Ocean Acidification and Direct Interactions Affect Coral, Macroalga, and Sponge Growth in the Florida Keys
Abstract
:1. Introduction
2. Methods
2.1. Specimen Collection
2.2. Experimental System (CAOS) and Design
2.3. Monitoring Environmental Conditions
2.4. Coral, Macroalgae, and Sponge Growth
2.5. Statistical Analyses
3. Results
3.1. Environmental Conditions
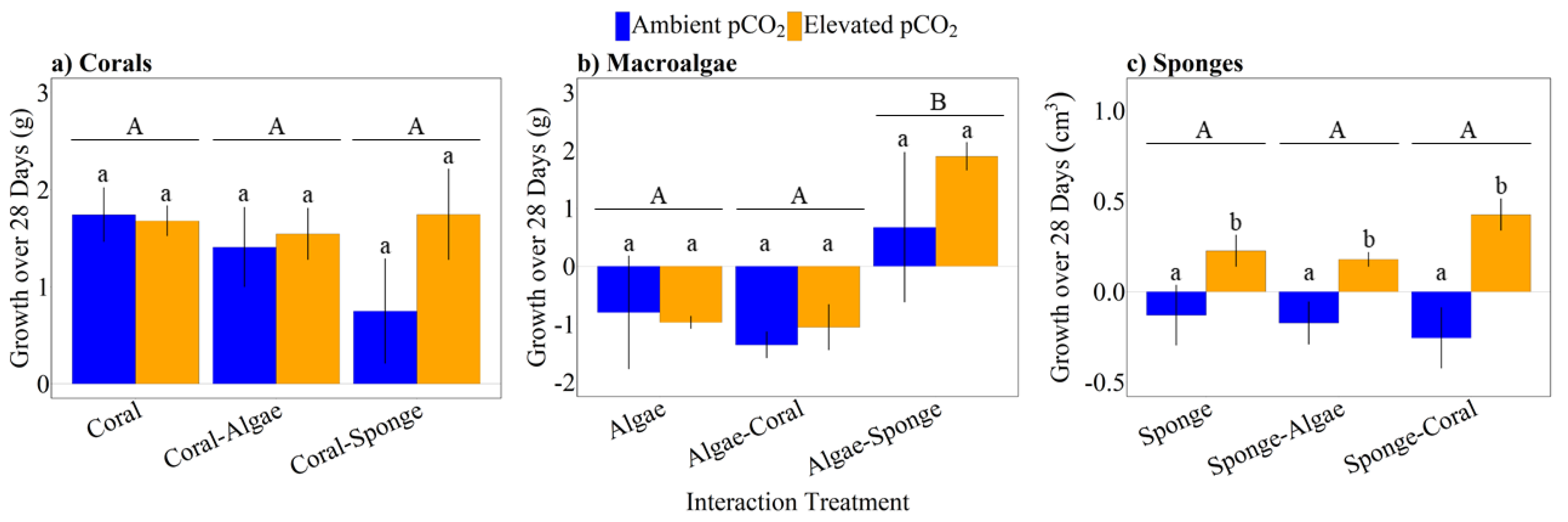
3.2. Growth Responses of Corals, Macroalgae, and Sponges
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.-D.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Andersson, A.J.; MacKenzie, F.T. Revisiting four scientific debates in ocean acidification research. Biogeosciences 2012, 9, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.J.; Davy, S.; Jones, T.; Taylor, M.W.; Webster, N. Could some coral reefs become sponge reefs as our climate changes? Glob. Chang. Biol. 2013, 19, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.C.S.; Connolly, S.R. Sensitivity of coral calcification to ocean acidification: A meta-analysis. Glob. Chang. Biol. 2013, 19, 282–290. [Google Scholar] [CrossRef]
- Fang, J.K.H.; Mello-Athayde, M.A.; Schönberg, C.H.L.; Kline, D.; Hoegh-Guldberg, O.; Dove, S. Sponge biomass and bioerosion rates increase under ocean warming and acidification. Glob. Chang. Biol. 2013, 19, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.-H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Price, N.; Smith, J.E. Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ 2014, 2, e411. [Google Scholar] [CrossRef] [Green Version]
- Bennett, H.M.; Altenrath, C.; Woods, L.; Davy, S.K.; Webster, N.S.; Bell, J.J. Interactive effects of temperature and pCO2 on sponges: From the cradle to the grave. Glob. Chang. Biol. 2017, 23, 2031–2046. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Comeau, S.; Lantz, C.; Andersson, A.; Briggs, C.; Cohen, A.; Gattuso, J.-P.; Grady, J.M.; Gross, K.; Johnson, M.; et al. Integrating the effects of ocean acidification across functional scales on tropical coral reefs. BioScience 2016, 66, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Pulido, G.; Gouezo, M.; Tilbrook, B.; Dove, S.; Anthony, K. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 2010, 14, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Monaco, C.; Hay, M.E.; Gartrell, P.; Mumby, P.; Diaz-Pulido, G. Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci. Rep. 2017, 7, srep41053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.E.; Sneed, J.M.; Johnston, L.; Paul, V.J. Effects of ocean acidification and contact with the brown alga Stypopodium zonale on the settlement and early survival of the coral Porites astreoides. Mar. Ecol. Prog. Ser. 2017, 577, 67–77. [Google Scholar] [CrossRef]
- McCook, L.; Jompa, J.; Diaz-Pulido, G. Competition between corals and algae on coral reefs: A review of evidence and mechanisms. Coral Reefs 2001, 19, 400–417. [Google Scholar] [CrossRef]
- Bell, J.J. The functional roles of marine sponges. Estuar Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Burkepile, D.E.; Thurber, R.V. A vicious cycle? Altered carbon and nutrient cycling may explain the low resilience of Caribbean coral reefs. BioScience 2016, 66, 470–476. [Google Scholar] [CrossRef]
- De Goeij, J.; Van Oevelen, D.; Vermeij, M.J.A.; Osinga, R.; Middelburg, J.; De Goeij, A.F.P.M.; Admiraal, W. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Rix, L.; de Goeij, J.; Mueller, C.E.; Struck, U.; Middelburg, J.; Van Duyl, F.C.; Al-Horani, F.A.; Wild, C.; Naumann, M.S.; Van Oevelen, D. Coral mucous fuels the sponge loop in warm- and cold-water coral reef ecosystems. Sci. Rep. 2016, 6, 18715. [Google Scholar] [CrossRef]
- Campana, S.; Hudspith, M.; Lankes, D.; De Kluijver, A.; Demey, C.; Schoorl, J.; Absalah, S.; Van der Meer, M.T.J.; Mueller, B.; De Goeij, J.M. Processing of naturally sourced macroalgal- and coral-dissolved organic matter (DOM) by high and low microbial abundance encrusting sponges. Front. Mar. Sci. 2021, 8, 640583. [Google Scholar] [CrossRef]
- Rix, L.; De Goeij, J.; Van Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M. Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar. Ecol. Prog. Ser. 2018, 589, 85–96. [Google Scholar] [CrossRef] [Green Version]
- McMurray, S.; Stubler, A.; Erwin, P.; Finelli, C.; Pawlik, J. A test of the sponge-loop hypothesis for emergent Caribbean reef sponges. Mar. Ecol. Prog. Ser. 2018, 588, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.C.; Ward, B.B. Sponge-mediated nitrification in tropical benthic communities. Mar. Ecol. Prog. Ser. 1997, 156, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Southwell, M.W.; Weisz, J.B.; Martens, C.S.; Lindquist, N. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol. Oceanogr. 2008, 53, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Silbiger, N.J. Impacts of Sponge Produced Dissolved Inorganic Nitrogen on Caribbean Coral Reef Seaweed Communities. Master’s Thesis, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2009. [Google Scholar] [CrossRef]
- Smith, J.E.; Shaw, M.; Edwards, R.A.; Obura, D.; Pantos, O.; Sala, E.; Sandin, S.A.; Smriga, S.; Hatay, M.; Rohwer, F.L. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol. Lett. 2006, 9, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Barott, K.L.; Rohwer, F.L. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 2012, 20, 621–628. [Google Scholar] [CrossRef]
- Aerts, L.A.M. Sponge/coral interactiosn in Caribbean reefs: Analysis of overgrowth patterns in relation to species identity and cover. Mar. Ecol. Prog. Ser. 1998, 175, 241–249. [Google Scholar] [CrossRef]
- Glynn, P.W.; Manzello, D.P. Bioerosion and coral reef growth: A dynamic balance. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Loh, T.-L.; McMurray, S.E.; Henkel, T.P.; Vicente, J.; Pawlik, J.R. Indirect effects of overfishing on Caribbean reefs: Sponges overgrow reef-building corals. PeerJ 2015, 3, e901. [Google Scholar] [CrossRef] [Green Version]
- Wisshak, M.; Schönberg, C.H.L.; Form, A.; Freiwald, A. Ocean acidification accelerates reef bioerosion. PLoS ONE 2012, 7, e45124. [Google Scholar] [CrossRef] [Green Version]
- González-Rivero, M.; Yakob, L.; Mumby, P.J. The role of sponge competition on coral reef alternative steady states. Ecol. Model. 2011, 222, 1847–1853. [Google Scholar] [CrossRef]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef] [Green Version]
- Schutte, V.G.W.; Selig, E.R.; Bruno, J.F. Regional spatio-temporal trends in Caribbean coral reef benthic communities. Mar. Ecol. Prog. Ser. 2010, 402, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Aronson, R.B.; Precht, W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs 2006, 25, 441–450. [Google Scholar] [CrossRef]
- Lirman, D.; Manzello, D.; Maciá, S. Back from the dead: The resilience of Siderastrea radians to severe stress. Coral Reefs 2002, 21, 291–292. [Google Scholar] [CrossRef]
- Chartrand, K.M.; Durako, M.J.; Blum, J.E. Effect of hyposalinity on the photophysiology of Siderastrea radians. Mar. Biol. 2009, 156, 1691–1702. [Google Scholar] [CrossRef]
- Muller, E.M.; Sartor, C.; Alcaraz, N.I.; Van Woesik, R. Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci. 2020, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, P.J. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 2011, 56, 2402–2410. [Google Scholar] [CrossRef]
- Brown, N.E.M.; Bernhardt, J.R.; Anderson, K.M.; Harley, C.D.G. Increased food supply mitigates ocean acidification effects on calcification but exacerbates effects on growth. Sci. Rep. 2018, 8, 9800. [Google Scholar] [CrossRef]
- North Pacific Marine Science Organization. Guide to Best Practices for Ocean CO2 Measurement; Dickson, A.G., Sabine, C.L., Christian, J.R., Eds.; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007; p. 191. [Google Scholar]
- Gattuso, J.-P.; Epitalon, J.-M.; Lavigne, H.; Orr, J. Seacarb: Seawater Carbonate Chemistry. R Package Version 3.2.10. Available online: http://CRAN.R-project.org/package=seacarb (accessed on 1 April 2020).
- Lueker, T.J.; Dickson, A.G.; Keeling, C.D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 2000, 70, 105–119. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Maragos, J.E.; Franzisket, L. Coral growth: Buoyant weight technique. In Coral Reefs: Research Methods; Stoddart, D.R., Johannes, R.E., Eds.; UNESCO: Paris, France, 1978; p. 581. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 10 March 2020).
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 10 March 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 3 March 2020).
- Meléndez, M.; Salisbury, J.; Gledhill, D.; Langdon, C.; Morell, J.M.; Manzello, D.; Rodriguez-Abudo, S.; Musielewicz, S.; Sutton, A. Seasonal variations of carbonate chemistry at two western Atlantic coral reefs. J. Geophys. Res. Oceans 2020, 125, 016108. [Google Scholar] [CrossRef]
- Millero, F.J.; Hiscock, W.T.; Huang, F.; Roche, M.; Zhang, J.Z. Seasonal variation of the carbonate system in Florida Bay. Bull. Mar. Sci. 2001, 68, 101–123. [Google Scholar]
- Zhang, J.-Z.; Fischer, C.J. Carbon dynamics of Florida Bay: Spatiotemporal patterns and biological control. Environ. Sci. Technol. 2014, 48, 9161–9169. [Google Scholar] [CrossRef]
- Enochs, I.C.; Manzello, D.P.; Jones, P.R.; Stamates, S.J.; Carsey, T.P. Seasonal carbonate chemistry dynamics on southeast Florida coral reefs: Localized acidification hotspots from navigational inlets. Front. Mar. Sci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Crook, E.D.; Potts, D.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Calcifying coral abundance near low-pH springs: Implications for future ocean acidification. Coral Reefs 2012, 31, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, R.R.; Towle, E.K.; Hooidonk, R.; Mor, C.; Winter, R.N.; Piggot, A.M.; Cunning, R.; Baker, A.; Klaus, J.S.; Swart, P.K.; et al. Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs. Glob. Chang. Biol. 2017, 23, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.R.; Swart, P.; Langdon, C. Stress-tolerant corals of Florida Bay are vulnerable to ocean acidification. Coral Reefs 2013, 32, 671–683. [Google Scholar] [CrossRef]
- Rivest, E.B.; Comeau, S.; Cornwall, C.E. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Chang. Rep. 2017, 3, 271–281. [Google Scholar] [CrossRef]
- Schoepf, V.; Jury, C.P.; Toonen, R.J.; McCulloch, M. Coral calcification mechanisms facilitate adaptive responses to ocean acidificiation. Proc. R. Soc. B Boil. Sci. 2017, 284, 20172117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyronak, T.; Takeshita, Y.; Courtney, T.A.; DeCarlo, E.H.; Eyre, B.D.; Kline, D.I.; Martz, T.; Page, H.; Price, N.N.; Smith, J.; et al. Diel temperature and pH variability scale with depth across diverse coral reef habitats. Limnol. Oceanogr. Lett. 2020, 5, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Van Der Loos, L.M.; Schmid, M.; Leal, P.P.; McGraw, C.M.; Britton, D.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecol. Evol. 2019, 9, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Cornwall, C.E.; Revill, A.T.; Hall-Spencer, J.M.; Milazzo, M.; Raven, J.A.; Hurd, C.L. Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci. Rep. 2017, 7, srep46297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platz, M.C.; Takeshita, Y.; Bartels, E.; Arias, M.E. Evaluating the potential for autonomous measurements of net community production and calcification as a tool for monitoring coral restoration. Ecol. Eng. 2020, 158, 106042. [Google Scholar] [CrossRef]
- Asnaghi, V.; Chiantore, M.; Mangialajo, L.; Gazeau, F.; Francour, P.; Alliouane, S.; Gattuso, J.-P. Cascading effects of ocean acidification in a rocky subtidal community. PLoS ONE 2013, 8, e61978. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.; Carpenter, R.C. Differential growth responses to water flow and reduced pH in tropical marine macroalgae. J. Exp. Mar. Biol. Ecol. 2017, 491, 58–65. [Google Scholar] [CrossRef]
- Diaz, M.C.; Rütler, K. Sponges: An essential component of Caribbean coral reefs. Bull. Mar. Sci. 2001, 69, 535–546. [Google Scholar]
- Bell, J.J.; Bennett, H.M.; Rovellini, A.; Webster, N.S. Sponges to be winners under near-future climate scenarios. BioScience 2018, 68, 955–968. [Google Scholar] [CrossRef]
- Duckworth, A.; West, L.; Vansach, T.; Stubler, A.; Hardt, M. Effects of water temperature and pH on growth and metabolite biosynthesis of coral reef sponges. Mar. Ecol. Prog. Ser. 2012, 462, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, A.R.; Peterson, B.J. Effects of seawater temperature and pH on the boring rates of the sponge Cliona celata in scallop shells. Mar. Biol. 2013, 160, 27–35. [Google Scholar] [CrossRef]
- Ribes, M.; Calvo, E.; Movilla, J.I.; Logares, R.; Coma, R.; Pelejero, C. Restructuring of the sponge microbiome favors tolerance to ocean acidification. Environ. Microbiol. Rep. 2016, 8, 536–544. [Google Scholar] [CrossRef]
- Goodwin, C.; Rodolfo-Metalpa, R.; Picton, B.; Hall-Spencer, J. Effects of ocean acidification on sponge communities. Mar. Ecol. 2013, 35, 41–49. [Google Scholar] [CrossRef]
- Morrow, K.M.; Bourne, D.G.; Humphrey, C.; Botté, E.S.; Laffy, P.; Zaneveld, J.; Uthicke, S.; E Fabricius, K.; Webster, N.S. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J. 2015, 9, 894–908. [Google Scholar] [CrossRef] [Green Version]
- Kandler, N.M.; Wahab, M.A.A.; Noonan, S.H.; Bell, J.J.; Davy, S.K.; Webster, N.S.; Luter, H.M. In situ responses of the sponge microbiome to ocean acidification. FEMS Microbiol. Ecol. 2018, 94, 205. [Google Scholar] [CrossRef]
- Lesser, M.P.; Fiore, C.; Slattery, M.; Zaneveld, J. Climate change stressors destabilize the microbiome of the Caribbean barrel sponge, Xestospongia muta. J. Exp. Mar. Biol. Ecol. 2016, 475, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Bennett, H.; Bell, J.J.; Davy, S.K.; Webster, N.S.; Francis, D.S. Elucidating the sponge stress response; lipids and fatty acids can facilitate survival under future climate scenarios. Glob. Chang. Biol. 2018, 24, 3130–3144. [Google Scholar] [CrossRef]
- Achlatis, M.; Van Der Zande, R.M.; Schönberg, C.H.L.; Fang, J.K.H.; Hoegh-Guldberg, O.; Dove, S. Sponge bioerosion on changing reefs: Ocean warming poses physiological constraints to the success of a photosymbiotic excavating sponge. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Schönberg, C.H.L.; Fang, J.K.H.; Carreiro-Silva, M.; Tribollet, A.; Wisshak, M. Bioerosion: The other ocean acidification problem. ICES J. Mar. Sci. 2017, 74, 895–925. [Google Scholar] [CrossRef]
- Brown, K.T.; Bender-Champ, D.; Kenyon, T.M.; Rémond, C.; Hoegh-Guldberg, O.; Dove, S. Temporal effects of ocean warming and acidification on coral-algal competition. Coral Reefs 2019, 38, 297–309. [Google Scholar] [CrossRef]
- Wisshak, M.; Schönberg, C.H.L.; Form, A.U.; Freiwald, A. Sponge bioerosion accelerated by ocean acidification across species and latitudes? Helgol. Mar. Res. 2014, 68, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Enochs, I.C.; Manzello, D.P.; Donham, E.; Kolodziej, G.; Okano, R.; Johnston, L.H.; Young, C.; Iguel, J.; Edwards, C.B.; Fox, M.D.; et al. Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat. Clim. Chang. 2015, 5, 1083–1088. [Google Scholar] [CrossRef]
- Webb, A.E.; Van Heuven, S.M.A.C.; De Bakker, D.M.; Van Duyl, F.C.; Reichart, G.-J.; De Nooijer, L.J. Combined effects of experimental acidification and eutrophication on reef sponge bioerosion rates. Front. Mar. Sci. 2017, 4, 311. [Google Scholar] [CrossRef] [Green Version]
- Stubler, A.D.; Furman, B.T.; Peterson, B.J. Sponge erosion under acidification and warming scenarios: Differential impacts on living and dead coral. Glob. Chang. Biol. 2015, 21, 4006–4020. [Google Scholar] [CrossRef]
- Wisshak, M.; Schönberg, C.H.L.; Form, A.U.; Freiwald, A. Effects of ocean acidification and global warming on reef bioerosion—Lessons from a clionaid sponge. Aquat. Biol. 2013, 19, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Stubler, A.D.; Furman, B.T.; Peterson, B.J. Effects of pCO2 on the interaction between an excavating sponge, Cliona varians, and a hermatypic coral, Porties furcate. Mar. Biol. 2014, 161, 1851–1859. [Google Scholar] [CrossRef]
- McCook, L.J.; Folke, C.; Hughes, T.P.; Nyström, M.; Obura, D.; Salm, R. Ecological resilience, climate change and the Great Barrier Reef. In Climate Change and the Great Barrier Reef; Johnson, J., Marshall, P., Eds.; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2007; pp. 75–96. [Google Scholar]
- Fang, J.K.H.; Schönberg, C.H.L.; Mello-Athayde, M.A.; Achlatis, M.; Hoegh-Guldberg, O.; Dove, S. Bleaching and mortality of a photosymbiotic bioeroding sponge under future carbon dioxide emission scenarios. Oceologia 2018, 187, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jonas, L.; Lin, H.; Hill, R.T. Microbially mediated nutrient cycles in marine sponges. FEMS Microbiol. Ecol. 2019, 95, 155. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.; de Goeij, J.; Vermeij, M.; Mulders, Y.; Van Der Ent, E.; Ribes, M.; Van Duyl, F.C. Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PLoS ONE 2014, 9, e90152. [Google Scholar] [CrossRef] [Green Version]
- Corredor, J.E.; Wilkinson, C.R.; Vicente, V.P.; Morell, J.M.; Otero, E. Nitrate release by Caribbean reef sponges. Limnol. Oceanogr. 1988, 33, 114–120. [Google Scholar] [CrossRef]
| T (°C) | Salinity | DIC (μmol kg−1) | TA (μmol kg−1) | pHT | pCO2 (μatm) | ΩArag. | |
|---|---|---|---|---|---|---|---|
| Reservoir Tanks | |||||||
| Ambient pCO2 | 28.6 ± 0.1 | 37.74 ± 1.74 | 1953 ± 56 | 2169 ± 73 | 7.82 ± 0.04 | 676 ± 65 | 2.46 ± 0.28 |
| Elevated pCO2 | 28.8 ± 0.2 | 37.60 ± 1.52 | 2040 ± 67 | 2152 ± 70 | 7.58 ± 0.02 | 1263 ± 76 | 1.52 ± 0.09 |
| Experimental Aquaria | |||||||
| Ambient pCO2 | 27.1 ± 0.3 | 37.61 ± 1.37 | 1926 ± 58 | 2149 ± 63 | 7.86 ± 0.05 | 603 ± 88 | 2.50 ± 0.24 |
| Elevated pCO2 | 27.2 ± 0.3 | 37.54 ± 1.31 | 2021 ± 56 | 2144 ± 67 | 7.63 ± 0.04 | 1105 ± 89 | 1.59 ± 0.18 |
| Organism | Treatments | df | F-Value | p-Value | |
|---|---|---|---|---|---|
| Coral | Seawater pCO2 | 1 | 0.749 | 0.487 | |
| Interaction | 2 | 1.327 | 0.264 | ||
| Seawater pCO2 × Interaction | 2 | 1.107 | 0.352 | ||
| Macroalgae | Seawater pCO2 | 1 | 0.630 | 0.438 | |
| Interaction | 2 | 7.523 | 0.004 | * | |
| Seawater pCO2 × Interaction | 2 | 0.516 | 0.605 | ||
| Sponge | Seawater pCO2 | 1 | 21.911 | <0.001 | * |
| Interaction | 2 | 0.235 | 0.793 | ||
| Seawater pCO2 × Interaction | 2 | 1.221 | 0.318 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, H.N.; Hewett, C.; Tompkins, H.; Hall, E.R. Ocean Acidification and Direct Interactions Affect Coral, Macroalga, and Sponge Growth in the Florida Keys. J. Mar. Sci. Eng. 2021, 9, 739. https://doi.org/10.3390/jmse9070739
Page HN, Hewett C, Tompkins H, Hall ER. Ocean Acidification and Direct Interactions Affect Coral, Macroalga, and Sponge Growth in the Florida Keys. Journal of Marine Science and Engineering. 2021; 9(7):739. https://doi.org/10.3390/jmse9070739
Chicago/Turabian StylePage, Heather N., Clay Hewett, Hayden Tompkins, and Emily R. Hall. 2021. "Ocean Acidification and Direct Interactions Affect Coral, Macroalga, and Sponge Growth in the Florida Keys" Journal of Marine Science and Engineering 9, no. 7: 739. https://doi.org/10.3390/jmse9070739
APA StylePage, H. N., Hewett, C., Tompkins, H., & Hall, E. R. (2021). Ocean Acidification and Direct Interactions Affect Coral, Macroalga, and Sponge Growth in the Florida Keys. Journal of Marine Science and Engineering, 9(7), 739. https://doi.org/10.3390/jmse9070739







