Seasonal Sources and Cycling of Nitrogen Revealed by Stable Isotopes in the Northeastern Beibu Gulf, China
Abstract
:1. Introduction
2. Materials and Methods
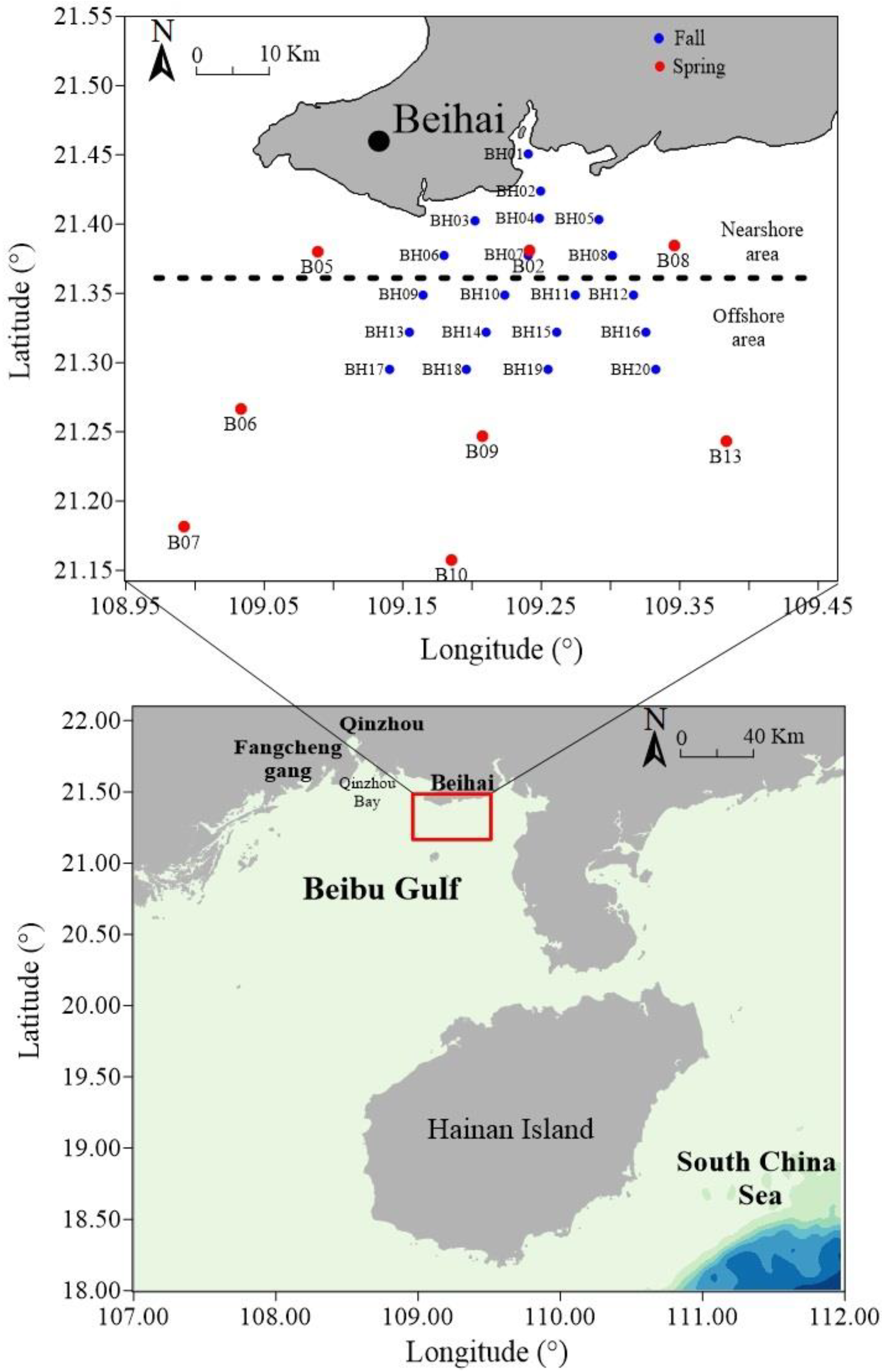
2.1. Study Area and Sampling
2.2. Chemical Analysis
2.3. Mixing Model
2.4. Quantification of the Mixing Model
3. Results
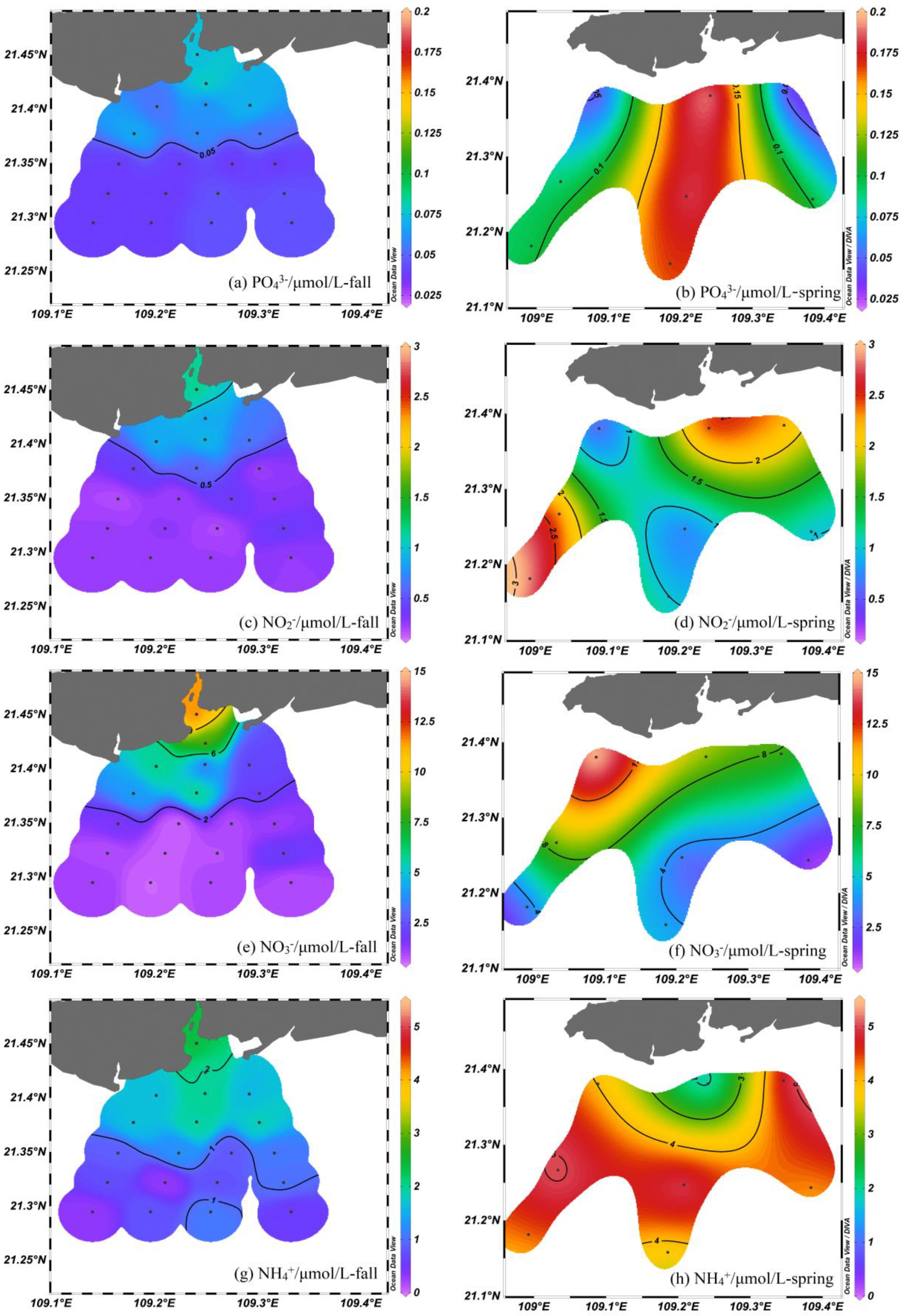
3.1. Chemical Parameters
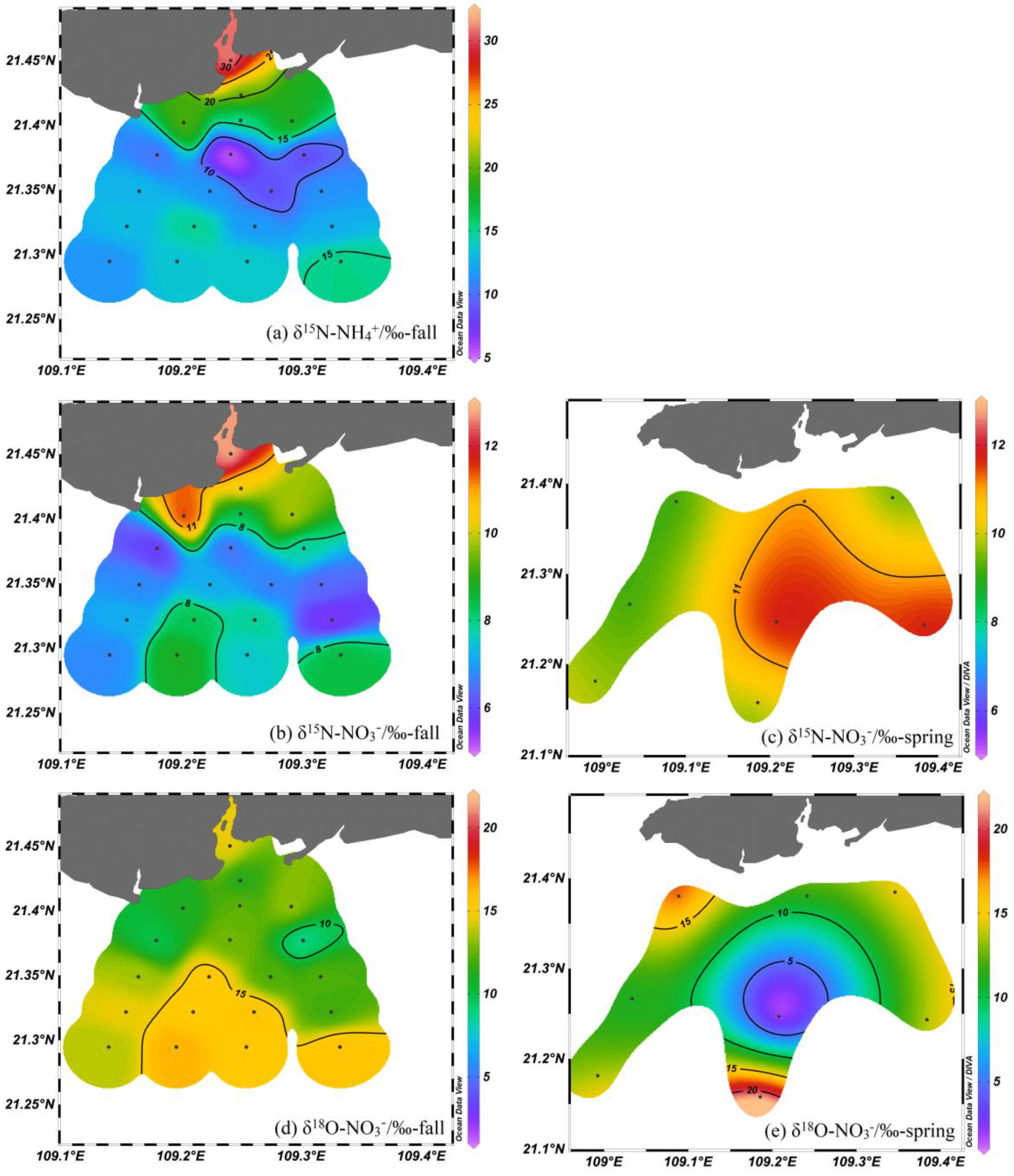
3.2. Isotopic Composition
4. Discussion
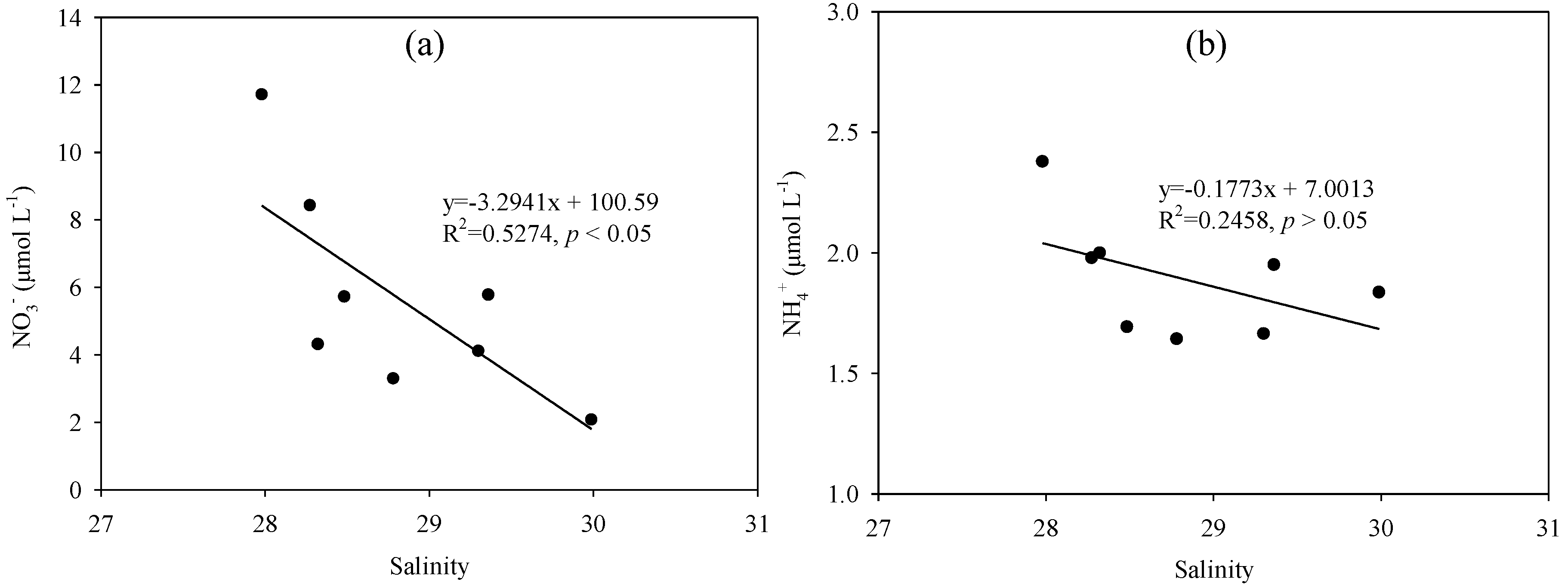
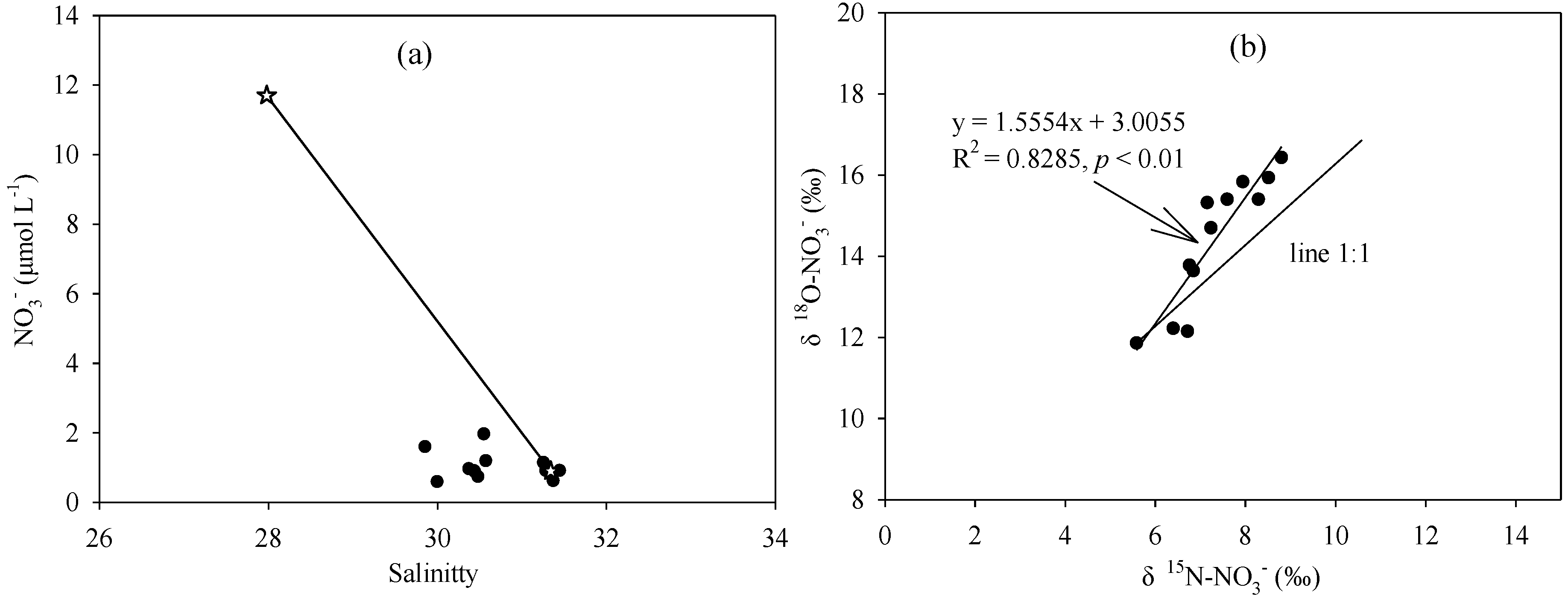
4.1. Hydrographic Characteristics
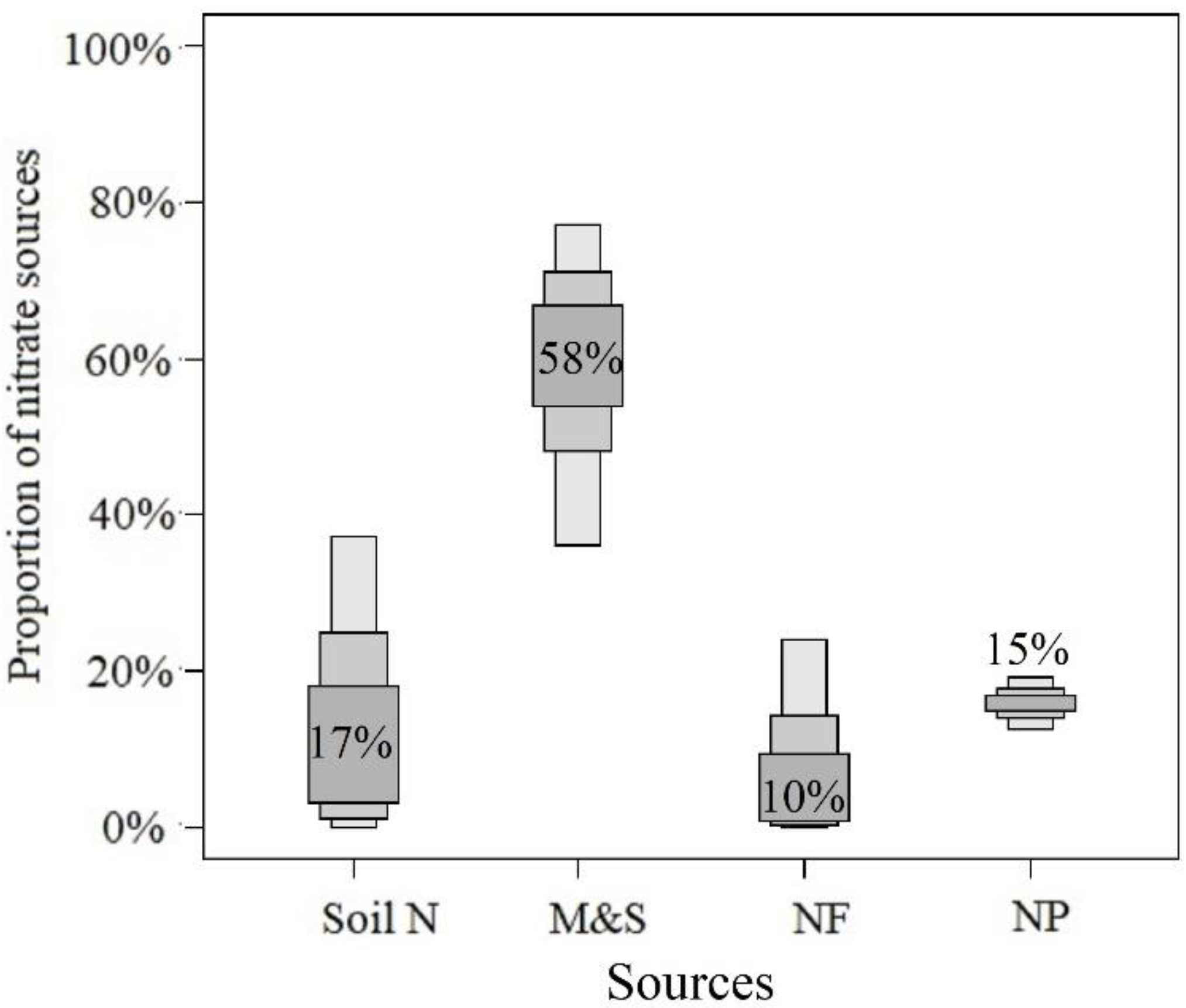
4.2. Nutrient Sources in the Nearshore Area
4.3. Nitrate Biogeochemical Process in the Offshore Area
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar]
- Sharples, J.; Middelburg, J.J.; Fennel, K.; Jickells, T.D. What proportion of riverine nutrients reaches the open ocean? Glob. Biogeochem. Cycles 2017, 31, 39–58. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chen, J.; Li, H.; Jin, H.; Gao, S.; Ji, Z.; Zhu, Y.; Ran, L.; Zhang, J.; Liao, Y. Sources of nitrate in xiangshan bay (China), as identified using nitrogen and oxygen isotopes. Estuar. Coast. Shelf Sci. 2018, 207, 109–118. [Google Scholar] [CrossRef]
- Wong, W.W.; Cartwright, I.; Chee Poh, S.; Cook, P. Sources and cycling of nitrogen revealed by stable isotopes in a highly populated large temperate coastal embayment. Sci. Total. Environ. 2022, 806, 150408. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lao, Q.; Zhang, S.; Bian, P.; Jin, G.; Zhu, Q.; Chen, C. Nitrate sources and biogeochemical processes identified using nitrogen and oxygen isotopes on the eastern coast of Hainan Island. Cont. Shelf Res. 2020, 207, 104209. [Google Scholar] [CrossRef]
- Howe, S.; Miranda, C.; Hayes, C.T.; Letscher, R.T.; Knapp, A.N. The Dual Isotopic Composition of Nitrate in the Gulf of Mexico and Florida Straits. J. Geophys. Res. Ocean. 2020, 125, e2020JC016047. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Bermanfrank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Seitzinger, S.P.; Kroeze, C.; Bouwman, A.F.; Caraco, N.; Dentener, F.; Styles, R.V. Global patterns of dissolved inorganic and particulate nitrogen inputs to coastal systems: Recent conditions and future projections. Estuaries 2002, 25, 640–655. [Google Scholar] [CrossRef]
- Ye, F.; Jia, G.; Xie, L.; Wei, G.; Xu, J. Isotope constraints on seasonal dynamics of dissolved and particulate n in the pearl river estuary, south china. J. Geophys. Res. Ocean. 2016, 121, 8689–8705. [Google Scholar]
- Yan, X.; Xu, M.N.; Wan, X.S.; Yang, J.Y.T.; Trull, T.W.; Dai, M.; Kao, S.J. Dual isotope measurements reveal zoning of nitrate processing in the summer Changjiang (Yangtze) River Plume. Geophys. Res. Lett. 2017, 44, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Morimoto, A.; Zhou, F.; Yoshie, N.; Damar, A.; Takikawa, T. Coastal ecosystem change in Asia: Hypoxia, eutrophication, and nutrient conditions. J. Oceanogr. 2021, 90. [Google Scholar] [CrossRef]
- Geeraert, N.; Archana, A.; Xu, M.N.; Kao, S.J.; Bake, D.M.; Thibodeau, B. Investigating the link between Pearl River-induced eutrophication and hypoxia in Hong Kong shallow coastal waters. Sci. Total. Environ. 2021, 772, 145007. [Google Scholar] [CrossRef]
- Liu, X.; Stock, C.A.; Dunne, J.P.; Lee, M.; Shevliakova, E.; Malyshev, S.; Milly, P.C.D. Simulated Global Coastal Ecosystem Responses to a Half-Century Increase in River Nitrogen Loads. Geophys. Res. Lett. 2021, 48, e2021GL094367. [Google Scholar] [CrossRef]
- Li, J.; Cao, R.; Lao, Q.; Chen, F.; Chen, C.; Zhou, X.; Meng, Y.; Zhu, Q. Assessing seasonal nitrate contamination by nitrate dual isotopes in a monsoon-controlled bay with intensive human activities in South China. Int. J. Environ. Res. Public Health 2020, 17, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, R.E.; O’Neil, J.R.; Surkov, A.V. Biogeochemical cycling of phosphorus: Insights from oxygen isotope effects of phosphoenzymes. Am. J. Sci. 2005, 305, 596–620. [Google Scholar] [CrossRef]
- Chen, F.J.; Jia, G.D.; Chen, J.Y. Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, South China. Biogeochemistry 2009, 94, 163–174. [Google Scholar] [CrossRef]
- Sigman, D.M.; Granger, J.; Difiore, P.J.; Lehmann, M.F.; Ho, R.; Cane, G.; Geen, A.V. Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin. Glob. Biogeochem. Cycles 2005, 19, GB4022. [Google Scholar] [CrossRef]
- Wankel, S.D.; Kendall, C.; Pennington, J.T.; Chavez, F.P.; Paytan, A. Nitrification in the euphotic zone as evidenced by nitrate dual isotopic composition: Observations from monterey bay, california. Glob. Biogeochem. Cycles 2007, 21, GB2009. [Google Scholar] [CrossRef] [Green Version]
- Kendall, C. Tracing nitrogen sources and cycling in catchments. In Isotope Tracers in Catchment Hydrology; Kendall, C., McDonnel, J.J., Eds.; Elsevier: New York, NY, USA, 1998; pp. 519–576. [Google Scholar]
- Xue, D.; Botte, J.; Baets, B.D.; Accoe, F.; Nestler, A.; Taylor, P.; Cleemput, O.V.; Berglund, M.; Boeckx, P. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.T.; Hsu, S.C.; Dai, M.; Hsiao, S.S.Y.; Kao, S.J. Isotopic composition of water-soluble nitrate in bulk atmospheric deposition at dongsha island: Sources and implications of external n supply to the northern south china sea. Biogeosciences 2014, 11, 9661–9695. [Google Scholar] [CrossRef] [Green Version]
- Lao, Q.; Chen, F.; Liu, G.; Chen, C.; Jin, G.; Zhu, Q.; Wei, C.; Zhang, C. Isotopic evidence for the shift of nitrate sources and active biological transformation on the western coast of guangdong province, south china. Mar. Pollut. Bull. 2019, 142, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; De Baets, B.; Van Cleemput, O.; Hennessy, C.; Berglund, M.; Boeckx, P. Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ. Pollut. 2012, 161, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhi, Y.; Shi, J.; Wu, L. Apportionment and uncertainty analysis of nitrate sources based on the dual isotope approach and a bayesian isotope mixing model at the watershed scale. Sci. Total. Environ. 2018, 639, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.; Sigman, D.M.; Needoba, J.A.; Harrison, P.J. Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol. Oceanogr. 2004, 49, 1763–1773. [Google Scholar] [CrossRef] [Green Version]
- Sigman, D.M.; Robinson, R.; Knapp, A.N.; Geen, A.V.; Mccorkle, D.C.; Brandes, J.A.; Thunell, R.C. Distinguishing between water column and sedimentary denitrification in the santa barbara basin using the stable isotopes of nitrate. Geochem. Geophys. 2003, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Granger, J.; Sigman, D.M.; Lehmann, M.F.; Tortell, P.D. Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria. Limnol. Oceanogr. 2008, 53, 2533–2545. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Jia, G.; Jin, H.; Jie, X.; Zhi, Y.; Zhuang, Y.; Liu, X.; Zhang, H. Nitrate δ 15N and δ 18 O evidence for active biological transformation in the changjiang estuary and the adjacent east china sea. Acta Oceanol. Sin. 2013, 32, 11–17. [Google Scholar]
- Chen, F.; Lin, J.; Qian, B.; Wu, Z.; Huang, P.; Chen, K.; Li, T.; Cai, M. Geochemical assessment and spatial analysis of heavy metals in the surface sediments in the eastern beibu gulf: A reflection on the industrial development of the south china coast. Int. J. Environ. Res. Public Health 2018, 15, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, H.; Lin, J.; Liang, K.; Xia, Z. Selected trace metals (as, cd and hg) distribution and contamination in the coastal wetland sediment of the northern Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2013, 66, 252–258. [Google Scholar] [CrossRef]
- Kaiser, D.; Unger, D.; Qiu, G. Particulate organic matter dynamics in coastal systems of the northern beibu gulf. Cont. Shelf Res. 2014, 82, 99–118. [Google Scholar] [CrossRef]
- Lai, J.; Jiang, F.; Ke, K.; Xu, M.; Lei, F.; Chen, B. Nutrients distribution and trophic status assessment in the northern beibu gulf, China. Chin. J. Oceanol. Limnol. 2014, 32, 1128–1144. [Google Scholar] [CrossRef]
- Lao, Q.; Su, Q.; Liu, G.; Shen, Y.; Chen, F.; Lei, X.; Qing, S.; Wei, C.; Zhang, C.; Gao, J. Spatial distribution of and historical changes in heavy metals in the surface seawater and sediments of the beibu gulf, China. Mar. Pollut. Bull. 2019, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Zhao, M.; Chen, B.; Dada, O.A.; Chu, Z. Spatial distribution and historical trends of heavy metals in the sediments of petroleum producing regions of the beibu gulf, China. Mar. Pollut. Bull. 2015, 91, 87–95. [Google Scholar] [PubMed]
- Yang, J.; Zhang, R.; Zhao, Z.; Weng, S.; Fenghua, L.I. Temporal and spatial distribution characteristics of nutrients in the coastal seawater of guangxi beibu gulf during the past 25 years. Ecol. Environ. Sci. 2015, 24, 1493–1498. (In Chinese) [Google Scholar]
- Lao, Q.; Liu, G.; Shen, Y.; Su, Q.; Lei, X. Biogeochemical processes and eutrophication status of nutrients in the northern Beibu Gulf, South China. J. Earth Syst. Sci. 2021, 130, 199. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Zhao, H.; Tang, J.; Jiang, G.; Li, Z.; Li, X.; Chen, S.; Zou, S.; Dong, K.; et al. Spatial distribution and functional profile of the bacterial community in response to eutrophication in the subtropical Beibu Gulf, China. Mar. Pollut. Bull. 2020, 161, 111742. [Google Scholar]
- Zeng, J.; Chen, M.; Zheng, M.; Qiu, Y.; He, W.; He, Y.; Liu, X. Effects of particles on potential denitrification in the coastal waters of the Beibu Gulf in China. Sci. Total. Environ. 2018, 624, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, T.; Zhou, J. Historical occurrence of algal blooms in the northern Beibu Gulf of china and implications for future trends. Front. Microbiol. 2019, 10, 451. [Google Scholar]
- Chen, B.; Dong, D.; Qin, X.; Xu, M.; Chen, X.; Shi, M. Relationship between the occurrence of red tides and the dynamic response mechanisms in the northern Beibu Gulf. J. Coast. Res. 2019, 93, 185–193. [Google Scholar] [CrossRef]
- Lao, Q.; Liu, G.; Zhou, X.; Chen, F.; Zhang, S. Sources of polychlorinated biphenyls (pcbs) and dichlorodiphenyltrichloroethanes (DDTs) found in surface sediment from coastal areas of Beibu Gulf: A reflection on shipping activities and coastal industries. Mar. Pollut. Bull. 2021, 167, 112318. [Google Scholar] [CrossRef]
- Lao, Q.; Liu, G.; Shen, Y.; Su, Q.; Gao, J.; Chen, F. Distribution characteristics and fluxes of nutrients in the rivers of the Beibu Gulf. Haiyang Xuebao 2020, 42, 93–100. (In Chinese) [Google Scholar]
- McIlvin, M.R.; Altabet, M.A. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal. Chem. 2005, 77, 5589–5595. [Google Scholar] [CrossRef]
- Zhang, L.; Altabet, M.A.; Wu, T.; Hadas, O. Sensitive measurement of nh4+ 15n/14n (delta 15nh4+) at natural abundance levels in fresh and saltwaters. Anal. Chem. 2007, 79, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Fry, B. Conservative mixing of stable isotopes across estuarine salinity gradients: A conceptual framework for monitoring watershed influences on downstream fisheries production. Estuaries 2002, 25, 264–271. [Google Scholar] [CrossRef]
- Dähnke, K.; Bahlmann, E.; Emeis, K. A nitrate sink in estuaries? An assessment by means of stable nitrate isotopes in the elbe estuary. Limnol. Oceanogr. 2008, 53, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wu, G.; Nguyen, K.-C.; Shi, M. Counter-wind deep current in the northern beibu gulf in boreal winter. J. Ocean. Univ. China 2019, 18, 57–68. [Google Scholar] [CrossRef]
- Gao, J.; Wu, G.; Ya, H. Review of the circulation in the beibu gulf, South China Sea. Cont. Shelf Res. 2017, 138, 106–119. [Google Scholar] [CrossRef]
- Li, B.; Lan, W.L.; Li, T.S.; Li, M.M. Variation of environmental factors during phaeocystis globosa blooms and its implications for the bloom decay. Chin. J. Ecol. 2015, 34, 1351–1358. [Google Scholar]
- Li, T.-S. Analyze red tide with automatic monitoring system of water quality in Lianzhou Gulf. Environ. Monit. China 2011, 27, 35–38. [Google Scholar]
- Herbert, R.A. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 1999, 23, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Guo, X.; Zhai, W.; Yuan, L.; Wang, B.; Wang, L.; Cai, P.; Tang, T.; Cai, W.J. Oxygen depletion in the upper reach of the Pearl River estuary during a winter drought. Mar. Chem. 2006, 102, 159–169. [Google Scholar] [CrossRef]
- Ye, F.; Ni, Z.; Xie, L.; Wei, G.; Jia, G. Isotopic evidence for the turnover of biological reactive nitrogen in the pearl river estuary, South China. J. Geophys. Res. Biogeosciences 2015, 120, 661–672. [Google Scholar] [CrossRef]
- Wankel, S.D.; Kendall, C.; Paytan, A. Using nitrate dual isotopic composition (δ15n and δ18o) as a tool for exploring sources and cycling of nitrate in an estuarine system: Elkhorn slough, california. J. Geophys. Res. Biogeosci. 2015, 114, 315–327. [Google Scholar]
- Lehmann, M.F.; Sigman, D.M.; Mccorkle, D.C.; Granger, J.; Hoffmann, S.; Cane, G.; Brunelle, B.G. The distribution of nitrate 15n/14n in marine sediments and the impact of benthic nitrogen loss on the isotopic composition of oceanic nitrate. Geochim. Et Cosmochim. Acta 2007, 71, 5384–5404. [Google Scholar] [CrossRef]
- Dai, M.; Wang, L.; Guo, X.; Zhai, W.; Li, Q.; He, B.; Kao, S.J. Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: The Pearl River Estuary, China. Biogeosciences 2008, 5, 1227–1244. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.R.; Ging, P.B.; Lee, R.W.; Ebbert, J.C.; Tesoriero, A.J.; Inkpen, E.L. Forensic applications of nitrogen and oxygen isotopes in tracing nitrate sources in urban environments. Environ. Forensics 2002, 3, 125–130. [Google Scholar] [CrossRef]
- Chen, F.; Lao, Q.; Jia, G.; Chen, C.; Zhu, Q.; Zhou, X. Seasonal variations of nitrate dual isotopes in wet deposition in a tropical city in China. Atmos. Environ. 2019, 196, 1–9. [Google Scholar] [CrossRef]


| Station | Salinity | PO43− [μmol L−1] | NH4+ [μmol L−1] | NO2− [μmol L−1] | NO3− [μmol L−1] | δ15N-NO3− [‰] | δ18O-NO3− [‰] |
|---|---|---|---|---|---|---|---|
| BH01 | 27.98 | 0.07 | 2.4 | 1.2 | 11.7 | 12.9 | 14.8 |
| BH20 | 31.45 | 0.05 | 0.6 | 0.3 | 0.9 | 8.5 | 15.9 |
| δ15N-NO3− | δ18O-NO3− | |||||
|---|---|---|---|---|---|---|
| Source | Range | Average ± SD | Reference | Range | Average ± SD | Reference |
| Soil N | −0.05–8.25 | 4.52 ± 2.67 | [20,26] | −5–15 | 4.08 ± 0.33 | [20,26] |
| M&S | 6.89–18.88 | 12.73 ± 3.4 | [20,26] | −5–15 | 4.08 ± 0.33 | [20,26] |
| NF | −1.87–2.96 | 0.04 ± 1.87 | [20,26] | −5–15 | 4.08 ± 0.33 | [20,26] |
| NP | −1.8– 4.1 | 0.8 ± 1.5 | [60] | 42.7–61.6 | 52.4 ± 5.1 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, Q.; Liu, G.; Gao, J.; Shen, Y.; Su, Q.; Chen, C.; Chen, F. Seasonal Sources and Cycling of Nitrogen Revealed by Stable Isotopes in the Northeastern Beibu Gulf, China. J. Mar. Sci. Eng. 2021, 9, 1123. https://doi.org/10.3390/jmse9101123
Lao Q, Liu G, Gao J, Shen Y, Su Q, Chen C, Chen F. Seasonal Sources and Cycling of Nitrogen Revealed by Stable Isotopes in the Northeastern Beibu Gulf, China. Journal of Marine Science and Engineering. 2021; 9(10):1123. https://doi.org/10.3390/jmse9101123
Chicago/Turabian StyleLao, Qibin, Guoqiang Liu, Jingsong Gao, Youli Shen, Qizhong Su, Chunqing Chen, and Fajin Chen. 2021. "Seasonal Sources and Cycling of Nitrogen Revealed by Stable Isotopes in the Northeastern Beibu Gulf, China" Journal of Marine Science and Engineering 9, no. 10: 1123. https://doi.org/10.3390/jmse9101123





