Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Stomach Content Analyses
2.3. Multivariate Analyses of Dietary Data
3. Results
3.1. Overall Dietary Compositions
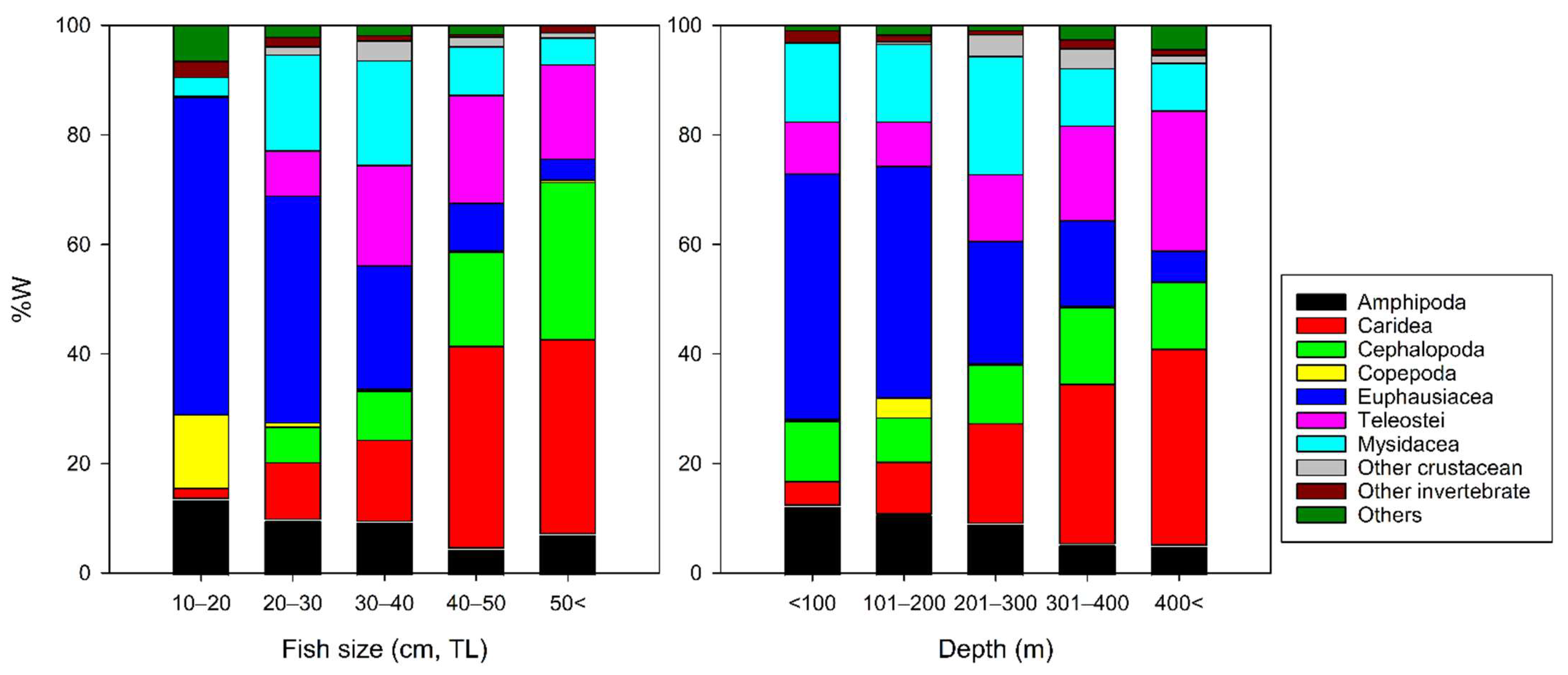
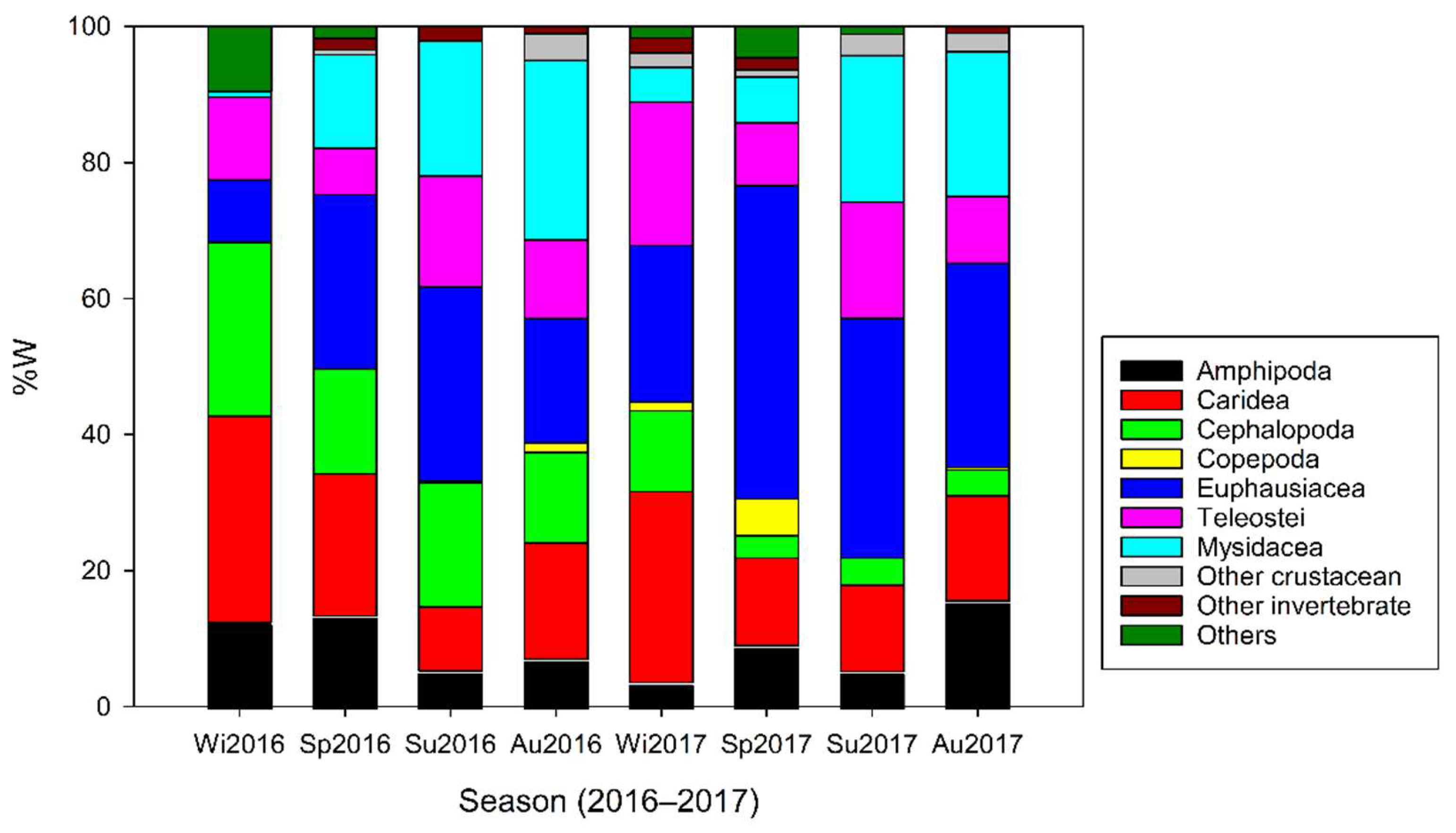
3.2. Ontogenetic, Depth-Dependent, and Temporal Changes in Dietary Composition
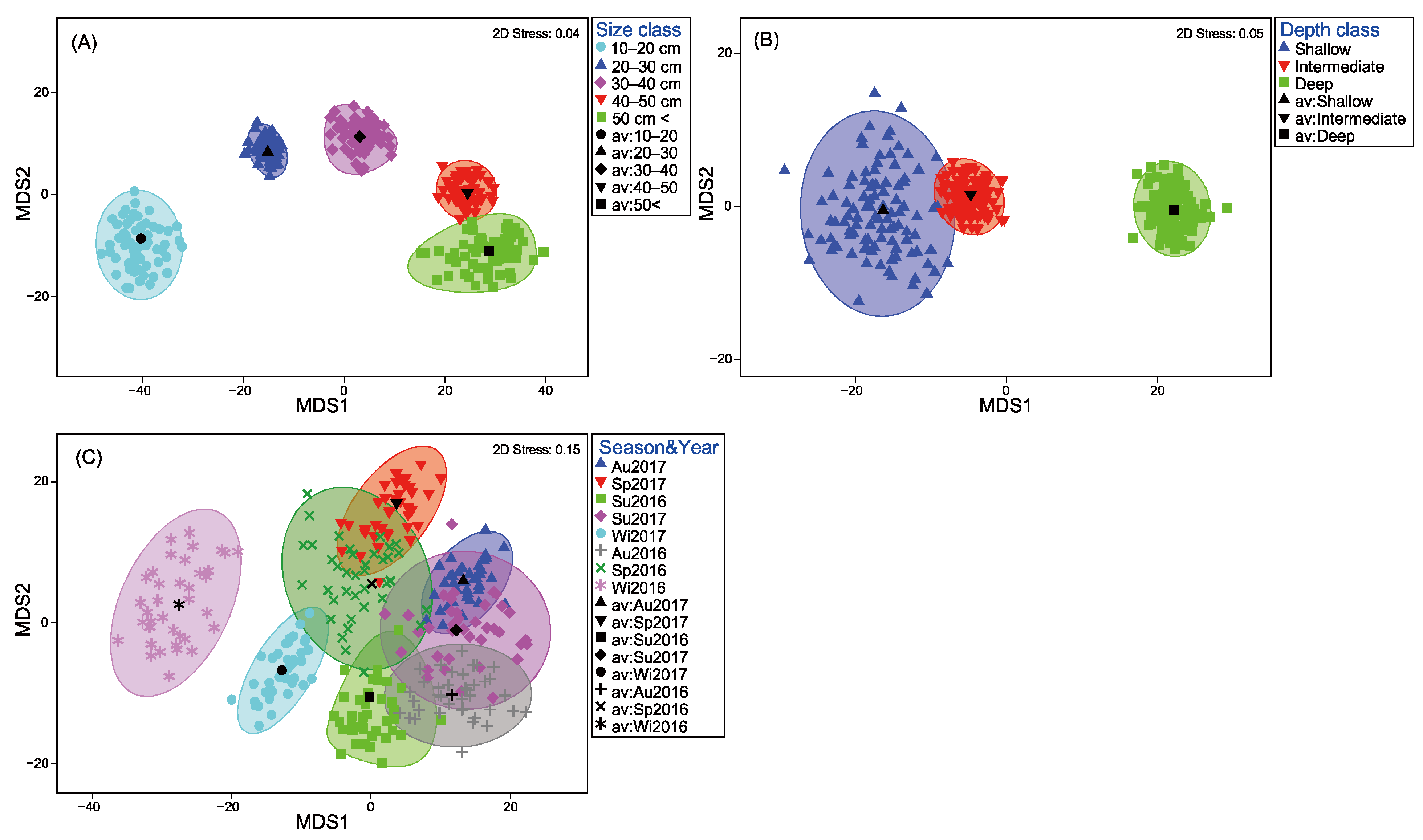
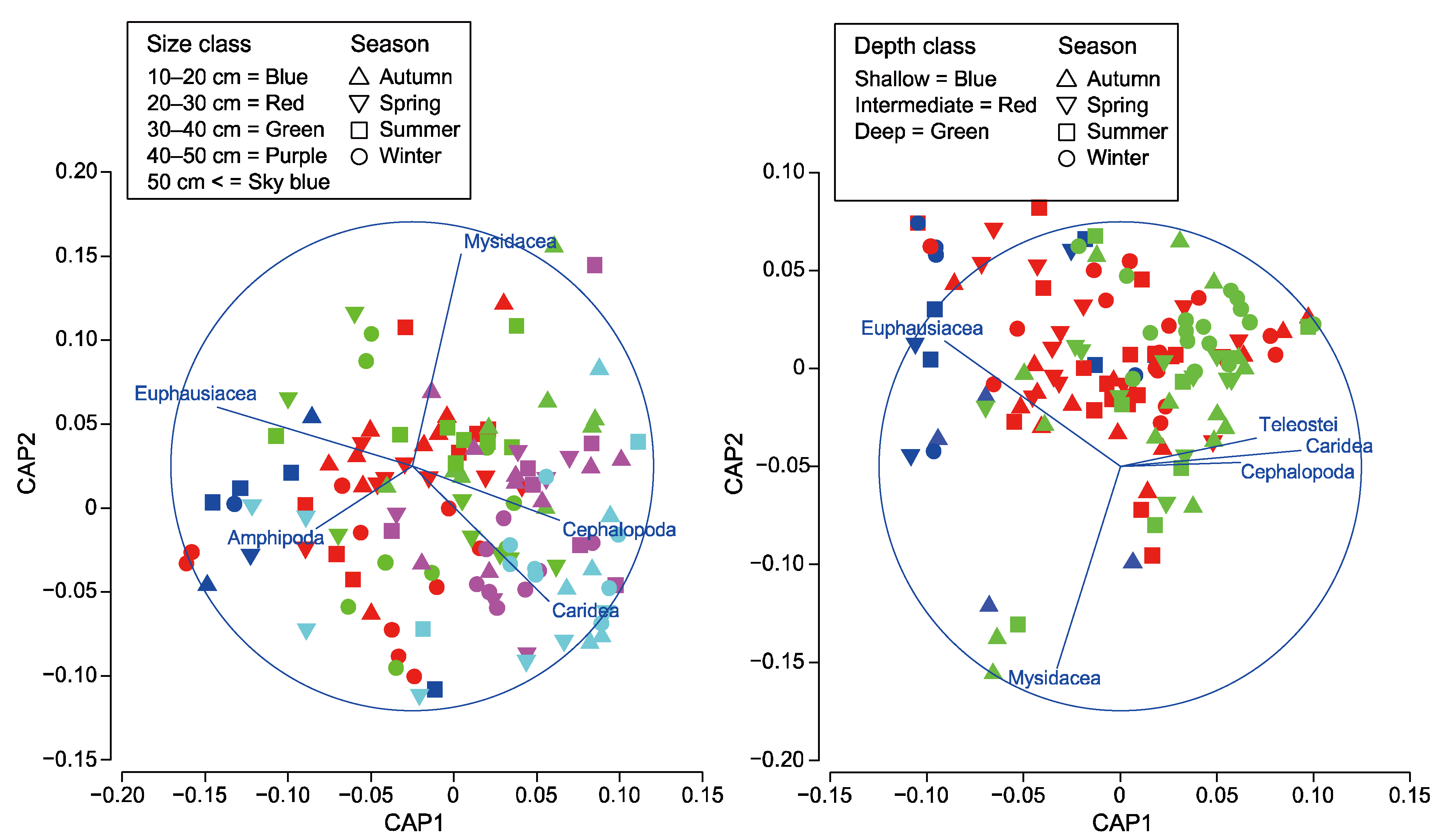
3.3. Dietary Pattern Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colwell, R.K.; Futuyma, D.J. On the measurement of niche breadth and overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef]
- Scharf, F.S.; Juanes, F.; Rountree, R.A. Predator size-prey size relationships of marine fish predators: Interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 2000, 208, 229–248. [Google Scholar] [CrossRef]
- Depczynski, M.; Bellwood, D.R. The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar. Ecol. Prog. Ser. 2003, 256, 183–191. [Google Scholar] [CrossRef]
- Zabel, R.W.; Harvey, C.J.; Katz, S.L.; Good, T.P.; Levin, P.S. Ecologically sustainable yield: Marine conservation requires a new ecosystem-based concept for fisheries management that looks beyond sustainable yield for individual fish species. Am. Sci. 2003, 91, 150–157. [Google Scholar]
- Bizzarro, J.J.; Yoklavich, M.M.; Wakefield, W.W. Diet composition and foraging ecology of US Pacific Coast groundfishes with applications for fisheries management. Environ. Biol. Fishes 2017, 100, 375–393. [Google Scholar] [CrossRef] [Green Version]
- Platell, M.; Potter, I. Partitioning of food resources amongst 18 abundant benthic carnivorous fish species in marine waters on the lower west coast of Australia. J. Exp. Mar. Biol. Ecol. 2001, 261, 31–54. [Google Scholar] [CrossRef]
- Lek, E.; Fairclough, D.V.; Platell, M.E.; Clarke, K.R.; Tweedley, J.R.; Potter, I.C. To what extent are the dietary compositions of three abundant, co-occurring labrid species different and related to latitude, habitat, body size and season? J. Fish Biol. 2011, 78, 1913–1943. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Gaston, T.F.; Williamson, J.E. Resource partitioning in gurnard species using trophic analyses: The importance of temporal resolution. Fish. Res. 2017, 186, 301–310. [Google Scholar] [CrossRef]
- Lamb, J.F.; Kimmel, D.G. The contribution of diet to the dramatic reduction of the 2013 year class of Gulf of Alaska walleye pollock (Gadus chalcogrammus). Fish. Oceanogr. 2021, 30, 1–15. [Google Scholar] [CrossRef]
- Kelly, D.J.; Schallenberg, M. Assessing food web structure in relation to nutrient enrichment, macrophyte collapse and lake resilience in shallow lowland lakes. N. Z. J. Mar. Freshw. Res. 2019, 53, 603–619. [Google Scholar] [CrossRef]
- Bowes, R.E.; Thorp, J.H.; Reuman, D.C. Multidimensional metrics of niche space for use with diverse analytical techniques. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roessig, J.M.; Woodley, C.M.; Cech, J.J.; Hansen, L.J. Effects of global climate change on marine and estuarine fishes and fisheries. Rev. Fish Biol. Fish. 2004, 14, 251–275. [Google Scholar] [CrossRef]
- Stewart, S.D.; Kelly, D.; Biessy, L.; Laroche, O.; Wood, S.A. Individual diet specialization drives population trophic niche responses to environmental change in a predator fish population. Food Webs 2021, 27, e00193. [Google Scholar] [CrossRef]
- Quast, J.C.; Hall, E.L. List of Fishes of Alaska and Adjacent Waters with a Guide to Some of Their Literature; NOAA Technical Report NMFS SSRF-658; US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Seattle, WA, USA, 1972; p. 47.
- Kotwicki, S.; Buckley, T.W.; Honkalehto, T.; Walters, G. Variation in the distribution of walleye pollock (Theragra chalcogramma) with temperature and implications for seasonal migration. Fish. Bull. 2005, 103, 574–587. [Google Scholar]
- Kim, Y.; Han, K.; Kang, C.; Kim, J. Commercial Fishes of the Coastal and Offshore Waters in Korea; Hangul Press: Busan, Korea, 2004. [Google Scholar]
- Beamish, R.; McFarlane, G.A. A discussion of the importance of aging errors, and an application to walleye pollock: The world’s largest fishery. In Recent Developments in Fish Otolith Research; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 545–565. [Google Scholar]
- Kang, S.; Kim, S. What caused the collapse of walleye pollock population in Korean waters? KMI Int. J. Marit. Aff. Fish. 2015, 7, 43–58. [Google Scholar] [CrossRef]
- Nishimura, A.; Yamada, J. Age and growth of larval and juvenile walleye pollock, Theragra chalcogramma (Pallas), as determined by otolith daily growth increments. J. Exp. Mar. Biol. Ecol. 1984, 82, 191–205. [Google Scholar] [CrossRef]
- Akira, N.; Yanagimoto, T.; Mito, K.; Katakura, S. Interannual variability in growth of walleye pollock, Theragra chalcogramma, in the central Bering Sea. Fish. Oceanogr. 2001, 10, 367–375. [Google Scholar] [CrossRef]
- Hinckley, S. The reproductive biology of walleye pollock, Theragra chalcogramma, in the Bering Sea, with reference to spawning stock structure. Fish. Bull. 1987, 85, 481–498. [Google Scholar]
- Hamatsu, T.; Yabuki, K.; Watanabe, K. Decadal changes in reproduction of walleye pollock (Theragra chalcogramma) off the Pacific coast of northern Japan. Fish. Oceanogr. 2004, 13, 74–83. [Google Scholar] [CrossRef]
- Yamamura, O.; Honda, S.; Shida, O.; Hamatsu, T. Diets of walleye pollock Theragra chalcogramma in the Doto area, northern Japan: Ontogenetic and seasonal variations. Mar. Ecol. Prog. Ser. 2002, 238, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.F.; Pinchuk, A.I.; Coyle, K.O. Seasonal changes in the diet composition and prey selection of walleye pollock (Theragra chalcogramma) in the northern Gulf of Alaska. Fish. Res. 2007, 84, 378–389. [Google Scholar] [CrossRef]
- Urban, D. Food habits of Pacific cod and walleye pollock in the northern Gulf of Alaska. Mar. Ecol. Prog. Ser. 2012, 469, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Purcell, J.E.; Sturdevant, M.V. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2001, 210, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.W.; Ortiz, I.; Kotwicki, S.; Aydin, K. Summer diet composition of walleye pollock and predator—Prey relationships with copepods and euphausiids in the eastern Bering Sea, 1987–2011. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 134, 302–311. [Google Scholar] [CrossRef]
- Ko, A.R.; Lee, S.J.; Yang, J.H.; Baeck, G.W. Diet of the walleye pollock Gadus chalcogrammus in the East Sea, Korea. Korean J. Fish. Aquat. Sci. 2020, 53, 456–463. [Google Scholar] [CrossRef]
- Park, H.J.; Park, T.H.; Lee, C.-I.; Kang, C.-K. Ontogenetic shifts in diet and trophic position of walleye pollock, Theragra chalcogramma, in the western East Sea (Japan Sea) revealed by stable isotope and stomach content analyses. Fish. Res. 2018, 204, 297–304. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Pearcy, W.G. Effects of environmental variability on trophic interactions and food web structure in a pelagic upwelling ecosystem. Mar. Ecol. Prog. Ser. 1992, 84, 101–119. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Chapman & Hall: New York, NY, USA, 1990. [Google Scholar]
- Ahlbeck, I.; Hansson, S.; Hjerne, O. Evaluating fish diet analysis methods by individual-based modelling. Can. J. Fish. Aquat. Sci. 2012, 69, 1184–1201. [Google Scholar] [CrossRef]
- Greenstreet, S.P.R.; Rogers, S.I. Indicators of the health of the North Sea fish community: Identifying reference levels for an ecosystem approach to management. ICES J. Mar. Sci. 2006, 63, 573–593. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S. Low functional redundancy in coastal marine assemblages. Ecol. Lett. 2005, 8, 391–400. [Google Scholar] [CrossRef]
- Park, J.M.; Kwak, S.; Choi, H.; Jawad, L.; Riedel, R. Diet patterns of the marbled flounder, Pseudopleuronectes yokohamae, in the mid-western coast of Korea. Sci. Int. 2016, 4, 94–100. [Google Scholar]
- Hyslop, E. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.K.; Rahman, S.M.; Kang, C.-K.; Park, S.-Y.; Lee, S.H.; Park, H.J.; Kim, H.-W.; Lee, C.I. The influence of climate regime shifts on the marine environment and ecosystems in the East Asian Marginal Seas and their mechanisms. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 110–120. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Plymouth Marine Laboratory: Plymouth, UK, 2008. [Google Scholar]
- White, W.; Platell, M.; Potter, I. Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: Implications for resource partitioning. Mar. Biol. 2004, 144, 439–448. [Google Scholar] [CrossRef]
- Linke, T. Trophic Interactions among Abundant Members of the Fish Fauna in a Permanently-Open and a Seasonally-Open Estuary in South-Western Australia; Murdoch University: Perth, Australia, 2011. [Google Scholar]
- Dunn, M.R. Feeding habits of the ommastrephid squid Nototodarus sloanii on the Chatham Rise, New Zealand. N. Z. J. Mar. Freshw. Res. 2009, 43, 1103–1113. [Google Scholar] [CrossRef]
- Jeong, J.M.; Kim, Y.; Song, S.-H.; Park, J.M. Feeding patterns of brown croaker, Miichthys miiuy (Basilewsky, 1855) from the south-western waters off Korea: Size-related and seasonal trends. Thalass. Int. J. Mar. Sci. 2019, 35, 413–420. [Google Scholar] [CrossRef]
- Coyle, K.O.; Eisner, L.B.; Mueter, F.J.; Pinchuk, A.I.; Janout, M.A.; Cieciel, K.D.; Farley, E.V.; Andrews, A.G. Climate change in the southeastern Bering Sea: Impacts on pollock stocks and implications for the oscillating control hypothesis. Fish. Oceanogr. 2011, 20, 139–156. [Google Scholar] [CrossRef]
- Lee, C.I.; Han, M.H.; Jung, H.K.; Park, H.J.; Park, J.M. Spawning season, and factors influencing allometric growth pattern and body condition of walleye pollock Gadus chalcogrammus in the middle East Sea, Korea. Korean J. Ichthyol. 2019, 31, 141–149. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Trenkel, V.M.; Tidd, A.N.; Dawson, W.A.; Du Buit, M.H. Does diet in Celtic Sea fishes reflect prey availability? J. Fish Biol. 2003, 63, 197–212. [Google Scholar] [CrossRef]
- Yamamura, O.; Yabuki, K.; Shida, O.; Watanabe, K.; Honda, S. Spring cannibalism on 1 year walleye pollock in the Doto area, northern Japan: Is it density dependent? J. Fish Biol. 2001, 59, 645–656. [Google Scholar] [CrossRef]
- Bailey, K.M. Interaction between the vertical distribution of juvenile walleye pollock Theragra chalcogramma in the eastern Bering Sea, and cannibalism. Mar. Ecol. Prog. Ser. 1989, 53, 205–213. [Google Scholar] [CrossRef]
- Jung, H.K.; Lee, C.I.; Park, H.J.; Park, J.M. Influences of oceanographic features on spatial and temporal distributions of size spectrum of walleye pollock, Gadus chalcogrammus inhabiting middle eastern coast of Korea. Korean J. Ichthyol. 2020, 32, 148–159. [Google Scholar] [CrossRef]
- Gerking, S.D. Feeding Ecology of Fish; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Langton, R. Diet overlap between Atlantic cod, Gadus morhua, silver hake, Merluccius bilinearis, and fifteen other northwest Atlantic finfish. Fish. Bull. 1982, 80, 745–759. [Google Scholar]
- Barnes, L.M.; Leclerc, M.; Gray, C.A.; Williamson, J.E. Dietary niche differentiation of five sympatric species of Platycephalidae. Environ. Biol. Fishes 2011, 90, 429–441. [Google Scholar] [CrossRef]
- Brodeur, R.D. Prey selection by age-0 walleye pollock, Theragra chalcogramma, in nearshore waters of the Gulf of Alaska. Environ. Biol. Fishes 1998, 51, 175–186. [Google Scholar] [CrossRef]
- Costello, J.H.; Colin, S.P. Morphology, fluid motion and predation by the scyphomedusa Aurelia aurita. Mar. Biol. 1994, 121, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Hirota, Y.; Nemoto, T. Relationship between feeding and vertical distribution of euphausiids in the western Pacific Ocean. Bull. Plankton Soc. Jpn. 1990, 36, 127–135. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Version (06/2021). Available online: www.fishbase.org (accessed on 4 September 2021).
- Palomares, M.L.D.; Pauly, D. SeaLifeBase. World Wide Web Electronic Publication. Version (08/2021). Available online: www.sealifebase.org (accessed on 4 September 2021).
- Sogawa, S.; Sugisaki, H.; Saito, H.; Okazaki, Y.; Ono, T.; Shimode, S.; Kikuchi, T. Seasonal and regional change in vertical distribution and diel vertical migration of four euphausiid species (Euphausia pacifica, Thysanoessa inspinata, T. longipes, and Tessarabrachion oculatum) in the northwestern Pacific. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 109, 1–9. [Google Scholar] [CrossRef]
- Kim, H.S.; Yamaguchi, A.; Ikeda, T. Abundance, biomass and life cycle patterns of euphausiids (Euphausia pacifica, Thysanoessa inspinata and T. longipes) in the Oyashio region, western subarctic Pacific. Plankton Benthos Res. 2009, 4, 43–52. [Google Scholar] [CrossRef] [Green Version]

| Taxa | Prey Items | Prey Type | %F | %N | %W | %IRI |
|---|---|---|---|---|---|---|
| Sipuncula * | Unidentified | Benthic | 0.08 | 0.14 | 0.05 | <0.01 |
| Nematoda | Unidentified | Parasite | 9.26 | 0.44 | 0.53 | 0.16 |
| Polychaeta * | Total | Benthic | 0.72 | 0.02 | 0.48 | 0.01 |
| Nereidae | 0.16 | <0.01 | 0.07 | |||
| Polynoidae | 0.08 | <0.01 | 0.01 | |||
| Unidentified | 0.48 | 0.01 | 0.40 | |||
| Arrow worm * | Unidentified | Pelagic | 0.32 | 0.05 | 0.17 | <0.01 |
| Gastropoda * | Unidentified | Epibenthic | 0.08 | <0.01 | 0.08 | <0.01 |
| Bivalvia * | Unidentified | Epibenthic | 0.32 | 0.01 | 0.03 | <0.01 |
| Cephalopoda * | Total | Bentho-pelagic | 18.36 | 0.82 | 10.86 | 3.70 |
| Berryteuthis magister | 0.81 | 0.10 | 0.71 | |||
| Loliginidae | 0.24 | <0.01 | 0.09 | |||
| Loligo bleekeri | 0.08 | <0.01 | 0.04 | |||
| Octopodidae | 0.32 | 0.01 | 0.31 | |||
| Sepiolidae | 0.08 | <0.01 | 0.04 | |||
| Todarodes pacificus | 0.24 | <0.01 | 0.24 | |||
| Watasenia scintillans | 2.58 | 0.12 | 1.54 | |||
| Unidentified | 14.57 | 0.56 | 7.88 | |||
| Copepoda * | Total | Pelagic | 5.15 | 3.14 | 1.36 | 0.40 |
| Calanus sinicus | 0.89 | 0.75 | 0.39 | |||
| Neocalanus copepodite | 0.24 | 0.05 | <0.01 | |||
| Paraeuchaeta elongata | 1.77 | 1.58 | 0.52 | |||
| Tortanus discaudatus | 0.16 | <0.01 | <0.01 | |||
| Unidentified | 2.42 | 0.76 | 0.44 | |||
| Cumacea * | Unidentified | Epibenthic | 0.16 | <0.01 | <0.01 | <0.01 |
| Ostracoda * | Unidentified | Epibenthic | 0.08 | <0.01 | <0.01 | <0.01 |
| Isopoda * | Unidentified | Epibenthic | 0.08 | <0.01 | <0.01 | <0.01 |
| Euphausiacea * | Total | Bentho-pelagic | 35.99 | 61.20 | 27.30 | 55.04 |
| Euphausia sp. A | 0.08 | 0.01 | 0.04 | |||
| Euphausia sp. B | 0.08 | <0.01 | <0.01 | |||
| Euphausia pacifica | 14.41 | 46.90 | 12.29 | |||
| Thysanoessa spp. | 2.33 | 0.45 | 0.79 | |||
| Unidentified | 20.29 | 13.83 | 14.17 | |||
| Mysidacea * | Total | Bentho-pelagic | 25.04 | 13.36 | 14.03 | 11.85 |
| Neomysis spp. | 10.79 | 9.23 | 5.64 | |||
| Unidentified | 14.65 | 4.13 | 8.39 | |||
| Amphipoda * | Total | Epibenthic | 27.78 | 18.28 | 8.60 | 12.90 |
| Ampelisca sp. | 0.08 | <0.01 | <0.01 | |||
| Anonyx sp. | 0.97 | 0.11 | 0.32 | |||
| Gammeridae spp. | 2.66 | 1.19 | 1.05 | |||
| Primno abyssalis | 0.32 | 0.02 | <0.01 | |||
| Themisto spp. | Semi-pelagic | 24.88 | 16.96 | 7.21 | ||
| Caridea * | Total | Hyper-benthic | 29.47 | 1.44 | 18.82 | 10.31 |
| Argis lar | 0.32 | <0.01 | 0.18 | |||
| Crangon dalli | 0.08 | <0.01 | <0.01 | |||
| Crangonidae spp. | 6.12 | 0.22 | 2.92 | |||
| Hippolytidae | 1.13 | 0.04 | 0.41 | |||
| Lebbeus polaris | 0.24 | 0.01 | 0.22 | |||
| Neocrangon communis | 5.96 | 0.27 | 3.17 | |||
| Nephropidae | 0.08 | <0.01 | 0.03 | |||
| Pandalidae spp. | 4.03 | 0.19 | 1.85 | |||
| Pandalus borealis | 6.60 | 0.29 | 4.75 | |||
| Spirontocaris spinus | 0.24 | <0.01 | 0.12 | |||
| Unidentified | 9.10 | 0.39 | 5.17 | |||
| Other crustacea | Unidentified | Epibenthic | 2.82 | 0.09 | 1.98 | 0.10 |
| Ophiuroidae * | Unidentified | Epibenthic | 0.48 | 0.02 | <0.01 | <0.01 |
| Teleostei * | Total | Bentho-pelagic | 21.58 | 0.89 | 13.64 | 5.42 |
| Arctoscopus japonicus | 0.56 | 0.02 | 0.50 | |||
| Bothrocara hollandi | 6.92 | 0.27 | 5.04 | |||
| Clupeidae | 0.40 | 0.02 | 0.23 | |||
| Gadus chalcogrammus | 0.64 | 0.03 | 0.47 | |||
| Lycodes toyamensis | 0.08 | <0.01 | 0.01 | |||
| Unidentified fish egg | 0.08 | 0.03 | 0.08 | |||
| Unidentified | 13.37 | 0.52 | 7.32 | |||
| Seaweed * | Unidentified | Benthic | 0.08 | <0.01 | <0.01 | <0.01 |
| Other materials | Unidentified | 2.66 | 0.08 | 2.08 | 0.10 |
| Source | df | MS | Pseudo-F | COV | P (perm) |
|---|---|---|---|---|---|
| Size | 4 | 8286.8 | 6.123 | 15.894 | 0.001 |
| Depth | 4 | 6688.3 | 4.742 | 13.702 | 0.001 |
| Season | 7 | 4122.8 | 3.047 | 14.127 | 0.001 |
| Size × Depth | 13 | 1321.9 | 0.977 | 6.698 | 0.512 |
| Size × Season | 25 | 2081.4 | 1.538 | 13.896 | 0.006 |
| Depth × Season | 26 | 2122.3 | 1.561 | 10.573 | 0.016 |
| Size × Depth × Season | 51 | 1919.9 | 1.419 | 16.340 | 0.016 |
| Residuals | 221 | 1353.3 | 35.270 |
| Predictor Variables | Pseudo-F | R2 | p | Prop |
|---|---|---|---|---|
| Marginal test | ||||
| Total length (TL) | 109.510 | 0.001 | 0.081 | |
| Maturity | 96.972 | 0.001 | 0.072 | |
| Depth | 79.843 | 0.001 | 0.060 | |
| Year | 15.669 | 0.001 | 0.012 | |
| Season | 19.142 | 0.001 | 0.015 | |
| Month | 25.786 | 0.001 | 0.021 | |
| Conditional Test | ||||
| +TL | 109.510 | 0.081 | 0.001 | 0.081 |
| +Depth | 28.274 | 0.102 | 0.001 | 0.021 |
| +Month | 20.042 | 0.116 | 0.001 | 0.014 |
| +Year | 13.451 | 0.126 | 0.001 | 0.010 |
| +Maturity | 4.621 | 0.129 | 0.001 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Jung, H.-K.; Lee, C.-I. Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast. J. Mar. Sci. Eng. 2021, 9, 1154. https://doi.org/10.3390/jmse9111154
Park J-M, Jung H-K, Lee C-I. Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast. Journal of Marine Science and Engineering. 2021; 9(11):1154. https://doi.org/10.3390/jmse9111154
Chicago/Turabian StylePark, Joo-Myun, Hae-Kun Jung, and Chung-Il Lee. 2021. "Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast" Journal of Marine Science and Engineering 9, no. 11: 1154. https://doi.org/10.3390/jmse9111154
APA StylePark, J.-M., Jung, H.-K., & Lee, C.-I. (2021). Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast. Journal of Marine Science and Engineering, 9(11), 1154. https://doi.org/10.3390/jmse9111154








