The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China
Abstract
:1. Introduction
2. Materials and Methods
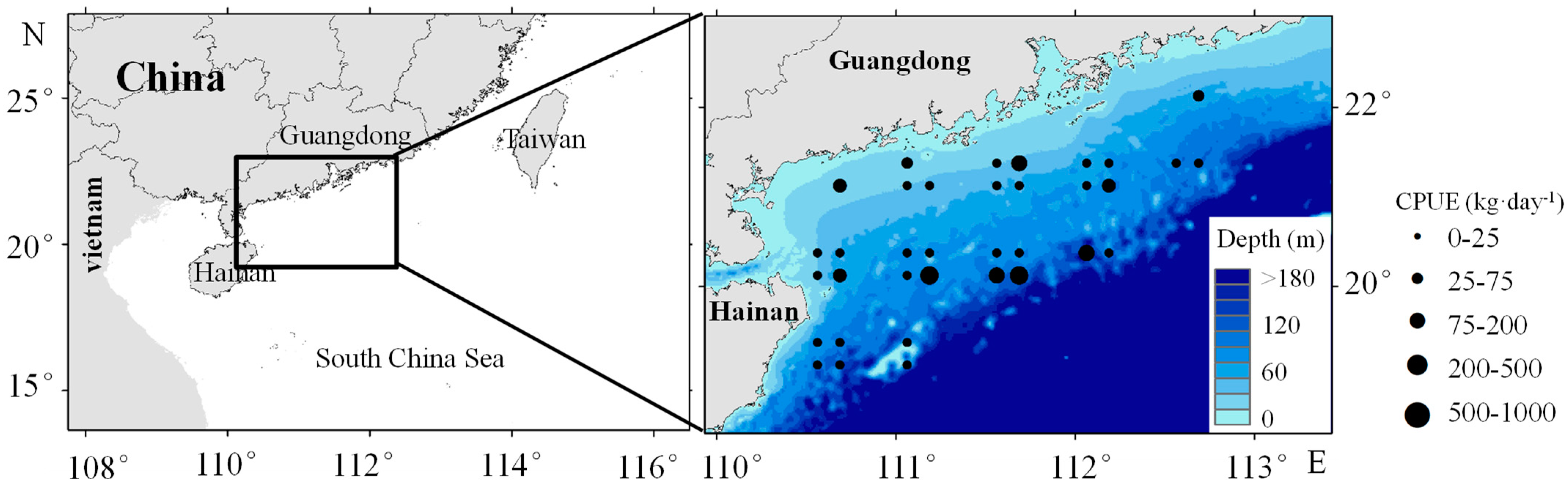
2.1. Fishery Data
2.2. Satellite Remote Sensing Dataremote Sensing Data
2.3. Catch Per Unit Effort
2.4. Clustering Fishing Tactics
2.5. GAM Analysis
3. Results
3.1. Analysis of Fishing Tactics
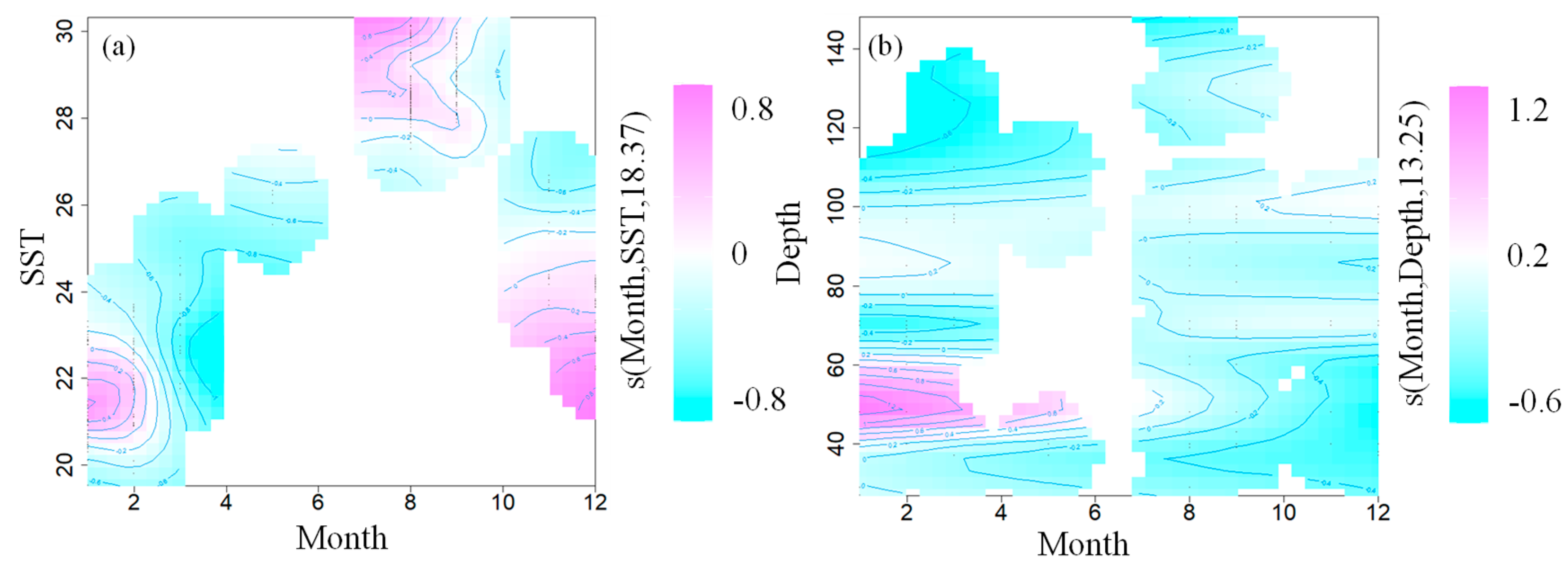
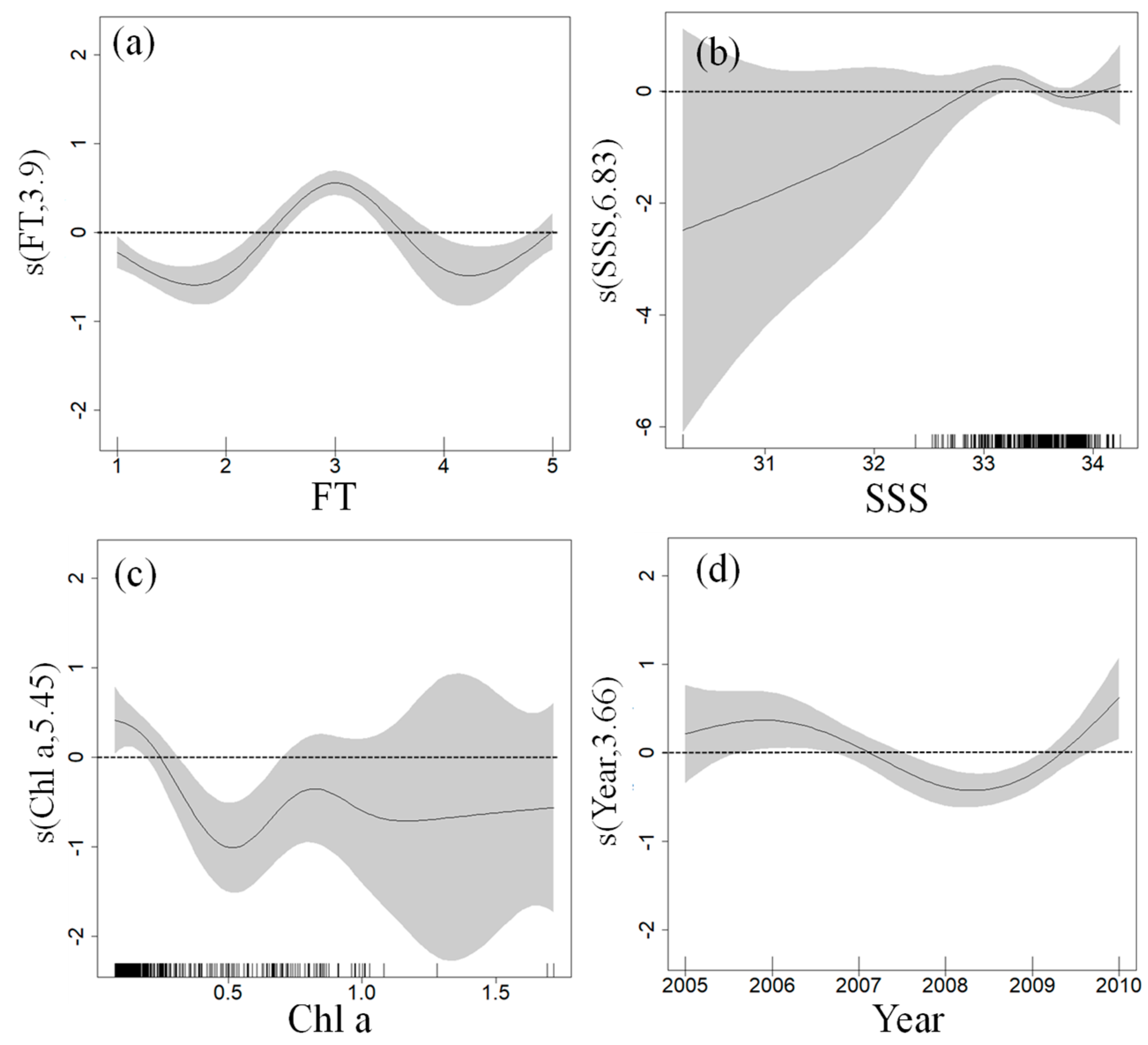
3.2. GAM Analysis
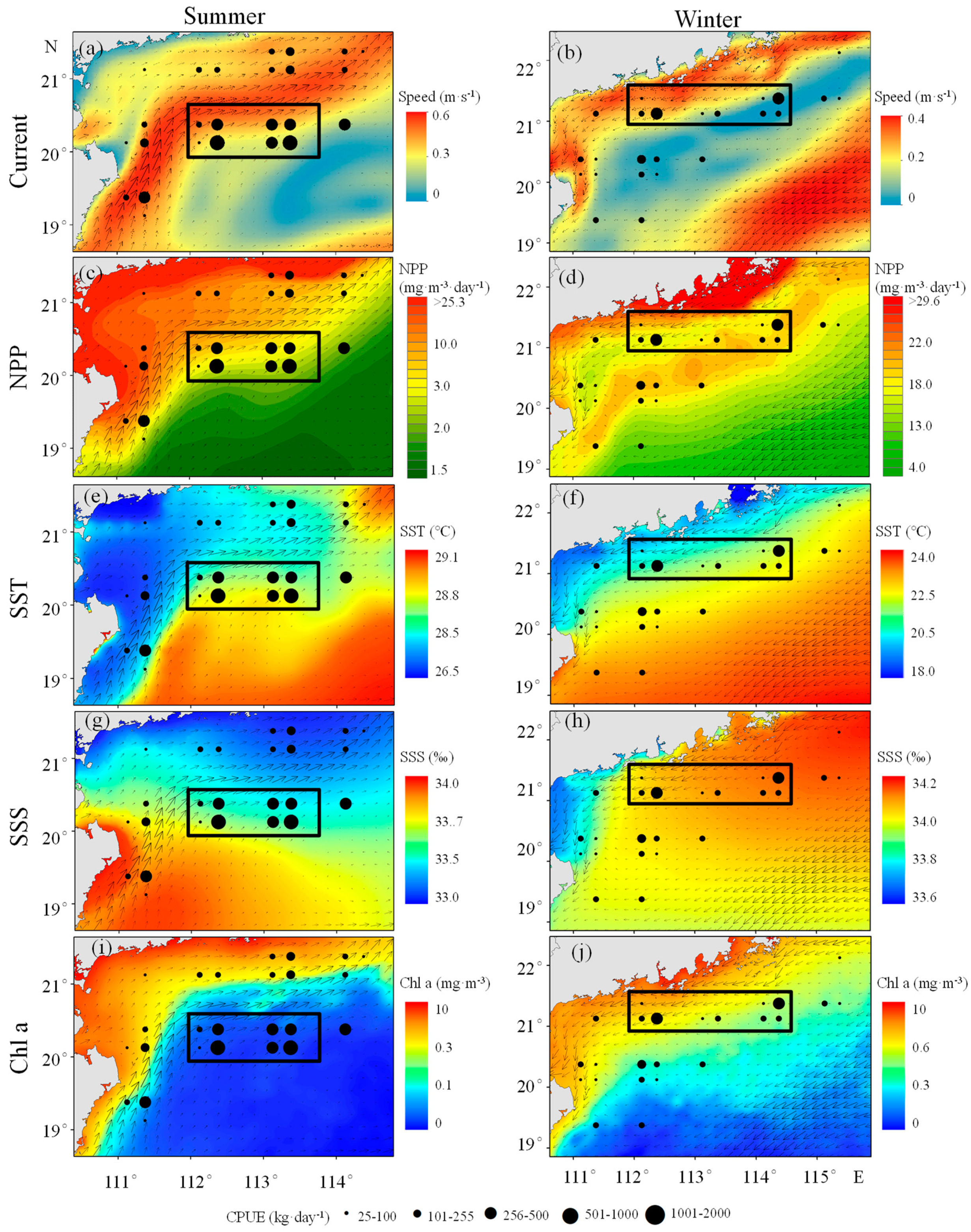
3.3. Seasonal Variation of U. chinensis
4. Discussion
4.1. Relationship between the U. chinensis and Marine Environment
4.2. Relationship between U. chinensis and CFT
4.3. Spatiotemporal Variation of U. chinensis
4.4. Analysis of Seasonal Variation of U. chinensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roper, C.F.E.; Sweeney, M.J.; Nauen, C.E. FAO Species Catalogue. Cephalopods of the World; FAO Fisheries Synopsis: Rome, Italy, 1984; Volume 3, pp. 1–247. [Google Scholar]
- Sukramongkol, N.; Tsuchiya, K.; Segawa, S. Age and maturation of Loligo duvauceli and l. Chinensis from Andaman sea of Thailand. Rev. Fish. Biol. Fish. 2006, 17, 237–246. [Google Scholar] [CrossRef]
- Editorial Committee of Fauna Sinica, Academia Sinica. Fauna Sinica: Phylum Mollusca Class Cephalopode; Science Press: Beijing, China, 1988; pp. 92–94. [Google Scholar]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World squid fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef] [Green Version]
- Robin, J.P.; Denis, V. Squid stock fluctuations and water temperature: Temporal analysis of English channel Loliginidae. J. Appl. Ecol. 1999, 36, 101–110. [Google Scholar] [CrossRef]
- Roberts, M.J.; Downey, N.J.; Sauer, W.H. The relative importance of shallow and deep shelf spawning habitats for the South African chokka squid (Loligo reynaudii). ICES J. Mar. Sci. 2012, 69, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Challier, L.; Royer, J.; Pierce, G.J.; Bailey, N.; Roel, B.; Robin, J.-P. Environmental and stock effects on recruitment variability in the english channel squidloligo forbesi. Aquat. Living. Resour. 2005, 18, 353–360. [Google Scholar] [CrossRef]
- Postuma, F.A.; Gasalla, M.A. On the relationship between squid and the environment: Artisanal jigging for loligo plei at São Sebastião island (24°s), southeastern brazil. ICES J. Mar. Sci. 2010, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Wang, J.T.; Chen, X.J.; Lei, L.; Guan, C.T. Spatio-temporal variation of Ommastrephes bartramii resources (winter & spring groups) in Northwest Pacific under different climate modes. South China Fish. Sci. 2020, 16, 62–69. [Google Scholar]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Wakabayashi, T.; Okamura, H.; Igarashi, H.; Inagake, D.; Okada, Y.J.M.E.P. Changes in abundance of the neon flying squid Ommastrephes bartramii in relation to climate change in the central North Pacific Ocean. Mar. Ecol. Prog. Ser. 2011, 441, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.M.; Pierce, G.J.; Zuur, A.F.; Martins, H.; Clara Martins, M.; Porteiro, F.; Rocha, F. Patterns of investment in reproductive and somatic tissues in the loliginid squid Loligo forbesii and Loligo vulgaris in Iberian and Azorean waters. Hydrobiologia 2011, 670, 201–221. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Q.; Tang, D.; Zhao, H.; Chen, P. Response of Sthenoteuthis oualaniensis to marine environmental changes in the north-central south China Sea based on satellite and in situ observations. PLoS ONE 2019, 14, e0211474. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Hu, Q.; Tang, D.; Chen, P. Environmental effects on the spatiotemporal variability of purpleback flying squid in Xishazhongsha waters, South China sea. Mar. Ecol. Prog. Ser. 2019, 623, 25–37. [Google Scholar] [CrossRef]
- David, J.A.; Simeon, H.; John, R.B. Predicting the recruitment strength of an annual squid stock: Loligo gahi around the falkland islands. Can. J. Fish. Aquat. Sci. 2000, 57, 2479–2487. [Google Scholar] [CrossRef]
- Lefkaditou, E.; Politou, C.-Y.; Palialexis, A.; Dokos, J.; Cosmopoulos, P.; Valavanis, V.D. Influences of environmental variability on the population structure and distribution patterns of the short-fin squid Illex coindetii (cephalopoda: Ommastrephidae) in the eastern Ionian sea. Hydrobiologia 2008, 612, 71–90. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, X.H.; Chen, Y. Influence of el niño/la niña on the western winter–spring cohort of neon flying squid (Ommastrephes bartramii) in the northwestern Pacific Ocean. ICES J. Mar. Sci. 2007, 64, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y.; Zhang, Y. Variability of suitable habitat of western winter-spring cohort for neon flying squid in the northwest pacific under anomalous environments. PLoS ONE 2015, 10, e0122997. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.R.; Li, Y.Y.; Yang, S.Y.; Wu, G.R.; Tao, Y.J.; Feng, Q.B.; Lu, H.S. Biological characteristics and spatial—Temporal distribution of mitre squid, Uroteuthis chinensis, in the Beibu gulf, South China Sea. J. Shellfish. Res. 2013, 32, 835–844. [Google Scholar] [CrossRef]
- Jin, Y.; Li, N.; Chen, X.; Liu, B.; Li, J. Comparative age and growth of Uroteuthis chinensis and Uroteuthis edulis from china seas based on statolith. Aquac. Fish. 2019, 4, 166–172. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, F.; Chen, X.; Liu, B.; Li, J. Microstructure comparison of hard tissues (statoliths, beaks, and eye lenses) of Uroteuthis chinensis in the South China Sea. B. Mar. Sci. 2019, 95, 13–26. [Google Scholar] [CrossRef]
- Chen, F.J. Survey of mitre squid resource in Minnan-Taiwan bank fishing ground and suggestions for sustainable utilization. Fish. Inf. Strategy 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, K.; Qian, W.; Punt, A.E.; Chen, X. Fatty acid comparison of four sympatric loliginid squids in the northern south china sea: Indication for their similar feeding strategy. PLoS ONE 2020, 15, e0234250. [Google Scholar] [CrossRef]
- Li, Y.; Sun, D.Y. Biological characteristics and stock changes of loligo chinensis gray in Beibu Gulf, South China Sea. Hubei Agric. Sci. 2011, 50, 2716–2719. [Google Scholar] [CrossRef]
- Gong, J.K. A study on the migratory distribution and biological characteristics of the Uroteuthis chinensis in Minnan-Taiwan Shoal fishing ground. J. Fujian Fish. 1981, 15–26. [Google Scholar] [CrossRef]
- Ji, X.; Sheng, J.; Zheng, J.; Zhang, W. Numerical study of seasonal circulation and variability over the inner shelf of the northern South China Sea. Ocean. Dynam. 2015, 65, 1103–1120. [Google Scholar] [CrossRef]
- Xu, J.D.; Cai, S.Z.; Xuan, L.L.; Qiu, Y.; Zhu, D.Y. Study on coastal upwelling in eastern Hainan Island and western Guangdong in summer, 2006. Acta. Oceanol. Sin. 2013, 35, 11–18. [Google Scholar]
- Shu, Y.; Wang, Q.; Zu, T. Progress on shelf and slope circulation in the northern South China Sea. Sci. China. Earth. Sci. 2018, 61, 560–571. [Google Scholar] [CrossRef]
- Ye, H.; Chen, C.; Sun, Z.; Tang, S.; Song, X.; Yang, C.; Tian, L.; Liu, F. Estimation of the primary productivity in pearl river estuary using modis data. Estuar. Coast. 2014, 38, 506–518. [Google Scholar] [CrossRef]
- Gan, J.; Li, L.; Wang, D.; Guo, X. Interaction of a river plume with coastal upwelling in the northeastern South China Sea. Cont. Shelf. Res. 2009, 29, 728–740. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, X.; Lao, Q.; Wang, S.; Jin, G.; Chen, C.; Zhu, Q. Dual isotopic evidence for nitrate sources and active biological transformation in the northern South China Sea in summer. PLoS ONE 2019, 14, e0209287. [Google Scholar] [CrossRef]
- Chen, X.J. Cephalopods of the World; China Ocean Press: Beijing, China, 2009; pp. 1178–1179. [Google Scholar]
- Wu, Z. Review and reflection on the ten years of fishing ban in the South China Sea. China Fish. 2008, 8, 4–6. [Google Scholar]
- Yu, J.; Hu, Q.W.; Yuan, H.R.; Chen, P.M. Effect assessment of summer fishing moratorium in Daya Bay based on remote sensing data. South China Fish. Sci. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Fu, D.Y.; Tang, D.L.; Levy, G. The impacts of 2008 snowstorm in China on the ecological environments in the Northern South China Sea. Geomat. Nat. Haz. Risk. 2017, 1–20. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Murie, D.J.; Hazin, F.H.V.; Hazin, H.G.; Leite-Mourato, B.; Travassos, P.; Burgess, G.H. Catch rates and size composition of blue sharks (Prionace glauca) caught by the Brazilian pelagic longline fleet in the southwestern Atlantic Ocean. Aquat. Living. Resour. 2011, 23, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Winker, H.; Kerwath, S.E.; Attwood, C.G. Comparison of two approaches to standardize catch-per-unit-effort for targeting behaviour in a multispecies hand-line fishery. Fish. Res. 2013, 139, 118–131. [Google Scholar] [CrossRef]
- Winker, H.; Kerwath, S.E.; Attwood, C.G. Proof of concept for a novel procedure to standardize multispecies catch and effort data. Fish. Res. 2014, 155, 149–159. [Google Scholar] [CrossRef]
- Pelletier, D.; Ferraris, J. A multivariate approach for defining fishing tactics from commercial catch and effort data. Can. J. Fish. Aquat. Sci. 2000, 57, 51–65. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Changes in marine communities: An approach to statistical analysis and interpretation. Mt. Sinai. J. Med. 2001, 40, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Tibshirani, R. Generalized Additive Models; Chapman and Hall: London, UK, 1990; pp. 587–602. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, J.; Lin, Z.; Chen, P. Environmental influence on the spatiotemporal variability of spawning grounds in the western Guangdong waters, South China Sea. J. Mar. Sci. Eng. 2020, 8, 607. [Google Scholar] [CrossRef]
- Bacha, M.; Jeyid, M.A.; Vantrepotte, V.; Dessailly, D.; Amara, R. Environmental effects on the spatio-temporal patterns of abundance and distribution of sardina pilchardusand sardinella off the Mauritanian coast (North-West Africa). Fish. Oceanogr. 2017, 26, 282–298. [Google Scholar] [CrossRef]
- Tweedie, M.C.K. An index which distinguishes between some important exponential families: Statistics: Applications and new directions. In Proceedings of the Indian Statistical Institute Golden Jubilee International Conference; Indian Statistical Institute: Calcutta, India, 1984; pp. 579–604. [Google Scholar]
- Sun, W.W. Application of generalized additive model to automobile insurance ratemaking based on tweedie distributions. J. Tianjin Univ. Commer. 2014, 34, 60–67. [Google Scholar] [CrossRef]
- Shono, H.J.F.R. Application of the tweedie distribution to zero-catch data in cpue analysis. Fish. Res. 2008, 93, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Walsh, W.A.; Kleiber, P.; Mccracken, M. Comparison of logbook reports of incidental blue shark catch rates by Hawaii-based longline vessels to fishery observer data by application of a generalized additive model. Fish. Res. 2002, 58, 79–94. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R; Chapman & Hall/CRC Press: Boca Ranton, FL, USA, 2006; Volume 66, p. 391. [Google Scholar]
- Moreno, A.; Pierce, G.J.; Azevedo, M.; Pereira, J.; Santos, A.M.P. The effect of temperature on growth of early life stages of the common squid Loligo vulgaris. J. Mar. Biol. Assoc. UK 2012, 92, 1619–1628. [Google Scholar] [CrossRef]
- Boavida-Portugal, J.; Moreno, A.; Gordo, L.; Pereira, J. Environmentally adjusted reproductive strategies in females of the commercially exploited common squid Loligo vulgaris. Fish. Res. 2010, 106, 193–198. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Chang, K.-Y.; Liao, C.-H.; Lee, M.-A.; Lee, K.-T. Growth strategies of the swordtip squid, Uroteuthis edulis, in response to environmental changes in the southern East China Sea—A cohort analysis. B. Mar. Sci. 2013, 89, 677–698. [Google Scholar] [CrossRef] [Green Version]
- Porcaro, R.R.; Zani-Teixeira, M.L.; Katsuragawa, M.; Namiki, C.; Ohkawara, H.M.; Favera, J.M. Spatial and temporal distribution patterns of Larval sciaenids in the estuarine system and adjacent continental shelf off Santos, southeastern Brazil. Braz. J. Oceanogr. 2014. [Google Scholar] [CrossRef] [Green Version]
- Vila, Y.; Silva, L.; Torres, M.A.; Sobrino, I. Fishery, distribution pattern and biological aspects of the common European squid Loligo vulgaris in the gulf of Cadiz. Fish. Res. 2010, 106, 222–228. [Google Scholar] [CrossRef]
- Zheng, Y.S.; Yang, G.L.; Zeng, J.Z.; Su, H.D.; Huang, L.Z.; Su, L. Investigation report on squid resources in Taiwan Strait. J. Fish. Res. 1988. [Google Scholar] [CrossRef]
- Hong, M.J. Investigation report on production and catch composition of light induced squid in Fujian Province. J. Fish. Res. 2002, 2, 28–33. [Google Scholar]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Phys. C. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Cinti, A.; Baron, P.J.; Rivas, A.L. The effects of environmental factors on the embryonic survival of the Patagonian squid Loligo gahi. J. Exp. Mar. Biol. Ecol. 2004, 313, 225–240. [Google Scholar] [CrossRef]
- Şen, H. Incubation of European squid (Loligo vulgaris lamarck, 1798) eggs at different salinities. Aquac. Res. 2005, 36, 876–881. [Google Scholar] [CrossRef]
- Pitchaikani, J.S.; Lipton, A.P. Nutrients and phytoplankton dynamics in the fishing grounds off tiruchendur coastal waters, Gulf of Mannar, India. SpringerPlus 2016, 5, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.L.; Ye, S.Z.; Hong, M.J.; Shen, C.C.; Su, X.H. Biological characteristics of the Chinese squid (Loligo chinensis) in Minnan-Taiwan shallow fishing ground. J. Fujian Fish. 2008, 116, 1–5. [Google Scholar]
- Fisheries Bureau of the Ministry of Agriculture. Animal Husbandry and Fisheries, Investigation and Division of Fishery Resources in South China Sea; Guangdong Science and Technology Press: Guangzhou, China, 1989. [Google Scholar]
- Zhang, J.; Qiu, Y.S.; Chen, Z.Z.; Zhang, P.; Zhang, K.; Fan, J.T.; Chen, G.B.; Cai, Y.C.; Sun, M.S. Advances in pelagic fishery resources survey and assessment in open South China Sea. South China Fish. Sci. 2018, 14, 118–127. [Google Scholar]
- Glazer, J.P.; Butterworth, D.S. Glm-based standardization of the catch per unit effort series for south african west coast hake, focusing on adjustments for targeting other species. S. Afr. J. Mar. Sci. 2010, 24, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Zeidberg, L.D.; Butler, J.L.; Ramon, D.; Cossio, A.; Stierhoff, K.L.; Henry, A. Estimation of spawning habitats of market squid (Doryteuthis opalescens) from field surveys of eggs off central and southern California. Mar. Ecol. 2012, 33, 326–336. [Google Scholar] [CrossRef]
- Sauer, W.H.H.; Goschen, W.S.; Koorts, A.S. A preliminary investigation of the effect of sea temperature fluctuations and wind direction on catches of Chokka squidloligo vulgaris reynaudiioff the eastern cape, south Africa. S. Afr. J. Marine. Sci. 2010, 11, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Jackson, G.D.J.F.B. Seasonal variation in reproductive investment in the tropical loliginid squid loligo chinensis and the small tropical Idiodepius pygmaeus. Fish. B NOAA 1993, 91, 260–270. [Google Scholar]
- Dawe, E.G.; Hendrickson, L.C.; Colbourne, E.B.; Drinkwater, K.F.; Showell, M.A. Ocean climate effects on the relative abundance of short-finned (Illex illecebrosus) and long-finned (Loligo pealeii) squid in the northwest Atlantic ocean. Fish. Oceanogr. 2007, 16, 303–316. [Google Scholar] [CrossRef]
- Maxwell, M.; Henry, A.; Elvidge, C.; Safran, J.; Hobson, V.; Nelson, I.; Tuttle, B.; Dietz, J.; Hunter, J. Fishery dynamics of the California market squid (Loligo opalescens), as measured by satellite remote sensing. Fish. B NOAA 2004, 102. [Google Scholar] [CrossRef]
- Vergani, D.F.; Labraga, J.C.; Stanganelli, Z.B.; Dunn, M. The effects of el nino la nina on reproductive parameters of elephant seals feeding in the Bellingshausen sea. J. Biogeogr. 2010, 35. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Watanabe, T.; Yatsu, A.; Inagake, D.; Okada, Y. Occurrence of jumbo flying squid Dosidicus gigas aggregations associated with the countercurrent ridge off the Costa Rica dome during 1997 el niño and 1999 la niña. Mar. Ecol. Prog. Ser. 2002, 231, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.Q.; Jia, X.L.; Wang, Y.G. Anomalies of ocean and general atmospheric circulation in 2008 and their impacts on climate anomalies in China. Meteorol. Mon. 2009, 35, 112–117. [Google Scholar] [CrossRef]
- Augustyn, C.J.; Lipinski, M.R.; Sauer, W.H.H.; Roberts, M.J.; Mitchell-Innes, B.A. Chokka squid on the agulhas bank: Life history and ecology. S. Afr. J. Sci. 1994, 90, 143–154. [Google Scholar]
- O’Dor, R.K.; Adamo, S.; Aitken, J.P.; Andrade, Y.; Finn, J.; Hanlon, R.T.; Jackson, G.D. Currents as environmental constraints on the behavior, energetics and distribution of squid and cuttlefish. B. Mar. Sci. 2002, 71, 601–617. [Google Scholar]
- Caley, T.; Kim, J.H.; Malaizé, B.; Giraudeau, J.; Laepple, T.; Caillon, N.; Charlier, K.; Rebaubier, H.; Rossignol, L.; Castañeda, I.S.; et al. High-latitude obliquity as a dominant forcing in the agulhas current system. Clim. Past. 2011, 7, 1285–1296. [Google Scholar] [CrossRef] [Green Version]
- Buckley, J.M.; Mingels, B.; Tandon, A. The impact of lateral advection on SST and SSS in the northern bay of Bengal during 2015. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2020, 172. [Google Scholar] [CrossRef]
- Mou, X.X.; Xu, B.D.; Xue, Y.; Zhang, C.L. Fish assemblage structure and fauna discrimination in the coastal waters of southern Yellow Sea. J. Fish. China 2017, 41, 1734–1743. [Google Scholar] [CrossRef]
- Bost, C.A.; Cotté, C.; Bailleul, F.; Cherel, Y.; Charrassin, J.B.; Guinet, C.; Ainley, D.G.; Weimerskirch, H. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 2009, 78, 363–376. [Google Scholar] [CrossRef]
- Roper, C.F.E. An overview of cephalopod systematics: Status, problems and recommendations. Mem. Natl. Mus. Vic. 1983, 44, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Brodersen, J.; Nilsson, P.A.; Ammitzboll, J.; Hansson, L.A.; Skov, C.; Bronmark, C. Optimal swimming speed in head currents and effects on distance movement of winter-migrating fish. PLoS ONE 2008, 3, e2156. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Yao, Z.; Zhou, L.; Bao, M.; Zang, Z. Numerical modeling of the seasonal circulation in the coastal ocean of the northern south china sea. Front. Earth. Sc. Switz. 2018, 14, 90–109. [Google Scholar] [CrossRef]
- Chen, X.Q.; Lin, R.C. Chlorophyll a and primary production in the Chinese Contract Area in the east-north Pacific. Acta. Oceanol. Sin. 2007, 29, 146–153. [Google Scholar] [CrossRef]
- Guan, W.J.; Chen, X.J.; Gao, F.; Li, G. Study on the dynamics of biomass of chub mackerel based on ocean net primary production in Southern East China Sea. Acta. Oceanol. Sin. 2013, 35, 121–127. [Google Scholar]
- Yu, W.; Chen, X.J.; Yi, Q. Relationship between spatio-temporal dynamics of neon flying squid Ommastrephes bartramii and net primary production in the northwest Pacific Ocean. Acta. Oceanol. Sin. 2016, 38, 64–72. [Google Scholar]
- Chen, X.; Li, J.; Liu, B.; Chen, Y.; Li, G.; Fang, Z.; Tian, S.J. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, off the Costa Rica dome. J. Mar. Biol. Assoc. UK 2013, 93, 567–573. [Google Scholar] [CrossRef]
- Medellín-Ortiz, A.; Cadena-Cárdenas, L.; Santana-Morales, O. Environmental effects on the jumbo squid fishery along Baja California’s west coast. Fish. Sci. 2016, 82, 851–861. [Google Scholar] [CrossRef]
- Li, K.Z.; Yin, J.Q.; Huang, L.M.; Tian, Y.H. Spatial and temporal variations of mesozooplankton in the Pearl River estuary, China. Estuar. Coast. Shelf. S. 2006, 67, 543–552. [Google Scholar] [CrossRef]
- Sun, D.R.; Li, Y.; Wang, X.H.; Wang, Y.Z.; Wu, Q.E. Biological characteristics and stock changes of Loligo edulis in Beibu Gulf, South China Sea. South China Fish. Sci. 2011, 7, 8–13. [Google Scholar] [CrossRef]
| Year | Month | Voyage | Number of Nets | Number of Stations | Number of Catch Species |
|---|---|---|---|---|---|
| 2005 | 8 | A1, A2, A3, A4, A5 | 25 | 10 | 6 |
| 9 | A5, A6, A7, A8 | 14 | 6 | 3 | |
| 2006 | 8 | B1, B2, B3, B4, B5 | 25 | 13 | 6 |
| 9 | B5, B6 | 8 | 4 | 4 | |
| 2007 | 1 | B14, B15 | 11 | 4 | 4 |
| 2 | B16 | 14 | 7 | 6 | |
| 3 | B18 | 3 | 3 | 3 | |
| 8 | C1, C2, C3, C4 | 23 | 10 | 6 | |
| 9 | C4, C5, C6 | 21 | 8 | 6 | |
| 12 | C11 | 9 | 4 | 4 | |
| 2008 | 1 | C12 | 11 | 3 | 5 |
| 2 | C14 | 1 | 1 | 3 | |
| 3 | C14 | 1 | 1 | 2 | |
| 8 | D1, D2, D4, D5 | 18 | 7 | 6 | |
| 9 | D5 | 7 | 3 | 6 | |
| 11 | D13 | 1 | 1 | 1 | |
| 12 | D13, D14, D15 | 17 | 9 | 6 | |
| 2009 | 1 | D16, D17, D18 | 14 | 7 | 7 |
| 2 | D18, D19, D20 | 20 | 4 | 8 | |
| 3 | D20, D21, D23 | 9 | 8 | 5 | |
| 5 | D23 | 4 | 4 | 4 | |
| 8 | E1, E2 | 21 | 6 | 6 | |
| 9 | E4, E5, E6 | 14 | 8 | 6 | |
| 11 | E11, E12 | 16 | 7 | 6 | |
| 12 | E13, E14 | 16 | 8 | 6 | |
| 2010 | 1 | E15 | 16 | 6 | 5 |
| 2 | E17, E18, E19 | 14 | 7 | 4 | |
| 3 | E20 | 6 | 2 | 5 |
| Species | FT1 | FT2 | FT3 | FT4 | FT5 |
|---|---|---|---|---|---|
| U. chinensis | 9.00 | 13.37 | 62.02 * | 30.98 | 13.77 |
| Scombridae | 1.88 | 1.40 | 2.52 | 0.00 | 49.04 * |
| Decapterus | 77.08 * | 8.43 | 18.22 | 0.00 | 8.08 |
| Trichiurus haumela | 2.39 | 75.57 * | 9.74 | 0.58 | 10.18 |
| Rastrelliger kanagurta | 5.86 | 0.00 | 0.63 | 0.00 | 2.17 |
| Formio niger | 0.32 | 0.38 | 0.78 | 0.00 | 11.04 |
| Sparidae | 1.69 | 0.00 | 1.32 | 0.00 | 1.08 |
| Navodon | 1.38 | 0.06 | 1.83 | 0.00 | 3.48 |
| unidentified species | 0.39 | 0.79 | 2.93 | 68.44 * | 1.17 |
| Influencing Factors (p = 1.7) | AIC | GCV | Adjusted R2 | Deviance Explained (%) | Residual Deviance |
|---|---|---|---|---|---|
| CPUE~NULL | 5122.37 | 63.02 | 0 | 0.0 | 22,498.85 |
| CPUE~s(Month, SST) | 4926.65 | 53.46 | 0.14 | 26.7 | 16,500.28 |
| CPUE~s(Month, SST) + s(Chl a) | 4896.35 | 52.05 | 0.17 | 30.7 | 15,581.08 |
| CPUE~s(Month, SST) + s(Chl a) + s(SSS) | 4831.30 | 48.19 | 0.23 | 38.4 | 13,864.01 |
| CPUE~s(Month, SST) + s(Chl a) + s(SSS) + s(Month, Depth) | 4715.83 | 41.56 | 0.37 | 51.6 | 10,885.54 |
| CPUE~s(Month, SST) + s(Chl a) + s(SSS) + s(Month, Depth) + s(FT) | 4616.75 | 34.62 | 0.48 | 60.1 | 8969.72 |
| CPUE~s(Month, SST) + s(Chl a)+s(SSS)+s(Month, Depth) + s(FT) + s(Year) | 4587.61 | 33.02 | 0.51 | 63.1 | 8295.15 |
| Variables | Contribution (%) | d.f. | Pr(F) | Pr(chi) |
|---|---|---|---|---|
| s(Month, SST) | 26.7 | 27.9 | < 0.001 | < 0.001 |
| s(Month, Depth) | 12.8 | 18.7 | < 0.001 | < 0.001 |
| s(FT) | 8.5 | 2.5 | < 0.001 | < 0.001 |
| s(SSS) | 7.7 | 7.5 | < 0.001 | < 0.001 |
| s(Chl a) | 4.0 | 6.3 | 0.015 | 0.013 |
| s(Year) | 3.1 | 5.0 | < 0.001 | < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Yao, L.; Yu, J.; Chen, P. The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China. J. Mar. Sci. Eng. 2021, 9, 131. https://doi.org/10.3390/jmse9020131
Wang D, Yao L, Yu J, Chen P. The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China. Journal of Marine Science and Engineering. 2021; 9(2):131. https://doi.org/10.3390/jmse9020131
Chicago/Turabian StyleWang, Dongliang, Lijun Yao, Jing Yu, and Pimao Chen. 2021. "The Role of Environmental Factors on the Fishery Catch of the Squid Uroteuthis chinensis in the Pearl River Estuary, China" Journal of Marine Science and Engineering 9, no. 2: 131. https://doi.org/10.3390/jmse9020131





