rhythm of the mole crab Emerita talpoida (Say). Biol. Bull. Mar. Biol. Lab. 2005, 209, 194–203. [Google Scholar] [CrossRef] [Green Version]
- McEward, L. Ecology of Marine Invertebrate Larvae, 1st ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 34–49. [Google Scholar]
- Hartnoll, R.G.; Mohamedeen, H. Laboratory growth of the larvae of six British crabs. J. Exp. Mar. Biol. Ecol. 1987, 107, 155–170. [Google Scholar] [CrossRef]
- Litulo, C. Population structure and reproductive biology of the fiddler crab Uca inverse (Hoffman, 1874) (Brachyura: Ocypodidae). Acta Oecol. 2005, 27, 135–141. [Google Scholar] [CrossRef]
- Calcagno, J.A.; Lovrich, G.A.; Thatje, S.; Nettelmann, U.; Anger, K. First year growth in the lithodids Lithodes santolla and Paralomis granulose reared at different temperatures. J. Sea Res. 2005, 54, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Shirley, S.M.; Shirley, T.C.; Rice, S.D. Latitudinal variation in the Dungeness crab. Cancer magister, zoeal morphology explained by incubation temperature. Mar. Biol. 1987, 95, 371–376. [Google Scholar]
- Efrizal, A.A.; Kamarudin, M.S.; Saad, C.R. Effects of temperature on the incubation period and reproductive performance of berried female blue swimming crab, portunus pelagicus (Linnaeus, 1758) under cultured conditions. J. Fish. Hydrobio. 2006, 1, 23–27. [Google Scholar]
- Dawirs, R.R. Temperature and larval development of Carcinus maenas (Decapoda) in the laboratory, predictions of larval dynamics in the sea. Mar. Ecol. Prog. Ser. 1985, 24, 297–302. [Google Scholar] [CrossRef]
- Ott, F.S.; Forward, R.B., Jr. The effect of temperature on phototaxis and geotaxis by larvae of the crab Rhithropanopeus harrisi (Gould). J. Exp. Mar. Biol. Ecol. 1976, 23, 97–107. [Google Scholar] [CrossRef]
- Forward, R.B., Jr. Behavioral responses of crustacean larvae to rates of salinity change. Biol. Bull. Mar. Biol. Lab. Woods Hole 1989, 176, 229–238. [Google Scholar] [CrossRef]
- Forward, R.B., Jr. Behavioral responses of crustacean larvae to rates of temperature change. Biol. Bull. 1990, 178, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hiu, Y.; Oh, I.K.; Iyooka, H.; Tagomori, K.; Hirata, M.; Lee, S.Y.; Yamanishi, H.; Kusuda, T. Changes of habitat characteristics and evaluation of habitat environment of Deiratonotus japonicus in the Kita River, Japan. In Proceedings of the IWA WWC 2004 Conference, Marrakeeh, Morocco, 19–24 September 2004. [Google Scholar]
- Perez-Miguel, M.; González-Ortegón, E.; Drake, P.; Navas, J.; Cuesta, J.A. Temperature and salinity tolerance of the larval stages of the African pea crab Afropinnotheres monodi Manning, 1993: Implications for its dispersal along European waters. Aquat. Invasions 2019, 14, 397–411. [Google Scholar] [CrossRef]


| Temp | Stage 1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zoea I | Zoea II | Zoea III | Zoea IV | Zoea V | ||||||||||||||
| °C | % | ±SD | % | ±SD | Cum % 2 | ±SD | % | ±SD | Cum % 2 | ±SD | % | ±SD | Cum % 2 | ±SD | % | ±SD | Cum % 2 | ±SD |
| 13 | 51.2 b | 3.0 | 57.8 | 2.1 | 29.5 b | 0.7 | ||||||||||||
| 18 | 89.4 a | 3.8 | 79.2 | 14.1 | 71.0 a | 15.6 | 72.5 | 7.6 | 50.9 ab | 5.9 | 67.6 | 4.7 | 34.5 a | 6.4 | ||||
| 23 | 93.5 a | 2.1 | 77.1 | 3.3 | 72.0 a | 1.4 | 83.4 | 0.4 | 60.0 a | 1.4 | 76.6 | 5.2 | 46.0 a | 4.2 | 2.1 | nd 3 | 1.0 a | nd 3 |
| 24 | 87.0 a | 14.1 | 77.8 | 18.2 | 69.0 a | 26.9 | 72.6 | 17.8 | 52.5 ab | 31.8 | 73.7 | 13.6 | 36.5 a | 16.3 | 11.3 | 4.7 | 4.5 a | 3.5 |
| 25 | 72.2 ab | 15.8 | 62.0 | 14.1 | 43.7 ab | 0.5 | 43.6 | 3.8 | 19.0 bc | 1.4 | 33.4 | 23.5 | 6.5 b | 5.0 | 16.7 | nd 3 | 0.5 a | nd 3 |
| 26 | 50.0 b | 14.1 | 59.1 | 4.9 | 29.2 b | 5.9 | 50.0 | 14.1 | 14.2 c | 1.2 | 50.9 | 34.2 | 7.0 b | 4.2 | ||||
| Temp °C | Days for Each Developmental Stage 1 | ||||
|---|---|---|---|---|---|
| Zoea II | Zoea III | Zoea IV | Zoea V | Megalopa | |
| 13 | 8.0 ± 0.0 a | 10.5 ± 2.1 a | |||
| 18 | 4.0 ± 0.0 b | 10.5 ± 2.1 a | 5.0 ± 0.0 a | 6.5 ± 2.1 ab | |
| 23 | 2.5 ± 0.7 c | 4.0 ± 2.8 b | 4.5 ± 0.7 a | 5.0 ± 0.0 ab | 13.0 2a |
| 24 | 2.0 ± 0.0 c | 3.0 ± 0.0 b | 5.5 ± 3.5 a | 8.0 ± 0.0 a | 8.0 ± 2.8 a |
| 25 | 2.0 ± 0.0 c | 3.0 ± 0.0 b | 5.5 ± 0.7 a | 6.5 ± 2.1 ab | 6.0 2a |
| 26 | 2.5 ± 0.7 c | 2.5 ± 0.7 b | 5.0 ± 0.0 a | 4.0 ± 1.4 ab | |
| Parameter | Zoea II | Zoea III | Zoea IV | Zoea V | Megalopa |
|---|---|---|---|---|---|
| Cum. dev | |||||
| a | 1436.9 | 2044.6 | 5938.7 | 1903.4 | 1198.5 |
| b | −2.026 | −1.814 | −1.982 | −1.487 | −1.195 |
| r2 | 0.985 | 0.898 | 0.975 | 0.897 | 0.995 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, I.-K.; Lee, S.-W. Effects of Temperature on the Survival and Larval Development of Deiratonotus Japonicus (Brachyura, Camptandriidae) as a Biological Indicator. J. Mar. Sci. Eng. 2020, 8, 213. https://doi.org/10.3390/jmse8030213
Oh I-K, Lee S-W. Effects of Temperature on the Survival and Larval Development of Deiratonotus Japonicus (Brachyura, Camptandriidae) as a Biological Indicator. Journal of Marine Science and Engineering. 2020; 8(3):213. https://doi.org/10.3390/jmse8030213
Chicago/Turabian StyleOh, Il-Kweun, and Seung-Woo Lee. 2020. "Effects of Temperature on the Survival and Larval Development of Deiratonotus Japonicus (Brachyura, Camptandriidae) as a Biological Indicator" Journal of Marine Science and Engineering 8, no. 3: 213. https://doi.org/10.3390/jmse8030213





