New Records of the Diatoms (Bacillariophyceae) from the Coastal Lagoons in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting and Fixation of Samples
2.2. Pretreatment and Observation of Diatoms
3. Results
3.1. Identification of Diatom Species in Korean Lagoons
3.2. Environmental Characteristics at the Sampling Sites
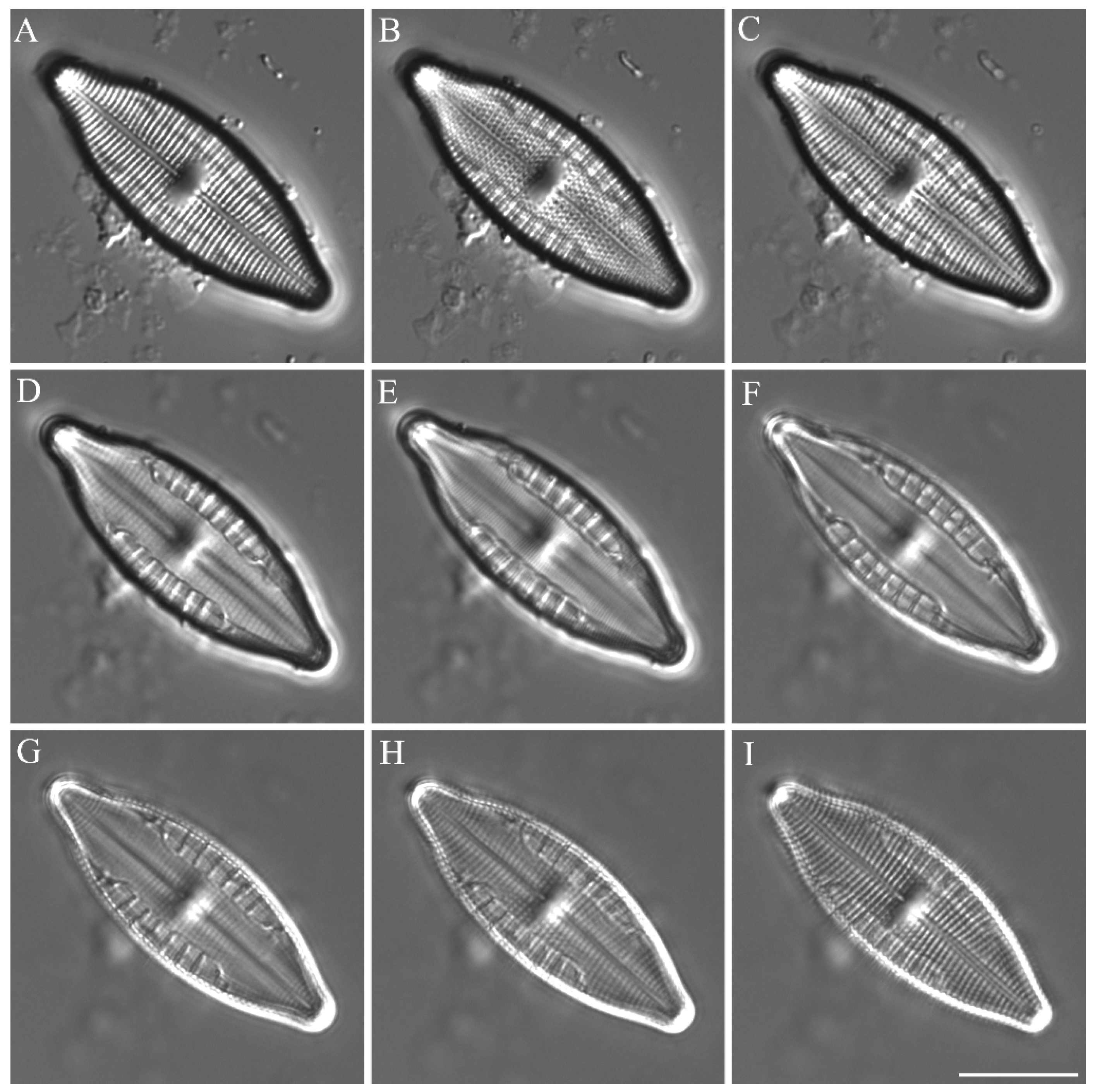
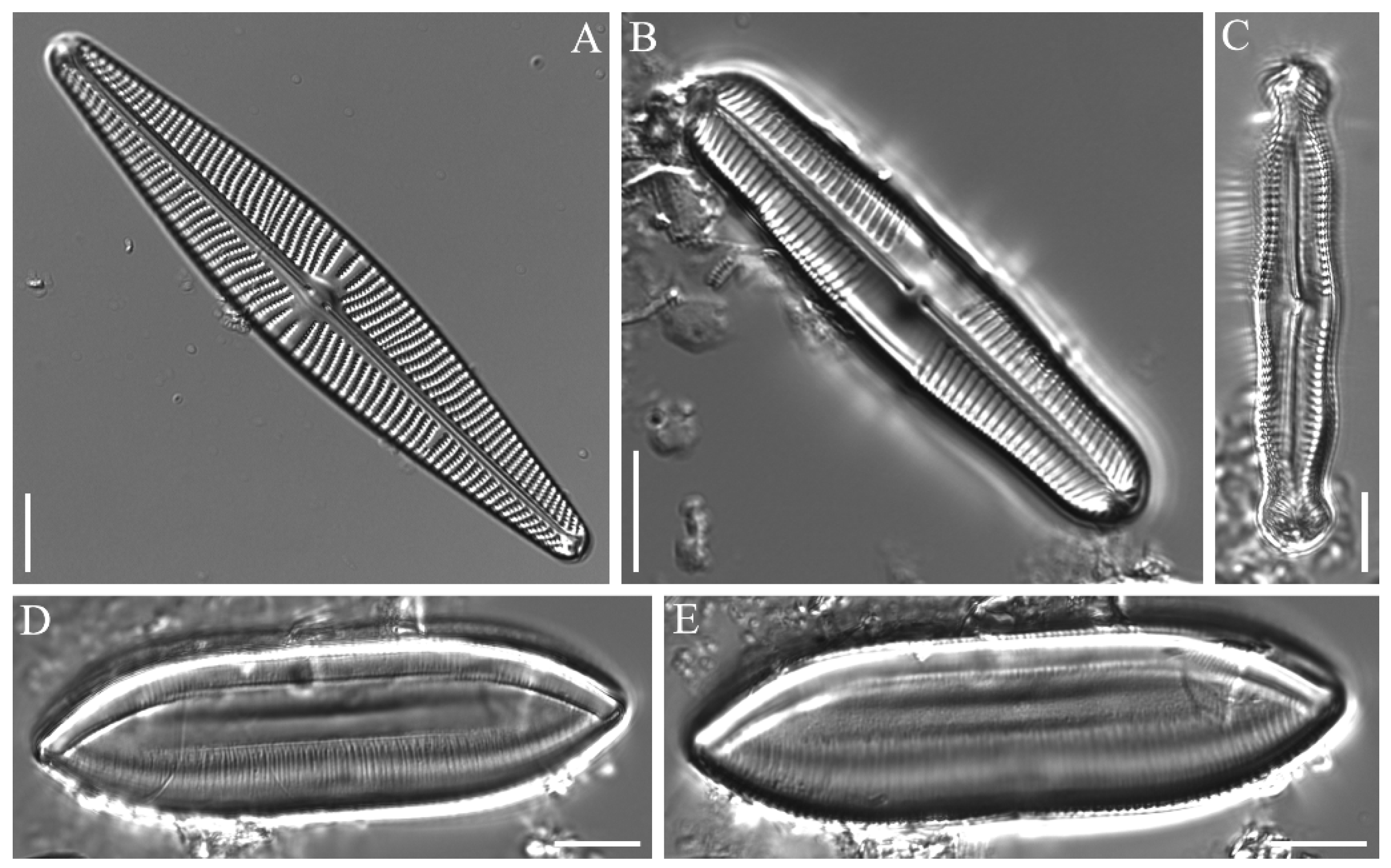
3.3. Species Descriptions
3.3.1. Cosmioneis citriformis R.L. Lowe & A.R. Sherwood 2010
3.3.2. Diploneis didyma (Ehrenberg) Ehrenberg 1839
3.3.3. Gomphonema guaraniarum Metzeltin & Lange-Bertalot 2007
3.3.4. Gomphonema italicum Kützing 1844
3.3.5. Gyrosigma sinense (Ehrenberg) Desikachary 1988
3.3.6. Haslea crucigera (W. Smith) Simonsen 1974
3.3.7. Mastogloia elliptica (C. Agardh) Cleve 1983
3.3.8. Navicula freesei R.M. Patrick & Freese 1961
3.3.9. Pinnularia bertrandii Krammer 2000
3.3.10. Pinnularia nodosa var. percapitata Krammer 2000
3.3.11. Tryblionella littoralis var. tergestina (Grunow) Snoeijs 1998
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dominguez, J.M.; Martin, L.; Bittencourt, A.C. Sea-Level History and Quaternary Evolution of River Mouth–Associated Beachridge Plains Along the East–Southeast Brazilian Coast: A Summary. In Sea-Level Fluctuation and Coastal Evolution; Nummedal, D., Pilkey, O.H., Howard, J.D., Price, W.A., Eds.; SEPM Special Publication: Broken Arrow, OK, USA, 1987; Volume 41, pp. 115–127. [Google Scholar]
- Müller, A.; Mathesius, U. The palaeoenvironments of coastal lagoons in the southern Baltic Sea, I. The application of sedimentary Corg/N ratios as source indicators of organic matter. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 145, 1–16. [Google Scholar] [CrossRef]
- Hwang, S.I.; Yoon, S.O. Geomorphic characteristics of coastal lagoons and river basins, and sedimentary environment at river mouths along the middle east coast in the Korean peninsular. Korean J. Geomorphol. Assoc. 2008, 15, 17–33. [Google Scholar]
- Moon, B.R.; Jeon, H.J.; Jeon, S.L.; Lee, J.S.; Shin, J.E.; Ahn, J.H.; Yang, Y.W.; Hyun, M.S.; Kim, M. Seasonal Variations of Water Quality and Phytoplankton of 4 Lagoons in the East Coast of Korea. Int. J. Environ. Sci. Technol. 2015, 24, 1101–1121. [Google Scholar]
- Yum, J.G.; Takemura, K.; Tokuoka, T.; Yu, K.M. Holocene environmental changes of the Hwajinpo Lagoon on the eastern coast of Korea. J. Paleolimnol. 2003, 29, 155–166. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Lee, J.I.; Kim, D.J.; Kim, B.C.; Heo, W.M. The limnological survey of a coastal lagoon in Korea (3): Lake Hwajinpo. Korean J. Ecol. Environ. 2004, 37, 12–25. [Google Scholar]
- Heo, W.M.; Kim, B.C.; Jun, M.S. Evaluation of eutrophication of lagoons in the eastern coast of Korea. Korean J. Limnol. 1999, 32, 141–151. [Google Scholar]
- Kwon, S.Y.; Lee, J.I.; Kim, D.J.; Kim, B.C.; Heo, W.M. The Limnological survey of a coastal lagoon in Korea (2): Lake Hyangho. Korean J. Ecol. Environ. 2004, 37, 1–11. [Google Scholar]
- Kim, H.Y. Change of the vegetation structure according to hydrosere processes and the evaluation of successional state of the 18 lagoon located in the east coastal region, Korea. Master’s Thesis, Gangneung University, Gangneung, Korea, 2009; pp. 1–137. [Google Scholar]
- Jeong, Y.I.; Hong, B.R.; Kim, Y.C.; Lee, K.S. Distribution, life history and growth characteristics of the Utricularia japonica Makino in the east coastal lagoon, Korea. Korean J. Ecol. Environ. 2016, 49, 110–123. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, C.S. An Analysis of Factors Influencing the Landscape of Gyeong Po Lake and the Establishment of Criteria for Height Control. J. Korean Inst. Landsc. Archit. 2009, 37, 104–113. [Google Scholar]
- Park, S.D.; Lee, S.; Shin, S.S.; Yoon, B.M. Effects of Operation of the Kyeongpo Retarding Basin on Flood Water Levelin Kyeongpo Lake. J. Wet. Res. 2016, 18, 413–423. [Google Scholar] [CrossRef][Green Version]
- Yoon, S.; Hwang, S.; Park, C.; Jin, M. Landscape changes during the 20th century of Ssangho, Gapy-eongri wetland, Gunggaeho and Yeongaeho, Yang yang-gun, Gangwon province. J. Korean Geomorph. Assoc. 2010, 17, 41–52. [Google Scholar]
- Falcão, M.; Vale, C. Nutrient dynamics in a coastal lagoon (Ria Formosa, Portugal): The importance of lagoon–sea water exchanges on the biological productivity. Cienc. Mar. 2003, 29, 425–433. [Google Scholar] [CrossRef]
- Bizzarro, J.J. A review of the physical and biological characteristics of the Bahia Magdalena lagoon complex (Baja California Sur, Mexico). Bull. South. Calif. Acad. Sci. 2008, 107, 1–24. [Google Scholar] [CrossRef]
- Salomoni, S.E. Diatomáceas epilíticas indicadoras da qualidade de água na bacia do Rio Gravataí, Rio Grande do Sul, Brasil. Ph.D. Thesis, Ppgern Ufscar, São Carlos, São Paulo, Brasil, 2004; pp. 1–230. [Google Scholar]
- Cho, I.H.; Kim, H.K.; Lee, M.H.; Kim, Y.J.; Lee, H.; Kim, B.H. The Effect of Monsoon Rainfall Patterns on Epilithic Diatom Communities in the Hantangang River, Korea. Water 2020, 12, 1471. [Google Scholar] [CrossRef]
- Wunsam, S.; Cattaneo, A.; Bourassa, N. Comparing diatom species, genera and size in biomonitoring: A case study from streams in the Laurentians (Quebec, Canada). Freshwater Biol. 2002, 47, 325–340. [Google Scholar] [CrossRef]
- Joung, S.H.; Park, H.K.; Lee, H.J.; Lee, S.H. Effect of climate change for diatom bloom at winter and spring season in Mulgeum Station of the Nakdong River, South Korea. J. Korean Soc. Water Environ. 2013, 29, 155–164. [Google Scholar]
- Sylvestre, F.; Beck-Eichler, B.; Duleba, W.; Debenay, J.P. Modern benthic diatom distribution in a hypersaline coastal lagoon: The Lagoa de Araruama (RJ), Brazil. Hydrobiologia 2001, 443, 213–231. [Google Scholar] [CrossRef]
- Park, J.S.; Yun, S.M.; Lee, S.D.; Lee, J.B.; Lee, J.H. New Records of the Diatoms (Bacillariophyta) in the Brackish and Coastal Waters of Korea. Korean J. Environ. Biol. 2017, 35, 215–226. [Google Scholar] [CrossRef]
- Lee, H.; Yun, S.M.; Lee, J.Y.; Lee, S.D.; Lim, J.; Cho, P.Y. Late Holocene climate changes from diatom records in the historical Reservoir Gonggeomji, Korea. J. Appl. Phycol. 2018, 30, 3205–3219. [Google Scholar] [CrossRef]
- Lee, S.D.; Lee, H.; Park, J.; Yun, S.M.; Lee, J.Y.; Lim, J.S.; Park, M.R.; Kwon, D.Y. Late Holocene diatoms in sediment cores from the Gonggeomji Wetland in Korea. Diatom Res. 2020, 35, 195–229. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.D.; Kang, S.E.; Lee, J.H. New records of the marine pennate diatoms in Korea. J. Ecol. Environ. 2014, 37, 231–244. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Park, J.S. Newly recorded diatom species in marine and fresh water of Korea. J. Ecol. Environ. 2015, 38, 545–562. [Google Scholar] [CrossRef]
- Sournia, A. Quelques nouvelles données sur le phytoplancton marin et la production primaire à Tuléar (Madagascar). Hydrobiologia 1968, 31, 545–560. [Google Scholar] [CrossRef]
- Hasle, G.R.; Fryxell, G.A. Diatoms: Cleaning and mounting for light and electron microscopy. Trans. Am. Microsc. Soc. 1970, 89, 469–474. [Google Scholar] [CrossRef]
- Anonymous. Proposals for a standardization of diatom terminology and diagnoses. Nova Hedwigia, Beiheft. 1975, 53, 323–354.
- Ross, R.; Cox, E.J.; Karayeva, N.I.; Mann, D.G.; Paddock, T.B.B.; Simonsen, R.; Sims, P.A. An amended terminologyfor the siliceous components of the diatom cell. Nova Hedwig. Beiheft. 1979, 64, 513–533. [Google Scholar]
- Round, E.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 1–747. [Google Scholar]
- Lowe, R.L.; Sherwood, A.R. Three new species of Cosmioneis (Bacillariophyceae) from Hawaii. Proc. Acad. Nat. Sci. Phila. 2010, 160, 21–28. [Google Scholar] [CrossRef]
- Ehrenberg, C.G. Über die Bildung der Kreidefelsen und des Kreidemergels durch unsichtbare Organismen. In Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin; Physikalische Klasse: Göttingen, Germany, 1839; Volume 1838, pp. 59–147, pls. [Google Scholar]
- Ehrenberg, C.G. Mittheilung über 2 neue Lager von Gebirgsmassen aus Infusorien als Meeres-Absatz in Nord-Amerika und eine Vergleichung derselben mit den organischen Kreide-Gebilden in Europa und Afrika. In Bericht über die zur Bekanntmachung geeigneten Verhandlungen der Königlich-Preussischen Akademie der Wissenschaften zu Berlin; Physikalische Klasse: Göttingen, Germany, 1844; Volume 1844, pp. 57–97. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H. Tropical Diatoms of South America II. Special remarks on biogeography disjunction. Iconogr. Diatomol. 2007, 18, 1–877. [Google Scholar]
- Kützing, F.T. Die Kieselschaligen Bacillarien oder Diatomeen, Nordhausen: Zu finden bei W.: Köhne, Germany, 1844; pp. [i-vii], [1]-152, pls 1–30.
- Desikachary, T.V. Marine diatoms of the Indian Ocean region. In Atlas of Diatoms; Madras: Madras Science Foundation: Tamil Nadu, India, 1988; Volume V, pp. 1–13. [Google Scholar]
- Ehrenberg, C.G. Mittheilung uber vor Kurzem von dem Preuß. Seehandlungs Schiffe, der Adler, aus Canton Mitgebrachte Verkaufliche Chinesische Blumen-Cultur-Erde; Bericht über die zur Bekanntmachtung geeigneten Verhandlungen der Koniglich Preussischen Akademie der Wissenschaften zu Berlin: Berlin, Germany, 1847; pp. 476–485. [Google Scholar]
- Simonsen, R. The diatom plankton of the Indian Ocean Expedition of R/V Meteor 1964-5. Meteor Forschungsergebnisse, Reihe D Biologie 1974, 19, 1–107. [Google Scholar]
- Smith, W. A Synopsis of the British Diatomaceae; with Remarks on Their Structure, Functions and Distribution; and Instructions for Collecting and Preserving Specimens. A–E; John van Voorst: London, UK, 1856; Volume 2, pp. [i-vi]–xxix, pls 32–60, 61–62. [Google Scholar]
- Schmidt, A. Atlas der Diatomaceen-kunde; ser. IV: Heft 47; O.R. Reisland: Leipzig, Germany, 1983; pp. 185–188. [Google Scholar]
- Agardh, C.A. Systema Algarum; Literis Berlingianis [Berling]: Lundae, Sweden, 1824; pp. [i]-xxxvii, [1]-312. [Google Scholar]
- Patrick, R.M.; Freese, L.R. Diatoms (Bacillariophyceae) from Northern Alaska. Proc. Acad. Nat. Sci. Phila. 1961, 112, 129–293. [Google Scholar]
- Krammer, K. The genus Pinnularia. In Diatoms of the European Inland Waters and Comparable Habitats; Lange-Bertalot, H., Ed.; Ruggell: A.R.G. Gantner Verlag K.G.: Berlin/Heidelberg, Germany, 2000; Volume 1, pp. 1–703. [Google Scholar]
- Snoeijs, P.; Balashova, N. Intercalibration and distribution of diatom species in the Baltic Sea. In The Baltic Marine Biologists Publication No. 16e; Opulus Press: Uppsala, Sweden, 1998; Volume 5, pp. 1–144. [Google Scholar]
- Cleve, P.T.; Grunow, A. Beiträge zur Kenntniss der arctischen Diatomeen. Kongliga Svenska Vetenskaps-Akademiens Handlingar 1880, 17, 1–121. [Google Scholar]
- Hällfors, G. Checklist of Baltic Sea Phytoplankton Species (Including Some Heterotrophic Protistan Groups); Helsinki Commission, Baltic Marine Environment Protection Commission: Helsinki, Finland, 2004; pp. 1–210. [Google Scholar]
- Sylvestre, F.; Guiral, D.; Debenay, J.P. Modern diatom distribution in mangrove swamps from the Kaw Estuary (French Guiana). Mar. Geol. 2004, 208, 281–293. [Google Scholar] [CrossRef]
- Marshall, H.G.; Burchardt, L.; Lacouture, R. A review of phytoplankton composition within Chesapeake Bay and its tidal estuaries. J. Plank. Res 2005, 27, 1083–1102. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Spaulding, S.A. Symmetrical naviculoid diatoms. In Freshwater Algae of the United States; Sheath, B., Wher, J., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 637–653. [Google Scholar]
- Massé, G.; Rincé, Y.; Cox, E.J.; Allard, G.; Belt, S.T.; Rowland, S.J. Haslea salstonica sp. nov. and Haslea pseudostrearia sp. nov.(Bacillariophyta), two new epibenthic diatoms from the Kingsbridge estuary, United Kingdom. Comptes Rendus de l’Académie des Sciences-Series III-Sciences de la Vie 2001, 324, 617–626. [Google Scholar] [CrossRef]
- Kheiri, S.; Solak, C.N.; Edlund, M.B.; Spaulding, S.; Nejadsattari, T.; Asri, Y.; Hamdi, S.M.M. Biodiversity of diatoms in the Karaj River in the Central Alborz, Iran. Diatom Res. 2018, 33, 355–380. [Google Scholar] [CrossRef]
- Jahn, R.; Sterrenburg, F.A. Gyrosigma sinense (Ehrenberg) desikachary: Typification and emended species description. Diatom Res. 2003, 18, 61–67. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.Y.; Kociolek, J.P.; Wang, L.Q. Gomphonema shanghaiensis sp. nov., a new diatom species (Bacillariophyta) from a river in Shanghai, China. Phytotaxa 2016, 278, 29–38. [Google Scholar] [CrossRef]
- Abarca, N.; Zimmermann, J.; Kusber, W.H.; Mora, D.; Van, A.T.; Skibbe, O.; Jahn, R. Defining the core group of the genus Gomphonema Ehrenberg with molecular and morphological methods. Bot. Lett. 2020, 167, 114–159. [Google Scholar] [CrossRef]
- Wojtal, A. Diatoms of the genus Gomphonema Ehr. [Bacillariophyceae] from a karstic stream in the Krakowsko-Czestochowska Upland. Acta Soc. Bot. Pol. 2003, 72. [Google Scholar] [CrossRef]
- Cox, E.J. Diatoms, Diatomeae (Bacillariophyceae sl, Bacillariophyta). Syllabus Plant Fam. 2015, 2, 64–103. [Google Scholar]
- Rybak, M.; Noga, T.; Poradowska, A. Diversity in anthropogenic environment–permanent puddle as a place for development of diatoms. J. Ecol. Eng. 2019, 20, 165–174. [Google Scholar] [CrossRef]
- Krammer, K. Bacillariophyceae. I. Teil. Naviculaceae. In Susswasserflora von Mitteleuropa. Springer: Berlin/Heidelberg, Germany, 1986; Volume 2, 876p.
- Vidaković, D.; Ćirić, M.; Krizmanić, J. First finding of a genus Haslea Simonsen in Serbia and new diatom taxa for the country’s flora in extreme and unique habitats in the Vojvodina Province. Bot. Serbica 2020, 44, 3–9. [Google Scholar] [CrossRef]
- Patrick, R.; Reimer, C.W. The diatoms of the United States. Exclusive of Alaska and Hawaii. Volume 1: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculaceae. Monogr. Acad. Nat. Sci. Phila. 1966, 13, 1–688. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süsswasserflora von Mitteleuropa, Band 2/1; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Jena, Germany, 1986; pp. 1–876. [Google Scholar]
- Pennesi, C.; Poulin, M.; De Stefano, M.; Romagnoli, T.; Totti, C. New insights to the ultrastructure of some marine Mastogloia species section Sulcatae (Bacillariophyceae), including M. neoborneensis sp. nov. Phycologia 2011, 50, 548–562. [Google Scholar] [CrossRef]
- Maltsev, Y.; Andreeva, S.; Podunay, J.; Kulikovskiy, M. Description of Aneumastus mongolotusculus sp. nov.(Bacillariophyceae, Mastogloiales) from Lake Hovsgol (Mongolia) on the basis of molecular and morphological investigations. Nova Hedwig. 2019, 148, 21–33. [Google Scholar] [CrossRef]
- Donadel, L.; Torgan, L. Subtropical Coastal Lagoon from Southern Brazil: Environmental Conditions and Phytobenthic Community Structure. In Lagoon Environments Around the World-A Scientific Perspective; IntechOpen: London, UK, 2019; pp. 189–205. [Google Scholar]
- Cartaxana, P.; Ruivo, M.; Hubas, C.; Davidson, I.; Serôdio, J.; Jesus, B. Physiological versus behavioral photoprotection in intertidal epipelic and epipsammic benthic diatom communities. J. Exp. Mar. Biol. Ecol. 2011, 405, 120–127. [Google Scholar] [CrossRef]
- Genkal, S.I.; Yarushina, M.I. Materials on the flora of Bacillariophyta in aquatic ecosystems of the Yarayakha River basin (Yamal Peninsula). Contemp. Probl. Ecol. 2016, 9, 306–317. [Google Scholar] [CrossRef]
- Medeiros, G.; Amaral, M.W.W.; Ferreira, P.C.; Ludwig, T.V.; Bueno, N.C. Gomphonema Ehrenberg (Bacillariophyceae, Gomphonemataceae) do rio São Francisco Falso, Paraná, Brasil. Biota Neotrop. 2018, 18, e20180568. [Google Scholar] [CrossRef]


| Site | Location | Latitude (N) | Longitude (E) | Collected Species |
|---|---|---|---|---|
| GPH | Jeo-dong, Gangneung-si, Gangwon-do | 34°47′32.43″ | 128°53′48.89″ | Diploneis didyma |
| HH | Hyangho-ri, Jumunjin-eup, Gangneung-si, Gangwon-do | 37°54′35.2″ | 128°48′22.2″ | Mastogloia elliptica; |
| Cosmioneis citriformis; | ||||
| Haslea crucigera; | ||||
| Pinnularia bertrandii | ||||
| CJH | Bongpo-ri, Toseong-myeon, Goseong-gun, Gangwon-do | 38°15′14.97″ | 128°33′20.47″ | Pinnularia nodosa var. percapitata |
| MH | Maeho-gil, Hyeonnam-myeon, Yangyang-gun, Gangwon-do | 37°57′8.17″ | 128°46′8.88″ | Gyrosigma sinense |
| GPW | Gapyeong-ri, Sonyang-myeon, Yangyang-gun, Gangwon-do | 38°6′8.46″ | 128°38′50.58″ | Gomphonema guaraniarum; |
| Gomphonema italicum | ||||
| YGH | Yeounpo-ri, Sonyang-myeon, Yangyang-gun, Gangwon-do | 38°2′14.12″ | 128°42′1.73″ | Navicula freesei; |
| Tryblionella littoralis var. tergestina |
| Site | Year | Temperature (°C) | DO (mg/L) | Conductivity (μS/cm3) | Salinity (PSU) | pH |
|---|---|---|---|---|---|---|
| GPH | 2018 | 20.8 | 8.5 | 35,261 | 22.1 | 8.4 |
| 2019 | 16.0 | 10.4 | 39,820 | 25.4 | 8.5 | |
| HH | 2018 | 17.8 | 11.0 | 8926 | 5.0 | 8.1 |
| 2020 | 28.6 | - | 60 | 0.03 | 8.2 | |
| CJH | 2019 | 16.0 | 9.5 | 360 | 0.2 | 8.7 |
| 2020 | 14.0 | 9.2 | 152 | 0.1 | 8.1 | |
| MH | 2018 | 20.2 | 10.9 | 15,969 | 9.4 | 8.1 |
| 2020 | 15.8 | 10.8 | 5767 | 3.9 | 7.9 | |
| GPW | 2020 | 13.3 | 16.2 | 1528 | 0.9 | 8.0 |
| YGH | 2020 | 8.3 | 11.5 | 288 | 0.1 | 7.6 |
| No. | Species | Habitat | Distribution | This Study | Reference | |
|---|---|---|---|---|---|---|
| Salinity | Habitat | |||||
| 1 | Diploneis didyma | Benthic | Marine | 22.1–25.4 | Epilithic | [64,65] |
| Freshwater | ||||||
| 2 | Mastogloia elliptica | Benthic | Marine | 0.03–5.0 | Epilithic | [59] |
| Epiphytic | Freshwater | |||||
| 3 | Cosmioneis citriformis | Benthic | Freshwater | 0.03–5.0 | Epilithic | [31] |
| 4 | Haslea crucigera | Benthic | Marine | 0.03–5.0 | Epilithic | [50] |
| 5 | Pinnularia bertrandii | Benthic | Freshwater | 0.03–5.0 | Epilithic | [51] |
| 6 | Pinnularia nodosa var. percapitata | Benthic | Freshwater | 0.1–0.2 | Epilithic | [66] |
| 7 | Gyrosigma sinense | Benthic | Marine | 3.9–9.4 | Epilithic | [52] |
| 8 | Gomphonema guaraniarum | Benthic | Freshwater | 0.9 | Epilithic | [67] |
| 9 | Gomphonema italicum | Benthic | Freshwater | 0.9 | Epilithic | [67] |
| Terrestrial | ||||||
| 10 | Navicula freesei | Benthic | Freshwater | 0.1 | Epilithic | [42] |
| 11 | Tryblionella littoralis var. tergestina | Benthic | Marine | 0.1 | Epilithic | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Park, M.; Lee, C.S.; Park, C.; Lee, S.D. New Records of the Diatoms (Bacillariophyceae) from the Coastal Lagoons in Korea. J. Mar. Sci. Eng. 2021, 9, 694. https://doi.org/10.3390/jmse9070694
Kwon D, Park M, Lee CS, Park C, Lee SD. New Records of the Diatoms (Bacillariophyceae) from the Coastal Lagoons in Korea. Journal of Marine Science and Engineering. 2021; 9(7):694. https://doi.org/10.3390/jmse9070694
Chicago/Turabian StyleKwon, Daeryul, Mirye Park, Chang Soo Lee, Chaehong Park, and Sang Deuk Lee. 2021. "New Records of the Diatoms (Bacillariophyceae) from the Coastal Lagoons in Korea" Journal of Marine Science and Engineering 9, no. 7: 694. https://doi.org/10.3390/jmse9070694
APA StyleKwon, D., Park, M., Lee, C. S., Park, C., & Lee, S. D. (2021). New Records of the Diatoms (Bacillariophyceae) from the Coastal Lagoons in Korea. Journal of Marine Science and Engineering, 9(7), 694. https://doi.org/10.3390/jmse9070694






