The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT)
Abstract
:1. Introduction
- Species living in colder water will tend to be larger than those living in warmer temperatures, other factors being equal.
- Within different populations of the same species, maximum size and mean size at first maturity should decline with temperature.
- The growth of chaetognaths should conform to the VBGF.
- 4.
- Arrow worms should remain small (compared, e.g., with other zooplanktivorous WBE, such as anchovies) and exhibit growth performances (requiring a high O2 supply) that are very low (again compared to fishes).
- 5.
- In a given population, chaetognaths reach maturity at a fraction of their maximum length that is similar to the fraction that would occur in fish of the same size.
2. Materials and Methods
2.1. Taxonomy and Compilation of Chaetognath Life Traits
2.2. Morphometrics
2.3. Growth, Growth Comparisons, and Longevity
2.4. Oxygen, Temperature, and Arrow Worms
2.5. Reproduction
3. Results
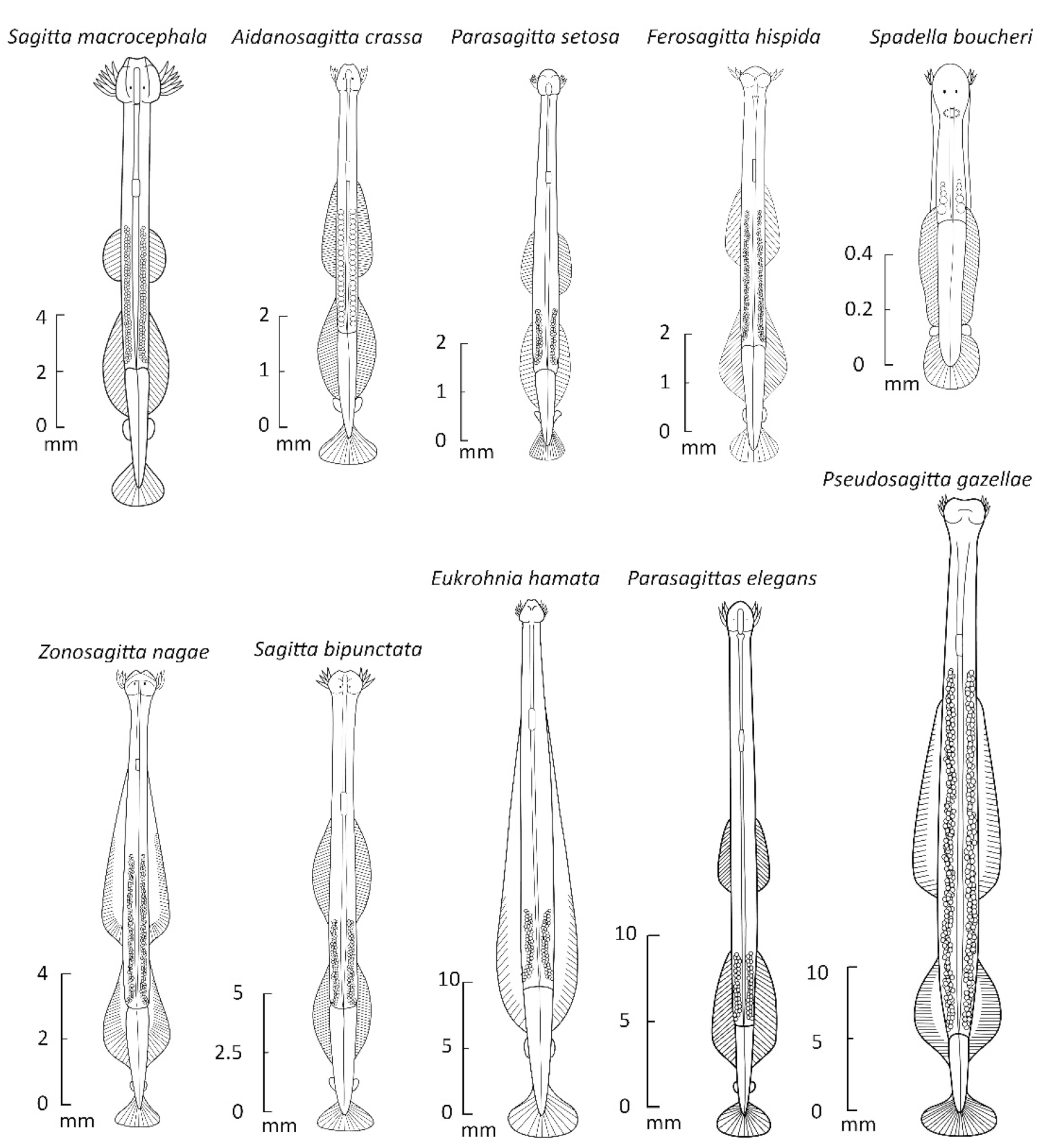
3.1. Early Illustrative and Taxonomic Work on the Chaetognatha, or Arrow Worms
3.2. Chinese Studies on Arrow Worms
3.3. Body Length in 132 Species: Relation to Temperature and Habitat
3.4. Body Lengths in Three Chaetognath Species Experiencing Different Water Temperatures
3.5. Morphometrics and Growth of Chaetognaths
3.6. Reproduction in Chaetognaths
4. Discussion
- Species occurring in colder waters are generally larger than those in warmer waters.
- Individual chaetognaths reach larger sizes in colder than in warmer waters.
- The growth of chaetognaths can be described by the VBGF.
- Chaetognaths remain small and exhibit low growth performance.
- Chaetognaths mature at a fraction of their maximum size that is similar to that of fish of the same size.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thuesen, E.V. Phylum Chaetognatha. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=2081 (accessed on 10 October 2021).
- Palomares, M.L.D.; Pauly, D. SeaLifeBase. Available online: www.sealifebase.org (accessed on 2 May 2021).
- Reeve, M.R. The biology of Chaetognatha. I. Quantitative aspects of growth and egg production in Sagitta hispida. In Marine Food Chains; Steele, J.H., Ed.; University of California Press: Berkeley, CA, USA, 1970; pp. 168–189. [Google Scholar]
- Casanova, J.-P.; Perez, Y. A Dwarf New Spadella (Chaetognatha) from Bora Bay (Miyako Island, Japan). Cah. Biol. Mar. 2000, 41, 137–141. [Google Scholar] [CrossRef]
- Ritter-Záhony, R. Die Chaetognathen der Gazelle Expedition. Zool. Anz. 1909, 34, 787–793. (In German) [Google Scholar]
- Ikeda, T.; Kirkwood, R. Metabolism and Elemental Composition of a Giant Chaetognath Sagitta gazellae from the Southern Ocean. Mar. Biol. 1989, 100, 261–267. [Google Scholar] [CrossRef]
- Feigenbaum, D.L. Food and feeding behaviour. In The Biology of Chaetognaths; Bone, Q., Kapp, H., Pierrot-Bults, A.C., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 45–54. ISBN 978-0-19-857715-7. [Google Scholar]
- Feigenbaum, D.; Reeve, M.R. Prey Detection in the Chaetognatha: Response to a Vibrating Probe and Experimental Determination of Attack Distance in Large Aquaria. Limnol. Oceanogr. 1977, 22, 1052–1058. [Google Scholar] [CrossRef]
- Kimmerer, W.J. Selective Predation and Its Impact on Prey of Sagitta enflata (Chaetognatha). Mar. Ecol. Prog. Ser. 1984, 15, 55–62. [Google Scholar] [CrossRef]
- Casanova, J.-P. Quatre Nouveaux Chaetognathes Atlantiques Abyssaux (Genre Heterokrohnia): Description, Remarques Éthologiques et Biogéographiques. Oceanol. Acta 1986, 9, 469–477. [Google Scholar]
- Casanova, J.-P.; Barthélémy, R.-M.; Duvert, M.; Faure, E. Chaetognaths Feed Primarily on Dissolved and Fine Particulate Organic Matter, Not on Prey: Implications for Marine Food Webs. Hypotheses Life Sci. 2012, 2, 20–29. [Google Scholar]
- Grigor, J.J.; Schmid, M.S.; Caouette, M.; St.-Onge, V.; Brown, T.A.; Barthélémy, R.-M. Non-Carnivorous Feeding in Arctic Chaetognaths. Prog. Oceanogr. 2020, 186, 102388. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. Available online: www.fishbase.org (accessed on 2 May 2021).
- Pauly, D. A Précis of Gill-Oxygen Limitation Theory (GOLT), with Some Emphasis on the Eastern Mediterranean. Mediterr. Mar. Sci. 2019, 20, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. Gasping Fish and Panting Squids: Oxygen Temperature and the Growth of Water Breathing Animals, 2nd ed.; Excellence in Ecology Series; International Ecology Institute: Oldendorf/Luhe, Germany, 2019; ISBN 978-3-946729-22-8. [Google Scholar]
- Pauly, D. The Gill-Oxygen Limitation Theory (GOLT) and Its Critics. Sci. Adv. 2021, 7, eabc6050. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. Observations on the Structure and Propagation of the Genus Sagitta. Ann. Mag. Nat. Hist. 1844, 13, 1–6. [Google Scholar] [CrossRef]
- Bullock, T.H. Chaetognatha, Pogonophora, Hemichordata, and Chordata Tunicata. In Structure and Function in the Nervous Systems of Invertebrates; Chapter 27; Bullock, T.H., Horridge, G.A., Eds.; W.H. Freeman: San Francisco, CA, USA, 1965; Volume II, pp. 1559–1592. [Google Scholar]
- Pauly, D. Beyond Our Original Horizons: The Tropicalization of Beverton and Holt. Rev. Fish Biol. Fish. 1998, 8, 307–334. [Google Scholar] [CrossRef]
- Shinn, F. Chaetognatha. In Microscopic Anatomy of Invertebrates, Volume 15: Hemichordata, Chaetognatha, and the Invertebrate Chordates; Harrison, F.W., Ruppert, E.E., Eds.; Wiley-Liss: New York, NY, USA, 1997; pp. 103–220. [Google Scholar]
- Bone, Q.; Duvert, M. Locomotion and Buoyancy. In The Biology of Chaetognaths; Bone, Q., Kapp, H., Pierrot-Bults, A.C., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 32–44. ISBN 978-0-19-857715-7. [Google Scholar]
- OBIS Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. Available online: www.obis.org (accessed on 1 June 2020).
- Kalfatovic, M.R.; Costantino, G.; Rinaldo, C.A. The Biodiversity Heritage Library: Unveiling a World of Knowledge About Life on Earth. In Proceedings of the Digital Libraries for Open Knowledge; Doucet, A., Isaac, A., Golub, K., Aalberg, T., Jatowt, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 352–355. [Google Scholar]
- Bailly, N.; Gerovasileiou, V.; Arvanitidis, C.; Legakis, A. Introduction to the Greek Taxon Information System (GTIS) in LifeWatchGreece: The Construction of the Preliminary Checklists of Species of Greece. Biodivers. Data J. 2016, 4, e7959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierrot-Bults, A.C. Chaetognatha of the World. Zoological Museum, University of Amsterdam. Available online: http://www.species-identification.org/species.php?species_group=chaetognatha&menuentry=index&selected=wetenschappelijk (accessed on 10 October 2021).
- Von Bertalanffy, L. Theoretische Biologie. Zweiter Band: Stoffwechsel, Wachstum; A. Francke Verlag: Bern, Germany, 1951. (In German) [Google Scholar]
- Von Bertalanffy, L. Principles and theory of growth. In Fundamental Aspects of Normal and Malignant Growth; Nowinski, W.W., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1960; pp. 137–259. [Google Scholar]
- Cox, M.M.; Nelson, D.L. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2008; ISBN 978-0-7167-7108-1. [Google Scholar]
- Grigor, J.J.; Schmid, M.S.; Fortier, L. Growth and Reproduction of the Chaetognaths Eukrohnia hamata and Parasagitta elegans in the Canadian Arctic Ocean: Capital Breeding versus Income Breeding. J. Plankton Res. 2017, 39, 910–929. [Google Scholar] [CrossRef] [Green Version]
- Welch, H.E.; Siferd, T.D.; Bruecker, P. Population Densities, Growth, and Respiration of the Chaetognath Parasagitta elegans in the Canadian High Arctic. Can. J. Fish. Aquat. Sci. 1996, 53, 520–527. [Google Scholar] [CrossRef]
- Zo, Z. Breeding and Growth of the Chaetognath Sagitta elegans in Bedford Basin. Limnol. Oceanogr. 1973, 18, 750–756. [Google Scholar] [CrossRef]
- Hirst, A.G.; Forster, J. When Growth Models are not Universal: Evidence from Marine Invertebrates. Proc. Royal Soc. B 2013, 280, 20131546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binohlan, C.; Pauly, D. The POPGROWTH Table. In FishBase 2000 Concepts, Design and Data Sources; Froese, R., Pauly, D., Eds.; ICLARM: Los Baños, Philippines, 2000; pp. 138–145. [Google Scholar]
- De Jager, S.; Dekkers, W.J. Relations Between Gill Structure and Activity in Fish. Neth. J. Zool. 1974, 25, 276–308. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. A Mechanism for the Juvenile-to-Adult Transition in Fishes. ICES J. Mar. Sci. 1984, 41, 280–284. [Google Scholar] [CrossRef]
- Meyer, K.A.; Schill, D.J. The Gill-Oxygen Limitation Theory and Size at Maturity/Maximum Size Relationships for Salmonid Populations Occupying Flowing Waters. J. Fish Biol. 2020. [Google Scholar] [CrossRef]
- Slabber, M. Natuurkundige Verlustigingen, Behelzende Microscopise Waarneemingen Van de in—En Uitlandse Water—En Land-Dieren; J. Bosch: Haarlem, The Netherlands, 1778. (In Dutch) [Google Scholar]
- Van der Hoeven, J. Eenige Aanteekeningen over Martinus Slabber’s Natuurkundige Verlustigingen; Benevens Opgave Der Systematische Namen van de Daarin Afgebeelde Diersoorten. Versl. Meded. Kon. Akad. Wet. 1862, 14, 270–285. (In Dutch) [Google Scholar]
- Van Benthem Jutting, W.S.S. Martinus Slabber (1740–1835): Amateur-zoöloog in Zeeland. Tijdschriftenbank Zeeland 1970, 1970, 45–66. (In Dutch) [Google Scholar]
- Welter-Schultes, F. Reference Summary for Slabber, M. 1769–1778. 12 September 2011. Available online: http://www.animalbase.uni-goettingen.de/zooweb/servlet/AnimalBase/home/reference?id=5303 (accessed on 10 October 2021).
- Peijnenburg, K.T.C.A.; Pierrot-Bults, A.C. Quantitative Morphological Variation in Sagitta setosa Müller, 1847 (Chaetognatha) and Two Closely Related Taxa. Contrib. Zool. 2004, 73, 305–315. [Google Scholar] [CrossRef] [Green Version]
- De Blainville, H.-M.D. Manuel de Malacologie et de Conchyliologie; F.G. Levrault, Edit.: Paris, France, 1825; pp. 1–668. (In French) [Google Scholar] [CrossRef]
- Eydoux, F.; Souleyet, M. Vers. In Voyage autour du Monde Exécuté pendant les Années 1836 et 1837 sur la Corvette “La Bonite”; Bertrand, A., Ed.; A. Bertrand: Paris, France, 1852; Volume 2, pp. 645–657. (In French) [Google Scholar]
- De Blainville, H.-M.D. [Accounts for] Sagitta, Flèche; Sagitella, Sagitelle. In Dictionnaire des Sciences Naturelles; Levrault, F.G., Ed.; Le Normant: Strasbourg, France, 1827; Volume T.47, pp. 4–8. (In French) [Google Scholar]
- Quoy, J.R.C.; Gaimard, J.P. Observations Zoologiques Faites à Bord de l’Astrolabe, en Mai 1826, dans le Détroit de Gibraltar (suite et fin). Description des genres Biphore, Carinaire, Hyale, Flèche, Cléodore, Anatife et Briarée. Ann. Sci. Nat. 1827, 10, 225–239. (In French) [Google Scholar]
- Keynes, R.D. (Ed.) Charles Darwin’s Zoology Notes & Specimen Lists from H.M.S. Beagle; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-46569-4. [Google Scholar]
- D’Orbigny, A.D. Mollusques. In Voyage dans l’Amérique Méridionale; Bertrand: Paris, France, 1836; Volume 5, pp. 1–758. (In French) [Google Scholar]
- Forbes, E. On the Addition of the Order Nucleobracha to the British Molluscous Fauna. In Report of the 13th meeting British Association for the Advancement of Science held at Cork in August 1943. Notices and Abstracts of Miscellaneous Communications to the Sections; John Murray: London, UK, 1844; pp. 72–73. [Google Scholar]
- Charles Darwin’s Notebooks, 1836-1844: Geology, Transmutation of Species, Metaphysical Enquiries; Barrett, P.H.; Gautrey, P.J.; Herbert, S.; Kohn, D.; Smith, S. (Eds.) British Museum (Natural History): London, UK; Cornell University Press: Ithaca, NY, USA, 1987. [Google Scholar]
- Leuckart, R. Bericht Über die Leistungen in der Naturgeschichte der Niederen Thiere Während des Jahres 1848–1853. Arch. Naturgesch. 1854, 20, 289–473. (In German) [Google Scholar]
- Leuckart, R. Nachträge und Berichtigungen zu dem ersten Bande von J. van Der Hoeven’s Handbuch der Zoologie. Eine Systematisch Geordnete Übersicht der Hauptsächlichste Neueren Leistungen:über die Zoologie der Wirbellosen Thiere; L. Voss: Leipzig, Germany, 1856. (In German) [Google Scholar] [CrossRef] [Green Version]
- Hertwig, O. Die Chaetognathen: Ihre Anatomie, Systematik und Entwicklungsgechichte: Eine Monographie. In Studien zur Blättertheorie; G. Fischer: Jena, Germany, 1880. (In German) [Google Scholar]
- Gegenbaur, C. Grundzüge der Vergleichenden Anatomie; Wilhelm Engelmann: Leipzig, Germany, 1859. (In German) [Google Scholar] [CrossRef] [Green Version]
- Harting, P. Wormen. In Leerboek van de Grondbeginselen der Dierkunde in Haren Geheelen Omvang: Derde Deel Ongewervelde Dieren; H.C.A. Campagne: Tiel, The Netherlands, 1871; pp. 489–796. (In Dutch) [Google Scholar]
- Grassi, B.J. I Chetognati. Anatomia e Sistematica con Aggiunte Embriologiche. Fauna und Flora des Golfes von Neapel, 5, Monographie: Die Chaetognathen; W. Engelmann: Leipzig, Germany, 1883; pp. 1–172. (In Italian) [Google Scholar]
- Marlétaz, F.; Martin, E.; Perez, Y.; Papillon, D.; Caubit, X.; Lowe, C.; Freeman, R.; Fasano, L.; Dossat, C.; Wincker, P.; et al. Chaetognath Phylogenomics: A Protostome with Deuterostome-like Development. Curr. Biol. 2006, 16, R577–R578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matus, D.Q.; Copley, R.R.; Dunn, C.W.; Hejnol, A.; Eccleston, H.; Halanych, K.M.; Martindale, M.Q.; Telford, M.J. Broad Taxon and Gene Sampling Indicate that Chaetognaths are Protostomes. Curr. Biol. 2006, 16, R575–R576. [Google Scholar] [CrossRef] [Green Version]
- Marlétaz, F.; Peijnenburg, K.T.C.A.; Goto, T.; Satoh, N.; Rokhsar, D.S. A New Spiralian Phylogeny Places the Enigmatic Arrow Worms among Gnathiferans. Curr. Biol. 2019, 29, 312–318.e3. [Google Scholar] [CrossRef] [Green Version]
- Barthélémy, R.-M.; Casanova, J.-P. Progress in the Knowledge of the Phylogeny of the Chaetognatha Needs Both Molecular Biology and Zoology. Int. J. Fauna Biol. Stud. 2019, 6, 21–26. [Google Scholar]
- Ritter-Záhony, R. Revision der Chätognathen. In Deutsche Südpolar-Expedition, 1901-1903, im Auftrage des Reichsamtes des Innern; von Drygalski, E., Ed.; Georg Reimer: Berlin, Germany, 1911; pp. 1–72. [Google Scholar]
- Tokioka, T. The Taxonomical Outline of Chaetognaths. Publ. Seto Mar. Biol. Lab. 1965, 12, 335–357. [Google Scholar] [CrossRef]
- Vinther, J.; Parry, L.A. Bilateral Jaw Elements in Amiskwia sagittiformis Bridge the Morphological Gap between Gnathiferans and Chaetognaths. Curr. Biol. 2019, 29, 881–888.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, J.-B.; Cheung, B. Amiskwia is a Large Cambrian Gnathiferan with Complex Gnathostomulid-like Jaws. Commun. Biol. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bieri, R. Six New Genera in the Chaetognath Family Sagittidae. Gulf Caribb. Res. 1991, 8, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Bieri, R. Systematics of the Chaetognatha. In The Biology of Chaetognaths; Bone, Q., Kapp, H., Pierrot-Bults, A.C., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 122–136. ISBN 978-0-19-857715-7. [Google Scholar]
- Briggs, D.E.G.; Caron, J.-B. A Large Cambrian Chaetognath with Supernumerary Grasping Spines. Curr. Biol. 2017, 27, 2536–2543.e1. [Google Scholar] [CrossRef]
- Shu, D.; Conway Morris, S.; Han, J.; Hoyal Cuthill, J.F.; Zhang, Z.; Cheng, M.; Huang, H. Multi-Jawed Chaetognaths from the Chengjiang Lagerstätte (Cambrian, Series 2, Stage 3) of Yunnan, China. Palaeontology 2017, 60, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Gasmi, S.; Nève, G.; Pech, N.; Tekaya, S.; Gilles, A.; Perez, Y. Evolutionary History of Chaetognatha Inferred from Molecular and Morphological Data: A Case Study for Body Plan Simplification. Front. Zool. 2014, 11, 84. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.C. Phylum Chaetognatha (Leuckart, 1894). In Checklist of Marine Biota of China Seas; Liu, R., Ed.; Science Press: Beijing, China, 2008; pp. 842–845. (In Chinese) [Google Scholar]
- Michael, E.L. Report on the Chaetognatha Collected by the United States Fisheries Steamer “Albatross” during the Philippine Expedition, 1907–1910; United States National Museum: Washington, DC, USA, 1919. (In Chinese) [Google Scholar]
- Hsu, F. On Some Species of Sagitta of China. Sinensia 1943, 14, 120–139. (In Chinese) [Google Scholar]
- Sproston, N.G. A preliminary survey of the plankton of the Chu-San region, with a review of the relevant literature. Sinensia 1949, 20, 58–161. (In Chinese) [Google Scholar]
- Xu, Z.L.; Dai, Y.F.; Chen, X.Q. Study on Species Composition and Diversity of Chaetognatha in the East China Sea. J. Shanghai Fish. Univ. 2004, 13, 304–309. (In Chinese) [Google Scholar]
- Wang, Y.L.; Yuan, Q. Ecological Study on Chaetognatha in the East China Sea. Ⅰ. Species Composition and Quantity Distribution. Mar. Fish. 2004, 26, 29–34. (In Chinese) [Google Scholar]
- Dai, Y.Y. A study on the Species Diversity of Chaetognaths in China Seas. Chin. Biodivers. 1995, 3, 69–73. (In Chinese) [Google Scholar]
- Dai, Y. Study on the Ecological Characters of Chaetognatha in Waters of Southern Huanghai Sea and East China Sea I. The Characteristics of Quantitative Distribution. Acta Oceanol. Sin. 2006, 28, 106–111. (In Chinese) [Google Scholar]
- Jiang, W.; Wang, Y.L.; Xu, Z.L. Studies on Distribution of Chaetognatha in Taiwan Strait. J. Oceanol. Taiwan Strait 2003, 22, 150–154. (In Chinese) [Google Scholar]
- Zhang, G.X.; Yi, J.Q. Diurnal Vertical Migration of Chaetognatha around Nansha Islands Waters Southern South China Sea. J. Trop. Oceanogr. 1995, 24, 49–56. (In Chinese) [Google Scholar]
- Du, F.Y.; Li, C.H.; Jia, X.P. A Preliminary Analysis of Chaetognatha Species Groups in Beibu Bay. J. Fish. China 2005, 29, 43–47. (In Chinese) [Google Scholar]
- Dai, Y.; Lin, M. Study on the Ecological Characters of Chaetognatha in Waters of Southern Huanghai Sea and East China Sea II. Community Characteristics. Acta Oceanol. Sin. 2007, 29, 90–97. (In Chinese) [Google Scholar]
- Xu, Z.L.; Dai, Y.F.; Chen, Y.Q. Relationship between Chaetognatha Abundance and Environmental Factors in the East China Sea. J. Shanghai Fish. Univ. 2004, 13, 203–208. (In Chinese) [Google Scholar]
- Xu, Z.L.; Chen, X.Q. Relationships between Dominant Species of Chaetognatha and Environmental Factors in the East China Sea. J. Fish. Sci. China 2005, 12, 76–82. (In Chinese) [Google Scholar]
- Liu, Q.; Qu, H.; Zhang, S.; Wei, J. Experimental Study on the Feeding Ecology of Sagitta crassa. J. Fish. China 2006, 30, 767–772. (In Chinese) [Google Scholar]
- Liu, Q.; Qu, H.; Zhang, S. Preliminary Studies on the Tolerance to Temperature and Salinity in Sagitta crassa. Trans. Oceanol. Limnol. 2007, 1, 111–116. (In Chinese) [Google Scholar]
- Liu, Q.; Zhu, H.Y.; Liu, F.; Ding, Z.Y. Effects of Temperature and Salinity on Oxygen Consumption Rate and Asphyxiation Point of Sagitta crassa. Chin. J. Appl. Ecol. 2011, 22, 3081–3086. (In Chinese) [Google Scholar]
- Huo, Y.Z.; Sun, S.; Yang, B. Life Cycle of the Chaetognath Sagitta crassa in the Southern Yellow Sea, China. Oceanol. Limnol. Sin. 2010, 41, 181–185. (In Chinese) [Google Scholar]
- Lindsey, C.C. Body Sizes of Poikilotherm Vertebrates at Different Latitudes. Evolution 1966, 20, 456–465. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.A.D.; Frank, K.T.; Leggett, W.C. Global Variation in Marine Fish Body Size and its Role in Biodiversity–Ecosystem Functioning. Mar. Ecol. Prog. Ser. 2010, 405, 1–13. [Google Scholar] [CrossRef] [Green Version]
- McLaren, I.A. Adaptive Significance of Large Size and Long Life of the Chaetognath Sagitta elegans in the Arctic. Ecology 1966, 47, 852–855. [Google Scholar] [CrossRef]
- Reeve, M.R.; Walter, M.A. Conditions of Culture, Food-Size Selection, and the Effects of Temperature and Salinity on Growth Rate and Generation Time in Sagitta hispida Conant. J. Exp. Mar. Biol. Ecol. 1972, 9, 191–200. [Google Scholar] [CrossRef]
- Reeve, M.R.; Baker, L.D. Production of Two Planktonic Carnivores (Chaetognath and Ctenophore) in South Florida Inshore Waters. Fish. Bull. 1975, 73, 238–248. [Google Scholar]
- Pearre, S. Feeding by Chaetognatha: Energy Balance and Importance of Various Components of the Diet of Sagitta Elegans. Mar. Ecol. Prog. Ser. 1981, 5, 45–54. [Google Scholar] [CrossRef]
- Harrison, R.J. Phosphorus and Iron in Sagitta setosa and Sagitta elegans. J. Mar. Biolog. Assoc. 1940, 24, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.Y. The Study of Size-Biomass Relationships of Dominant Species of Yellow Sea and East China Sea through Image Technology. Master’s Thesis, Graduate School, Institute of Oceanology, Chinese Academy of Science, Qingdao, China, 2013. (In Chinese). [Google Scholar]
- Reeve, M.R.; Raymont, J.E.G.; Raymont, J.K.B. Seasonal Biochemical Composition and Energy Sources of Sagitta hispida. Mar. Biol 1970, 6, 357–364. [Google Scholar] [CrossRef]
- Kiørboe, T. Zooplankton Body Composition. Limnol. Oceanogr. 2013, 58, 1843–1850. [Google Scholar] [CrossRef] [Green Version]
- Thuesen, E.V.; Childress, J.J. Enzymatic Activities and Metabolic Rates of Pelagic Chaetognaths: Lack of Depth-Related Declines. Limnol. Oceanogr. 1993, 38, 935–948. [Google Scholar] [CrossRef]
- Sameoto, D.D. Yearly Respiration Rate and Estimated Energy Budget for Sagitta elegans. J. Fish. Res. Bd. Can. 1972, 29, 987–996. [Google Scholar] [CrossRef]
- Kruse, S. Biology of Meso- and Bathypelagic Chaetognaths in the Southern Ocean. Ph.D. Thesis, Universität Bremen, Bremen, Germany, 2009. [Google Scholar]
- Palomares, M.L.D.; Pauly, D. The Growth of Jellyfishes. Hydrobiologia 2009, 616, 11–21. [Google Scholar] [CrossRef]
- Brey, T.; Müller-Wiegmann, C.; Zittier, Z.M.C.; Hagen, W. Body Composition in Aquatic Organisms—A Global Data Bank of Relationships between Mass, Elemental Composition and Energy Content. J. Sea Res. 2010, 64, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment; 5th ed.; Cambridge University Press: Cambridge, UK, 1997; ISBN 978-0-521-57098-5. [Google Scholar]
- Russell, F.S. On the Biology of Sagitta I. The Breeding and Growth of Sagitta elegans Verrill in the Plymouth Area, 1930–1931. J. Mar. Biolog. Assoc. U.K. 1932, 18, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Rhaesa, A.; Vieler, V. Spadella kappae, a New Small Benthic Chaetognath (Spadellidae) from Roscoff, France. Cah. Biol. Mar. 2018, 59, 257–265. [Google Scholar] [CrossRef]
- Schmidt-Rhaesa, A.; Vieler, V. Validation of Spadella kappae Schmidt-Rhaesa & Vieler, a Small Benthic Chaetognath from Roscoff, France. Zootaxa 2020, 4759, 300. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Johnson, M.W.; Fleming, R.H. The Oceans: Their Physics, Chemistry and General Biology; Prentice Hall: New York, NY, USA, 1942. [Google Scholar]
- Audzijonyte, A.; Barneche, D.R.; Baudron, A.R.; Belmaker, J.; Clark, T.D.; Marshall, C.T.; Morrongiello, J.R.; van Rijn, I. Is Oxygen Limitation in Warming Waters a Valid Mechanism to Explain Decreased Body Sizes in Aquatic Ectotherms? Glob. Ecol. Biogeogr. 2019, 28, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Pearre, S. Growth and reproduction. In The Biology of Chaetognaths; Bone, Q., Kapp, H., Pierrot-Bults, A.C., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 61–75. ISBN 978-0-19-857715-7. [Google Scholar]
- Russell, F.S. On the Biology of Sagitta. II. The Breeding and Growth of Sagitta Setosa J. Müller in the Plymouth Area, 1930–1931, with a Comparison with That of S. elegans Verrill. J. Mar. Biolog. Assoc. U.K. 1932, 18, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A. Rearing Experiments of a Chaetognath, Sagitta crassa. Inf. Bull. Planktol. Jpn. 1966, 13, 62–65. (In Japanese) [Google Scholar]
- Nagasawa, S.; Marumo, R. Seasonal Variation in Composition and Number of Epipelagic Chaetognaths in Sagami Bay, Japan. La Mer 1977, 15, 185–195. (In Japanese) [Google Scholar]
- David, P.M. The Distribution of Sagitta gazellae Ritter-Záhony. Discov. Rep. 1955, 27, 235–278. [Google Scholar]
- Kotori, M. The Biology of Chaetognatha in the Bering Sea and the Northern North Pacific Ocean, with Emphasis on Sagitta elegans. Mem. Fac. Fish. Hokkaido Univ. 1976, 23, 95–183. [Google Scholar]
- Kruse, S.; Hagen, W.; Bathmann, U. Feeding Ecology and Energetics of the Antarctic Chaetognaths Eukrohnia hamata, E. bathypelagica and E. bathyantarctica. Mar. Biol. 2010, 157, 2289–2302. [Google Scholar] [CrossRef]
- Bone, Q.; Kapp, H.; Pierrot-Bults, A.C. (Eds.) The Biology of Chaetognaths; Oxford University Press: Oxford, UK, 1991; ISBN 978-0-19-857715-7. [Google Scholar]
- Parry, D.A. Structure and Function of the Gut in Spadella cephaloptera and Sagitta setosa. J. Mar. Biolog. Assoc. U.K. 1944, 26, 16–36. [Google Scholar] [CrossRef] [Green Version]
- Bleich, S.; Müller, C.H.G.; Graf, G.; Hanke, W. Flow Generation by the Corona Ciliata in Chaetognatha—Quantification and Implications for Current Functional Hypotheses. Zoology 2017, 125, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kapp, H. Morphology and anatomy. In The Biology of Chaetognaths; Bone, Q., Kapp, H., Pierrot-Bults, A.C., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 5–17. ISBN 978-0-19-857715-7. [Google Scholar]
- Katsu-Kimura, Y.; Nakaya, F.; Baba, S.A.; Mogami, Y. Substantial Energy Expenditure for Locomotion in Ciliates Verified by Means of Simultaneous Measurement of Oxygen Consumption Rate and Swimming Speed. J. Exp. Biol. 2009, 212, 1819–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauly, D.; Cheung, W.W.L. Sound Physiological Knowledge and Principles in Modeling Shrinking of Fishes under Climate Change. Glob. Change Biol. 2017, 24, e15–e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.R. Relation Between Production and Biomass. J. Fish. Res. Bd. Can. 1971, 28, 1573–1581. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V. Ecosystem Models. Chapter 10. In Handbook of Fish Biology and Fisheries; Hart, P., Reynolds, J., Eds.; Blackwell Publishing: Oxford, UK, 2002; Volume 2, pp. 211–227. [Google Scholar]
- Colléter, M.; Valls, A.; Guitton, J.; Gascuel, D.; Pauly, D.; Christensen, V. Global Overview of the Applications of the Ecopath with Ecosim Modeling Approach Using the EcoBase Models Repository. Ecol. Model. 2015, 302, 42–53. [Google Scholar] [CrossRef]





| Parameter | Coeff. | SE | t-stat. | p-Value | Lower 95% | Upper 95% |
|---|---|---|---|---|---|---|
| Intercept | 0.632 | 0.153 | 4.124 | 0.000066 | 0.329 | 0.936 |
| log(LAT) | 0.445 | 0.114 | 3.880 | 0.000166 | 0.218 | 0.672 |
| B (benthic) | −0.580 | 0.086 | −6.711 | < 0.00001 | −0.752 | −0.408 |
| BP (benthopelagic) | −0.354 | 0.062 | −5.726 | < 0.00001 | −0.477 | −0.232 |
| Species | BL a | Location | % Dry Weight | Source |
|---|---|---|---|---|
| P. gazellae | 105 | Antarctica | 5.3 | Ikeda and Kirkwood [6] (Table 2) |
| P. elegans | 30 | NS, Canada | 9.0–10.9 | Pearre [92] (Table 4); Harrison [93] |
| Z. nagae | 25 | East China Sea | 7.7 | Feng [94], p. 56 |
| P. setosa | 14 | Off Plymouth | 9.3 | Harrison [93] (Table I) |
| F. hispida | 11 | Near Miami | 15.2 | Reeve et al. [95] (Table 1) |
| A. crassa | 10 | Yellow Sea | 14.4 | Feng [94], p. 51 |
| 12 species | -- | Various | 9.3 | Kiørboe [96] (Table 1) |
| Several spp. | -- | Various | 10.0 | Thuesen and Childress [97] (Table 1) |
| Case | Temp (oC) | W∞ (mg) a | K (year−1) | t0 (year) | Ø b | Data Sources |
|---|---|---|---|---|---|---|
| A | 21 | 16.7 | 31.09 | 0.0045 | 0.308 | Reeve and Baker [91] (Figure 1) |
| B | 31 | 7.5 | 88.07 | 0.0024 | 0.528 | Reeve and Baker [91] (Figure 1) |
| C | 17 | 13.1 | 41.75 | 0.0072 | 0.367 | Reeve and Walter [90] (Figure 3) |
| D | 21 | 14 | 43.79 | 0.0077 | 0.406 | Reeve and Walter [90] (Figure 3) |
| E | 23.5 | 9.2 | 72.51 | 0.0037 | 0.504 | Reeve and Walter [90] (Figure 3) |
| F | 26 | 10.7 | 83.67 | 0.0035 | 0.609 | Reeve and Walter [90] (Figure 3) |
| G | 31.5 | 7.1 | 97.00 | 0.0027 | 0.556 | Reeve and Walter [90] (Figure 3) |
| H | 21 | 9.8 | 53.37 | 0.0033 | 0.387 | Reeve [3] (Figure 2) |
| Species a | W∞ (g) | K (year −1) | Ø (logK+ 2/3logW∞) |
|---|---|---|---|
| Thunnus albacares | 198,940 | 0.250 | 2.93 |
| Morone saxatilis | 17,543 | 0.186 | 2.10 |
| Mugil cephalus | 13,890 | 0.110 | 1.80 |
| Trigla gurnardus | 534 | 0.312 | 1.31 |
| Callionymus lyra | 53 | 0.490 | 0.84 |
| Cottus bubalis | 102 | 0.230 | 0.70 |
| Pseudosagitta gazellaeb | ≈1 | (4.86) | (0.458) |
| Ferrosagitta hispidac | ≈0.01 | 55 | 0.458 |
| Species | Place/Time | Lm | Lmax | Source |
|---|---|---|---|---|
| P. elegans | Bedford Basin, May–June 1968 | 21 | 31 | Zo [31] (Figure 2) |
| P. elegans | Plymouth, September 1930 | 10 | 15 | Russell [103] (Plate I) |
| P. elegans | Plymouth, May 1930 & 1931 | 14 | 21 | Russell [103] (Plate I) |
| F. hispida | Laboratory, at ~30 °C, | 8.4 | 10 | Reeves and Walters [90] (Figures 3 and 4A) |
| F. hispida | Laboratory, at ~17 °C, | 11.5 | 14 | |
| E. bathypelagica | Lazarev Sea, Antarctica | 22 | 26 | Kruse [99] (Figure 1, p. 68) |
| E. bathyantarctica | Lazarev Sea, Antarctica | 25 | 30 | Kruse [99] (Figure 2, p. 69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, D.; Liang, C.; Xian, W.; Chu, E.; Bailly, N. The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT). J. Mar. Sci. Eng. 2021, 9, 1397. https://doi.org/10.3390/jmse9121397
Pauly D, Liang C, Xian W, Chu E, Bailly N. The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT). Journal of Marine Science and Engineering. 2021; 9(12):1397. https://doi.org/10.3390/jmse9121397
Chicago/Turabian StylePauly, Daniel, Cui Liang, Weiwei Xian, Elaine Chu, and Nicolas Bailly. 2021. "The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT)" Journal of Marine Science and Engineering 9, no. 12: 1397. https://doi.org/10.3390/jmse9121397
APA StylePauly, D., Liang, C., Xian, W., Chu, E., & Bailly, N. (2021). The Sizes, Growth and Reproduction of Arrow Worms (Chaetognatha) in Light of the Gill-Oxygen Limitation Theory (GOLT). Journal of Marine Science and Engineering, 9(12), 1397. https://doi.org/10.3390/jmse9121397







