Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations
Abstract
:1. Introduction
2. Methodology
2.1. Resource and Pre-Process Milling
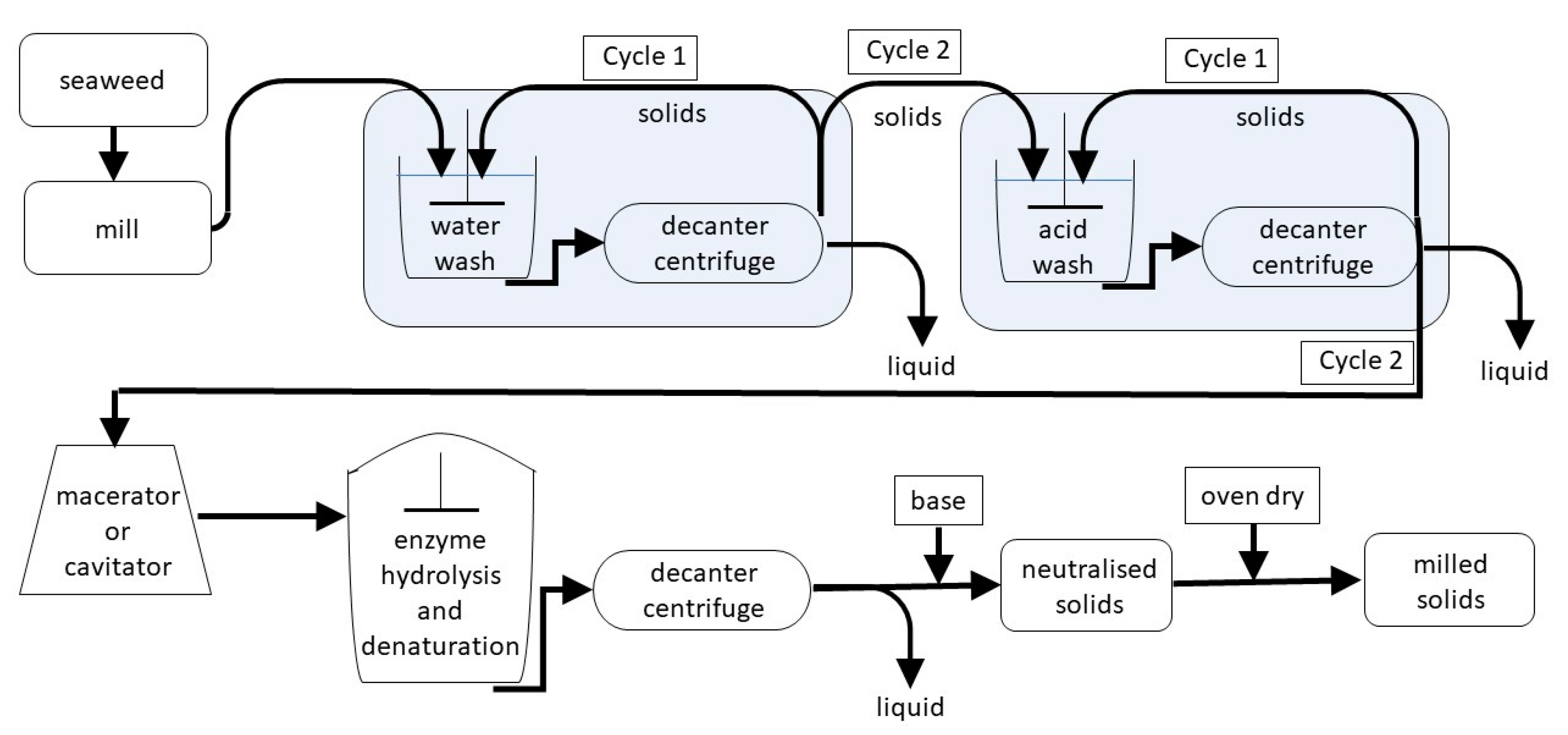
2.2. Batch Processing
3. Pre-Processing Considerations
3.1. Resource Diversity
3.2. Variation of Feedstock—Abiotic
3.3. Variation of Feedstock—Biotic
3.4. Supply Chain
3.4.1. Initial Processing
- Temperature control during harvest. Macroalgae can be held floating in the sea in nets using the water as a coolant and isotonic storage facilities to delay deterioration for extended periods. For example, deterioration of algae was only detected after two weeks in French L. hyperborea fragments [28].
- Temperature control on land. Keeping the macroalgae cool prior to processing is critical to reduce or prevent spoilage; simple steps such as using insulated bulk containers, packing nets of macroalgae with ice, or keeping out of strong sunlight all help reduce heating.
- Stabilisation prior to processing. There are a range of stabilisation options which will depend on downstream processes and end products. For short-term stabilisation: fresh macroalgae should be held between 1–8 °C before processing. If chopping macroalgae, storing the pieces in insulated tanks slows degradation as does anaerobic storage. For medium-term stabilisation, blast freezing is recommended. For long term storage, drying to <10 % moisture is recommended.
- Stabilisation during processing. Use Hazard Analysis and Critical Control Points (HACCP) analysis or other national food standard guidance [29] to identify critical control points to control microbial load during processing, including possible kill steps.
3.4.2. Final Product
4. Observations
4.1. Milling and Cutting
4.2. Equipment Considerations
4.3. Acids
5. Recommendations
5.1. Macroalgae Feedstock
- Optimise the condition of material, harvesting proximity and time of year harvested for the main extraction product of interest.
- Minimise level of contamination to acceptable levels, both for snails and stones and for epiphytes.
5.2. Equipment
- Consider the robustness and specifics of all equipment to be used, including vessels, centrifuges and pumps, to identify potential weak points or bottlenecks.
- Review alternatives, e.g., using plastic and non-metal presses, retrofitting existing equipment if needed. Review whether this will affect cleaning or processing regimes.
- Review working volumes for processing, including for future scale-up. One large vessel is often preferable to replicating steps using a smaller vessel.
5.3. Acid
- Consider the impact of selected acid on both the equipment used (especially if constructed of stainless steel or other metals) and the metals within the macroalgae, taking into account the final product use.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, J.M.M.; Bleathman, G.; Thomas, D.; Gallagher, J.A. The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. Environ. Boil. Fishes 2017, 29, 2463–2469. [Google Scholar] [CrossRef] [Green Version]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbo, R.; Amlund, H.; Heesch, S.; Lock, E.-J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortune Business Insights. In Commercial Seaweed Market Size, Share and COVID-19 Impact Analysis, by Type (Red Seaweed, Brown Seaweed, and Green Seaweed), form (Flakes, Powder, and Liquid), End-Uses (Food & Beverages, Agricultural Fertilizers, Animal Feed Additives, Pharmaceuticals, and Cosmetics & Personal Care), and Regional Forecast, 2020–2027; Grand View Research: San Francisco, CA, USA, 2021; Available online: https://www.fortunebusinessinsights.com/industry-reports/commercial-seaweed-market-100077 (accessed on 21 June 2021).
- FAO. Yearbook. Fishery and Aquaculture Statistics 2018; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- FAO. The Global Status of Seaweed Production, Trade and Utilization; FAO: Rome, Italy, 2018; p. 120. [Google Scholar]
- Sze, P. A Biology of the Algae, 3rd ed.; WCB McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- McHugh, D. A Guide to the Seaweed Industry; Fisheries technical paper 441; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2003; p. 105. [Google Scholar]
- Robic, A.; Sassi, J.-F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two Ulva species (Chlorophyta) from the brittany coast1. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Fletcher, H.; Biller, P.; Ross, A.; Adams, J. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res.-Biomass Biofuels Bioprod. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Black, W.A.P. The seasonal variation in weight and chemical composition of the common British Laminariaceae. J. Mar. Biol. Assoc. United Kingd. 1950, 29, 45–72. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, I.; Wiencke, C.; Bischof, K.; Buchholz, C.; Buck, B.H.; Eggert, A.; Feuerpfeil, P.; Hanelt, D.; Jacobsen, S.; Karez, R.; et al. The genus Laminaria sensu lato: Recent insights and developments. Eur. J. Phycol. 2008, 43, 1–86. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Sloth, J.; Holdt, S.L.; Hansen, M. Analysis and Risk Assessment of Seaweed; National Food Insititue, Technical University of Denmark: Lyngby-Taarbæk, Denmark, 2019; p. 11. [Google Scholar] [CrossRef] [Green Version]
- Visch, W.; Bergström, P.; Nylund, G.M.; Peterson, M.; Pavia, H.; Lindegarth, M. Spatial differences in growth rate and nutrient mitigation of two co-cultivated, extractive species: The blue mussel (Mytilus edulis) and the kelp (Saccharina latissima). Estuar. Coast. Shelf Sci. 2020, 246, 107019. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res.-Biomass Biofuels Bioprod. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Steinhagen, S.; Enge, S.; Larsson, K.; Olsson, J.; Nylund, G.; Albers, E.; Pavia, H.; Undeland, I.; Toth, G. Sustainable Large-Scale Aquaculture of the Northern Hemisphere Sea Lettuce, Ulva fenestrata, in an Off-Shore Seafarm. J. Mar. Sci. Eng. 2021, 9, 615. [Google Scholar] [CrossRef]
- Burnett, N.P.; Koehl, M.A.R. Mechanical properties of the wave-swept kelp, Egregia menziesii, change with season, growth rate, and herbivore wounds. J. Exp. Biol. 2019, 222, jeb.190595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.; Long, J.D. Geographic variation in the sensitivity of an herbivore-induced seaweed defense. Ecology 2018, 99, 1748–1758. [Google Scholar] [CrossRef]
- Haugland, B.T.; Armitage, C.S.; Kutti, T.; Husa, V.; Skogen, M.D.; Bekkby, T.; Carvajalino-Fernandez, M.A.; Bannister, R.J.; White, C.A.; Norderhaug, K.M.; et al. Large-scale salmon farming in Norway impacts the epiphytic community of Laminaria hyperborea. Aquac. Environ. Interact. 2021, 13, 81–100. [Google Scholar] [CrossRef]
- Rieper-Kirchner, M. Macroalgal decomposition: Laboratory studies with particular regard to microorganisms and meiofauna. Helgol. Meeresunters. 1990, 44, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Stévant, P.; Marfaing, H.; Rustad, T.; Sandbakken, I.; Fleurence, J.; Chapman, A. Nutritional value of the kelps Alaria esculenta and Saccharina latissima and effects of short-term storage on biomass quality. J. Appl. Phycol. 2017, 29, 2417–2426. [Google Scholar] [CrossRef]
- Sánchez-García, F.; Hernández, I.; Palacios, V.; Roldán, A. Freshness Quality and Shelf Life Evaluation of the Seaweed Ulva rigida through Physical, Chemical, Microbiological, and Sensory Methods. Foods 2021, 10, 181. [Google Scholar] [CrossRef]
- Bavington, C. Industry-led studies. 2021; Unpublished personal communication. [Google Scholar]
- Morris, D.J.; Pinnegar, J.; Maxwell, D.L.; Dye, S.R.; Fernand, L.J.; Flatman, S.; Williams, O.J.; Rogers, S.I. Over 10 million seawater temperature records for the United Kingdom Continental Shelf between 1880 and 2014 from 17 Cefas (United Kingdom government) marine data systems. Earth Syst. Sci. Data 2018, 10, 27–51. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.K.; Lewis, H.W.; Hyder, P.; Siddorn, J.; O’Dea, E. The Sensitivity of British Weather to Ocean Tides. Geophys. Res. Lett. 2021, 48, e2020GL090732. [Google Scholar] [CrossRef]
- De Bettignies, F.; Dauby, P.; Thomas, F.; Gobet, A.; Delage, L.; Bohner, O.; Loisel, S.; Davoult, D. Degradation dynamics and processes associated with the accumulation of Laminaria hyperborea (Phaeophyceae) kelp fragments: An in situ experimental approach. J. Phycol. 2020, 56, 1481–1492. [Google Scholar] [CrossRef]
- FSA. Hazard Analysis and Critical Control Point (HACCP). 2017. Available online: https://www.food.gov.uk/business-guidance/hazard-analysis-and-critical-control-point-haccp (accessed on 21 June 2021).
- Banach, J.L.; Hoek-van den Hil, E.F.; Van Der Fels-Klerx, H.J. Food safety hazards in the European seaweed chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Dyer, P.W.; Theodorou, M.K. Dewatering treatments to increase dry matter content of the brown seaweed, kelp (Laminaria digitata ((Hudson) JV Lamouroux)). Bioresour. Technol. 2017, 224, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Barrento, S.; Dyer, P.W.; Theodorou, M.K. Species variation in the effects of dewatering treatment on macroalgae. J. Appl. Phycol. 2018, 30, 2305–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J. GreenSeas Resouces. 2017. Available online: https://beaconwales.org/images/GreenSeas_Resources.pdf (accessed on 19 August 2021).
- Wirtanen, G.; Salo, S. Disinfection in Food Processing—Efficacy Testing of Disinfectants. Rev. Environ. Sci. Bio/Technol. 2003, 2, 293–306. [Google Scholar] [CrossRef]
- Berovič, M. Sterilisation in biotechnology. Biotechnol. Annu. Rev. 2005, 11, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Reactor engineering. In Bioprocess Engineering Principles, 2nd ed.; Doran, P.M., Ed.; Academic Press Ltd: London, UK, 2013; pp. 761–853. [Google Scholar]
- Stanton, D. ABEC Breaks Plastic Ceiling again with 6000 L Single-Use Bioreactor. BioProcess International. 2019. Available online: https://bioprocessintl.com/bioprocess-insider/upstream-downstream-processing/abec-breaks-plastic-ceiling-again-with-6000-l-single-use-bioreactor/ (accessed on 17 August 2021).
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2014, 27, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Łabowska, M.B.; Michalak, I.; Detyna, J. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field—A review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.; Bjerrum, J.; Buchardt, O.; Olsen, G.E.; Pedersen, C.; Toft, J. The Affinity of Some Divalent Metals for Different Types of Alginates. Acta Chem. Scand. 1961, 15, 1794–1795. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O. Strontium–Calcium Selectivity of Alginates. Nat. Cell Biol. 1967, 215, 757. [Google Scholar] [CrossRef]
- BSSA. Selection of Stainless Steels for Handling Hydrochloric Acid (HCl). 2021. Available online: https://bssa.org.uk/bssa_articles/9-selection-of-stainless-steels-for-handling-hydrochloric-acid-hcl/ (accessed on 23 June 2021).
- BSSA. Selection of Stainless Steels for Handling Sulphuric Acid (H2SO4). 2021. Available online: https://bssa.org.uk/bssa_articles/selection-of-stainless-steels-for-handling-sulphuric-acid-h2so4/ (accessed on 23 June 2021).
- European Commision. Commision Regulation 1881/2006 Setting Laximum Levels for Certain Contaminants in Foodstuffs; European Union: Brussels, Belgium, 2006. [Google Scholar]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marani, D.; Macchi, G.; Pagano, M. Lead precipitation in the presence of sulphate and carbonate: Testing of thermodynamic predictions. Water Res. 1995, 29, 1085–1092. [Google Scholar] [CrossRef]
- Nedwed, T.; Clifford, D.A. Feasibility of extracting lead from lead battery recycling site soil using high-concentration chloride solutions. Environ. Prog. 2000, 19, 197–206. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, J.M.M.; Morris, S.M.; Steege, L.; Robinson, J.; Bavington, C. Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations. J. Mar. Sci. Eng. 2021, 9, 1082. https://doi.org/10.3390/jmse9101082
Adams JMM, Morris SM, Steege L, Robinson J, Bavington C. Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations. Journal of Marine Science and Engineering. 2021; 9(10):1082. https://doi.org/10.3390/jmse9101082
Chicago/Turabian StyleAdams, Jessica M. M., S. Michael Morris, Laura Steege, Joanne Robinson, and Charles Bavington. 2021. "Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations" Journal of Marine Science and Engineering 9, no. 10: 1082. https://doi.org/10.3390/jmse9101082
APA StyleAdams, J. M. M., Morris, S. M., Steege, L., Robinson, J., & Bavington, C. (2021). Food-Grade Biorefinery Processing of Macroalgae at Scale: Considerations, Observations and Recommendations. Journal of Marine Science and Engineering, 9(10), 1082. https://doi.org/10.3390/jmse9101082





