Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems?
Abstract
:1. Introduction
2. Materials and Methods
3. Physical Properties of LNG and Hydrogen
4. Energy Density and Fuel Storage Conditions
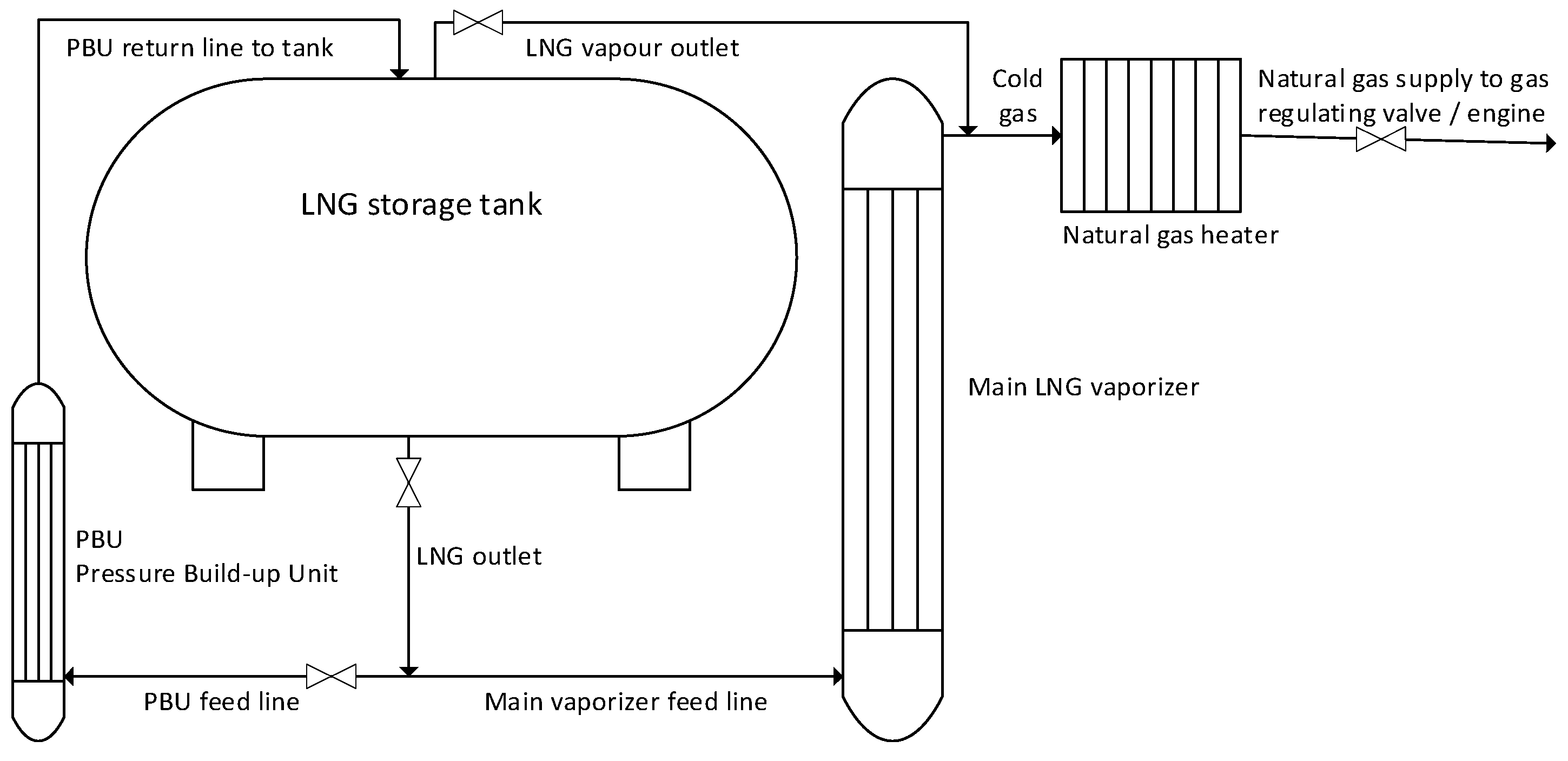
5. Existing Maritime Gas Fuel Systems Design
6. Energy Conversion from LH2 and LNG
7. Discussion
| Property | LH2 vs. LNG | Comment |
|---|---|---|
| Molecular size | ÷ | Smaller molecular size than methane. More difficult to avoid leaks [60]. |
| Temperature at 1 atm. | ÷ | −253 °C for LH2 vs. −163 °C for LNG [2,12]. Requires more efficient insulation. Higher risk of embrittlement. Requires higher material quality in pipes. |
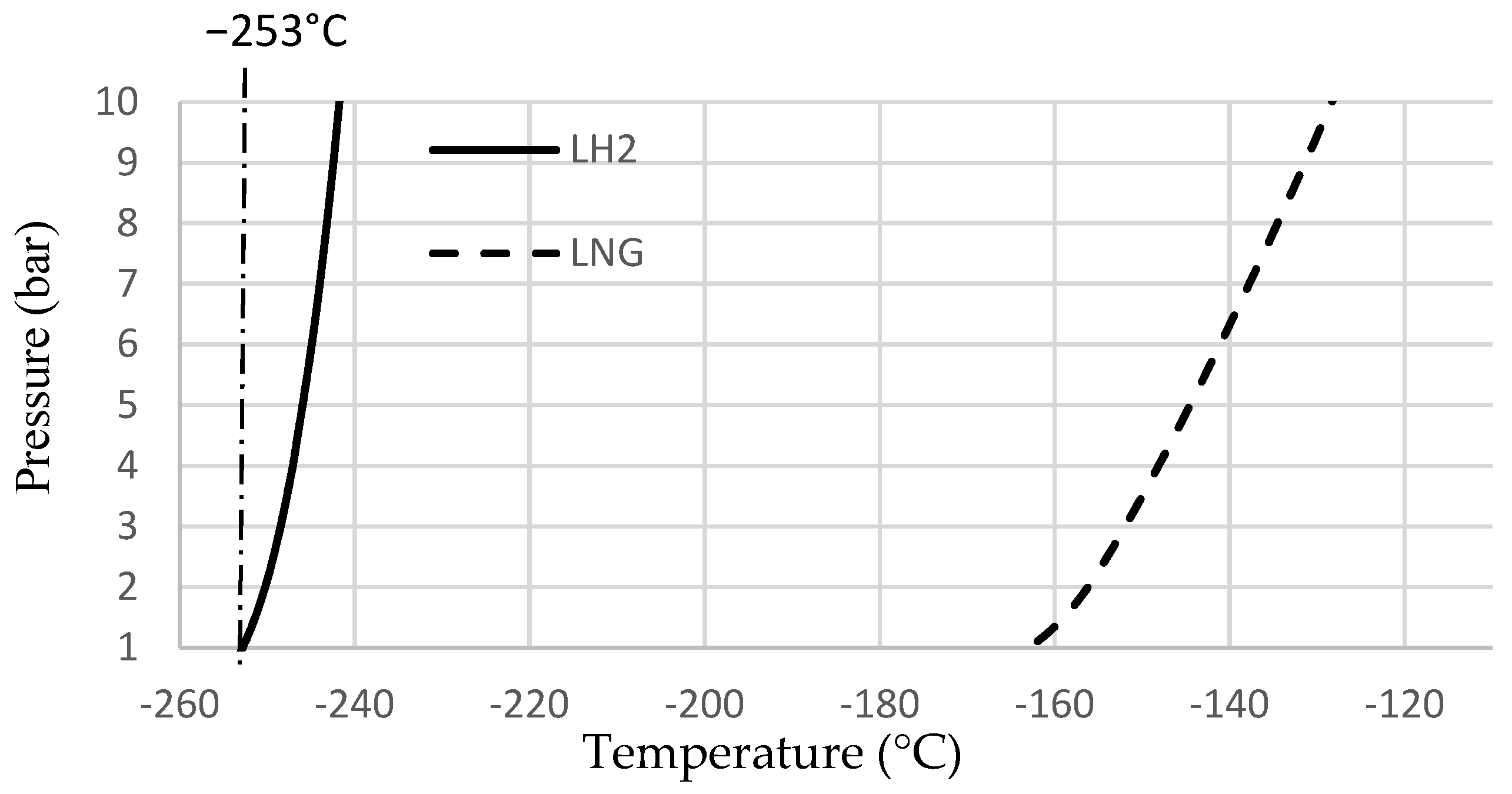
| Liquid phase temperature interval at 1–10 bar pressure | ÷ | ΔT = 13 °C for LH2 vs. ΔT = 30 °C for LNG [9,20]. Requires more efficient control of heat leak during reloading, transport and storage. |
| ΔT in processing equipment (Ambient temp. 17 °C) | ÷ | ΔT = 270 °C for LH2 vs. ΔT = 180 °C for LNG. Higher risk of fatigue and embrittlement. Requires higher material quality. |
| Reactivity | ÷ | Higher reactivity. Requires higher material quality [16,59] |
| Dense phase behavior of leakages | 0 | Similar to LNG systems [61,62]. Risk of gas with negative buoyancy returning to ship areas and potential ignition sources. |
| Energy content of liquid phase | ÷ | Lower volumetric energy content (ref. Table 1). Requires bigger fuel storage tank onboard the ship. |
| Heat capacity | + | Higher heat capacities [16,23]. Requires more energy to heat 1 kg gas 1 °C. Advantageous wrt. heating rate during storage. |
| Heat of vaporization | (÷) | Slightly lower than for LNG in kJ/kg [26,27]. |
| Heat flux from surroundings and into the tank | ÷ | Higher heat flux, if the insulation used is similar to what is used in LNG tanks. |
| Flammability range in air | ÷ | Wider, i.e., flammable conditions are more easily formed [14,28]. Higher risk of fire and/or explosion. |
| Ignition energy | ÷ | Low, i.e., more easily ignited than LNG [29]. |
| Auto-ignition temperature | (÷) | Slightly lower than methane [13,28]. |
| Maximum laminar flame speed in air | ÷ | Higher than for natural gas [22]. Greater risk of spreading of fire. |
| Space allocation of fuel cell | ÷ | Space allocation of fuel cell is twice the volume of combustion engines [66]. |
| Pressure drop due to sloshing in tank | 0 | Similar to LNG systems. |
| Purging and cooling of equipment | 0 | Helium can be used instead of nitrogen [70]. |
| Global Warming Potential (GWP) | + | 5.8 vs. 25 over 100 years [68]. Less damaging for the climate in case of leakage to the atmosphere. |
| Training of operating personnel | 0 | Required for both LH2 and LNG systems. |
| Availability as maritime fuel | ÷ | Supply chain of LH2 as maritime fuel is not established. Supply chains for LNG are well established in some regions [2]. |
8. Conclusions
- How can LH2 be maintained at low temperature, with a minimum of heat loss and a minimum of pressure increase? What are the implications for tank design, insulation and active cooling methods?
- What are the safety implications regarding tank localization onboard the ship?
- How can the relief valve and flare system be designed and localized to minimize the risk of fire and explosion?
- What are the material, system and design requirements for the piping and processing system?
- What are the total costs per kWh at propeller, as compared to other renewable or low carbon energy carriers?
- How to develop procedures for personnel handling LH2 in maritime fuel systems? Procedures must be developed for safe handling of LH2 during transport, distribution, transfer, bunkering, storage, and consumption on board the ship.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DNV. Alternative Fuels Insight Platform. Available online: https://afi.dnvgl.com/Statistics?repId=1 (accessed on 29 April 2021).
- IMO—International Maritime Organization. Studies on the Feasibility and Use of LNG as a Fuel for Shipping; Published by the International Maritime Organization, 2016. Available online: https://iwlearn.net/documents/32778 (accessed on 11 October 2020).
- dieBrennstoffzelle.de. Brennstoffzellen-Boot Hydra; dieBrennstoffzelle: Osterholz-Scharmbeck, Germany, 2021; Available online: http://www.diebrennstoffzelle.de/h2projekte/mobil/hydra.shtml (accessed on 20 February 2021).
- Prasad, R. The Zemship project—A case study in maritime applications. In Proceedings of the Maritime Hydrogen and Fuel Cells Conference, Bergen, Norway, 3 September 2019. [Google Scholar]
- Energy Observer. Available online: 2020.https://www.energy-observer.org/en/ (accessed on 20 February 2021).
- Stensvold, T. Norled Bygger Verdens første Hydrogen-Ferge; TU Maritim: Oslo, Norway, 2018; Available online: https://www.tu.no/artikler/norled-bygger-verdens-forste-hydrogen-ferge/452526. (accessed on 20 February 2021).
- Moore, R. Norled Project to Deploy Hydrogen ferry; Riviera: Enfield, UK, 2019; Available online: https://www.rivieramm.com/news-content-hub/news-content-hub/norled-project-to-deploy-hydrogen-ferry-54731 (accessed on 26 March 2020).
- NASA. Space Applications of Hydrogen and Fuel Cells; NASA—National Aeronautics and Space Administration: Dunbar, UK, 2017. Available online: https://www.nasa.gov/content/space-applications-of-hydrogen-and-fuel-cells (accessed on 20 July 2020).
- Katyal, A. Accurate prediction of phase equilibrium properties—Part 1. In Hydrocarbon Processing; Gulf Publishing Company LLC: Houston, TX, USA, 2019; Available online: https://www.hydrocarbonprocessing.com/magazine/2019/october-2019/process-optimization/accurate-prediction-of-phase-equilibrium-properties-part-1 (accessed on 1 June 2021).
- GIIGNL—International Group of LNG Importers. GIIGNL Annual Report; International Group of Liquefied Natural Gas Importers (GIIGNL): Neuilly-sur-Seine, France, 2018; Available online: https://giignl.org/sites/default/files/PUBLIC_AREA/Publications/rapportannuel-2018pdf (accessed on 11 October 2020).
- Desfa. LNG Quality Specifications; Desfa: Chalandri, Greece, 2020; Available online: https://www.desfa.gr/en/regulated-services/lng/users-information-lng/quality-specifications (accessed on 10 October 2020).
- Blackman, A.G. Aylward and Findlay’s SI Chemical Data, 7th ed.; Blackman, A.G., Gahan, L.R., Aylward, G., Findlay, T., Eds.; John Wiley & Sons: Milton, QLD, Australia, 2014; ISBN 9780730302469. [Google Scholar]
- IGU—International Gas Union. Natural Gas Conversion Guide; IGU, Office of the Secretary General: Oslo, Norway, 2012; Available online: http://agnatural.pt/documentos/ver/natural-gas-conversion-guide_cb4f0ccd80ccaf88ca5ec336a38600867db5aaf1.pdf (accessed on 10 October 2020).
- GIIGNL—The International Group of Liquefied Natural Gas Importers. LNG Information Paper #1—Basic Properties of LNG; International Group of Liquefied Natural Gas Importers (GIIGNL): Neuilly-sur-Seine, France, 2019; Available online: https://giignl.org/sites/default/files/PUBLIC_AREA/About_LNG/4_LNG_Basics/giignl2019_infopapers1.pdf (accessed on 11 October 2020).
- Benito, A. Accurate determination of LNG quality unloaded in receiving terminals—An innovative approach. In Proceedings of the 24th World Gas Conference, Buenos Aires, Argentina, 5–9 October 2009; Available online: http://www.iapg.org.ar/WGC09/admin/archivosNew/Special%20Projects/4.%20IGU%20Best%20Practices/4.%20IGU%20Best%20Practices%20FINAL%20-%20CD%20contents/8.%20LNG%20-%20Accurate%20determination%20of%20LNG%20quality%20unloaded%20in%20receiving%20terminals.pdf (accessed on 11 October 2020).
- NASA Safety Training Center. Safe Use of Hydrogen and Hydrogen Systems; Revision 2006; NTRS—NASA Technical Reports Server: Washington, DC, USA, 2006. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20070018005.pdf (accessed on 20 July 2020).
- Unitrove Limited. Compressed Natural Gas (CNG); Unitrove Limited: Leicestershire, UK, 2020; Available online: https://www.unitrove.com/engineering/gas-technology/compressed-natural-gas (accessed on 11 October 2020).
- Eswara, A.K.; Misra, S.C.; Ramesh, U.S. Introduction to natural gas: A comparative study of its storage, fuel costs and emissions for a harbor tug. In Proceedings of the Annual Meeting of Society of Naval Architects & Marine Engineers (SNAME), Bellevue, WA, USA, 8 November 2013; Available online: https://www.researchgate.net/publication/262691939_Introduction_to_natural_gas_A_comparative_study_of_its_storage_fuel_costs_and_emissions_for_a_harbor_tug/link/0a85e53abc9046525e000000/download (accessed on 11 October 2020).
- Sheffield, J.W.; Martin, K.B.; Folkson, R. Electricity and hydrogen as energy vectors for transportation vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance—Towards Zero Carbon Transportation; Woodhead Publishing Limited, Series in Energy; Elsevier: Oxford, UK, 2014; No. 57; ISBN 978-0-85709-522-0. [Google Scholar] [CrossRef]
- NIST Standard Reference Database Number 69. In NIST Chemistry WebBook; Thermophysical Properties of Fluids and Systems—Hydrogen Saturation Properties—Pressure Increments; National Institute of Standards and Technology, Department of Commerce: Washington, DC, USA, 2018. Available online: https://webbook.nist.gov/chemistry/ (accessed on 29 March 2021).
- IGU; BP. Guidebook to Gas Interchangeability and Gas Quality; BP: Sunbury on Thames, UK; IGU: Oslo, Norway, 2011; Available online: https://www.igu.org/app/uploads-wp/2011/08/Guidebook-to-Gas-Interchangeability-and-Gas-Quality-August-2011-min.pdf (accessed on 11 October 2020).
- Ogden, J.M. Hydrogen: The Fuel of the Future? Phys. Today 2002, 55, 69–75. [Google Scholar] [CrossRef]
- REN-Gasodutos. Gas Properties; Ren, Portugal, 2008. Document Number M-00000-SPC-MI-0002. Available online: http://www.mercado.ren.pt/PT/Gas/InfoMercado/Documentacao/BibOutros/GasProperties.pdf (accessed on 11 October 2020).
- Nuclear Power. Hydrogen—Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization; Nuclear Power (Website), 2019. Available online: https://www.nuclear-power.net/hydrogen-specific-heat-latent-heat-vaporization-fusion/ (accessed on 25 April 2020).
- NCEES. FE Reference Handbook; NCEES: Clemson, CA, USA, 2013; ISBN 978-1-932613-67-4. Available online: http://www.engineering.uco.edu/~aabuabed/index_files/fe_handbook.pdf (accessed on 11 October 2020).
- Włodek, T. Analysis of boil-off rate problem in Liquefied Natural Gas (LNG) receiving terminals. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 214, p. 012105. [Google Scholar]
- McAllister, S.; Chen, J.Y.; Fernandez-Pello, A.C. Thermodynamics of combustion. In Fundamentals of Combustion Processes. Mechanical Engineering Series; Springer: New York, NY, USA, 2011. [Google Scholar]
- Schmidtchen, U. Fuels—Safety, Hydrogen: Overview. In Encyclopedia of Electrochemical Power Sources; Garche, J., Dyer, C., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 519–527. ISBN 9780444527455. [Google Scholar]
- Verhelst, S.; Demuynck, J.; Sierens, R.; Scarcelli, R.; Matthias, N.S.; Wallner, T. Chapter 16—Update on the Progress of Hydrogen-Fueled Internal Combustion Engines. In Renewable Hydrogen Technologies; Elsevier: Oxford, UK, 2013; pp. 381–400. ISBN 9780444563521. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Zhou, Q.; Zhang, X.; Zhao, Q.; Xu, T.; Hui, S. Experimental study on the laminar flame speed of hydrogen/natural gas/air mixtures. Front. Chem. Eng. China 2010, 4, 417–422. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Sci. Nat. 2004, 91, 157–172. [Google Scholar] [CrossRef]
- DNV GL. Comparison of Alternative Marine Fuels; Report No. 2019-0567, Rev3, 05 July 2019; Sea\LNG Ltd.: Glasgow, Scotland, UK, 2019; Available online: https://sea-lng.org/reports/comparison-of-alternative-marine-fuels/ (accessed on 31 May 2021).
- Nerheim, A.R.; Oppedal, S.; Mortensen, A. LNG fuel systems for ships—Experiences, challenges and solutions. In Proceedings of the 6th Gas Fuelled Ships Conference, Hamburg, Germany, 10–12 November 2015. [Google Scholar]
- Rolls-Royce. Medium-Speed Generation Sets Portfolio; Booklet Edition 1-2020; Rolls-Royce Group: Bergen, Norway, 2020; Available online: https://bergen.rolls-royce.com/Portals/_default/assets/1/677/252_BookletEdition1-2020.pdf (accessed on 25 May 2021).
- Oppedal, S.; Mortensen, A.; Nerheim, A.R.; Chirkowski, J. Method and System for Conditioning of LNG. Norwegian Patent NO338906B1, 23 December 2014. [Google Scholar]
- Grotle, E.L. Thermodynamic Response Enhanced by Sloshing in Marine LNG Fuel Tanks—Experimental Work and Numerical Modelling. Ph.D. Thesis, NTNU—Norwegian University of Science and Technology, Faculty of Engineering, Department of Ocean Operations and Civil Engineering, Trondheim, Norway, April 2018. [Google Scholar]
- Wu, S.; Ju, Y. Numerical study of the boil-off gas (BOG) generation characteristics in a type C independent liquefied natural gas (LNG) tank under sloshing excitation. Energy 2021, 223, 120001. [Google Scholar] [CrossRef]
- Oppedal, S.; Mortensen, A.; Nerheim, A.R. Tank with Sloshing Bulkheads. Norwegian Patent NO338150B1, 25 November 2014. [Google Scholar]
- Nerheim, A.R.; Oppedal, S.; Mortensen, A. System and Method for Conditioning Pressure in an LNG Tank. Norwegian Patent NO339027B1, 15 July 2015. [Google Scholar]
- Kalyanaraman, A. Tug Project Leads the Way for 1 MW+ Hydrogen-Burning Engines; Riviera Maritime Media Ltd.: Enfield, UK, 1 November 2019; Available online: https://www.rivieramm.com/news-content-hub/news-content-hub/tug-project-leads-the-way-for-hydrogen-burning-mw-engines-56680 (accessed on 3 January 2020).
- Æsøy, V.; Einang, P.M.; Stenersen, D.; Hennie, E.; Valberg, I. LNG-Fuelled Engines and Fuel Systems for Medium-Speed Engines in Maritime Applications; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Æsøy, V.; Stenersen, D. Low emission LNG fuelled ships for environmental friendly operations in arctic areas. In Proceedings of the ASME 2013 32nd International Conference on Ocean, Offshore and Arctic Engineering, OMAE2013, Polar and Arctic Sciences and Technology; Offshore Geotechnics; Petroleum Technology Symposium, Nantes, France, 9–14 June 2013; Volume 6. V006T07A028. [Google Scholar] [CrossRef]
- Wärtsila. Leading the Way towards the World’s First Zero Emissions Supply Vessel; Wärtsila: Helsinki, Finland, 2020; Available online: https://www.wartsila.com/media/news/04-02-2020-leading-the-way-towards-the-world-s-first-zero-emissions-supply-vessel (accessed on 29 April 2021).
- Burheim, O.S. Hydrogen for Energy Storage; Elsevier Inc.: Oxford, UK, 2017; ISBN 9780128141007. [Google Scholar]
- Wang, L.; Husar, A.; Zhou, T.; Liu, H. A parametric study of PEM fuel cell performances. Int. J. Hydrogen Energy 2003, 28, 1263–1272. [Google Scholar] [CrossRef]
- Yan, Q.; Toghianib, H.; Causey, H. Steady state and dynamic performance of proton exchange membrane fuelcells (PEMFCs) under various operating conditions and load changes. J. Power Sources 2006, 161, 492–502. [Google Scholar] [CrossRef]
- Dell, R.M.; Moseley, P.T.; Rand, D.A.J. Chapter 8—Hydrogen, Fuel Cells and Fuel Cell Vehicles. In Towards Sustainable Road Transport; Dell, R.M., Moseley, P.T., Rand, D.A.J., Eds.; Academic Press: Oxford, UK, 2014; pp. 260–295. ISBN 9780124046160. [Google Scholar]
- Tafaoli-Masoule, M.; Bahrami, A.; Mohammadrezaei, D. Optimum Conditions for Maximum Power of a Direct Methanol Fuel Cell. In International Scholarly Research Notices; Hindawi Publishing Corporation, Hindawi Limited: London, UK, 2013; Volume 2013, Article ID 872873; Available online: https://www.hindawi.com/journals/isrn/2013/872873/ (accessed on 19 July 2020).
- Henke, M.; Willich, C.; Westner, C.; Leucht, F.; Leibinger, R.; Kallo, J.; Friedrich, K.A. Effect of pressure variation on power density and efficiency of solid oxide fuel cells. Electrochim. Acta 2012, 66, 158–163. [Google Scholar] [CrossRef]
- McLean, G. An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Song, R.-H.; Shin, D.R. Influence of CO concentration and reactant gas pressure on cell performance in PAFC. Int. J. Hydrogen Energy 2001, 26, 1259–1262. [Google Scholar] [CrossRef]
- Ubong, E.U.; Shi, Z.; Wang, X. Three-Dimensional Modeling and Experimental Study of a High Temperature PBI-Based PEM Fuel Cell. J. Electrochem. Soc. 2009, 156, B1276–B1282. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Park, S.H.; Lee, S.; Noh, Y.; Chang, D. Molten carbonate fuel cell (MCFC)-based hybrid propulsion systems for a liquefied hydrogen tanker. Int. J. Hydrogen Energy 2018, 43, 7525–7537. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, Y.; Lee, M.C. On the observation of high-order, multi-mode, thermo-acoustic combustion instability in a model gas turbine combustor firing hydrogen containing syngases. Int. J. Hydrogen Energy 2019, 44, 11111–11120. [Google Scholar] [CrossRef]
- Verma, S.; Kaushik, S.C.; Das, L.M. Exergy Analysis of Hydrogen-Fueled Spark Ignition Engine Based on Numerical Investigations. In Combustion for Power Generation and Transportation; Agarwal, A., De, S., Pandey, A., Singh, A., Eds.; Springer: Singapore, 2017; pp. 297–316. [Google Scholar]
- Castonguay, S. Green Technologies: Electric Cars with Hydrogen Fuel Cells. WIPO Magazine; World Intellectual Property Organization: Geneva, Switzerland, 2009. No. 2. Available online: https://www.wipo.int/wipo_magazine/en/2009/02/article_0009.html (accessed on 24 October 2020).
- Veziroglu, T.N.; Sherif, S.A.; Barbir, F. Chapter 7—Hydrogen Energy Solutions. In Environmental Solutions; Agardy, F.J., Nemerow, N.L., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 143–180. ISBN 978-0-12-088441-4. [Google Scholar]
- Schiro, F.; Stoppato, A.; Benato, A. Modelling and analyzing the impact of hydrogen enriched natural gas on domestic gas boilers in a decarbonization perspective. Carbon Resour. Convers. 2020, 3, 122–129. [Google Scholar] [CrossRef]
- Norsk Hydrogenforum (the Norwegian Hydrogen Association). Sikkerhet. 2020. Available online: https://www.hydrogen.no/ressurser/hva-er-hydrogen/sikkerhet (accessed on 3 January 2021).
- College of the Desert. Hydrogen Fuel Cell Engines and Related Technologies; Rev 0, December 2001—Module 1: Hydrogen Properties. Available online: https://www.energy.gov/sites/default/files/2014/03/f12/fcm01r0.pdf (accessed on 3 January 2021).
- Li, D.; Zhang, Q.; Ma, Q.; Shen, S. Comparison of explosion characteristics between hydrogen/air and methane/air at the stoichiometric concentrations. Int. J. Hydrogen Energy 2015, 40, 8761–8768. [Google Scholar] [CrossRef]
- Houf, W.; Winters, W. Simulation of high-pressure liquid hydrogen releases. Int. J. Hydrogen Energy 2013, 38, 8092–8099. [Google Scholar] [CrossRef]
- Zhu, D.-Z. Example of Simulating Analysis on LNG Leakage and Dispersion. Procedia Eng. 2014, 71, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Hansen, O.R. Design optimization reflecting risk for a hydrogen storage and fuel cell system. In Proceedings of the Maritime Hydrogen and Fuel Cells Conference, Bergen, Norway, 3 September 2019. [Google Scholar]
- Hansen, O.R. Liquid hydrogen releases show dense gas behavior. Int. J. Hydrogen Energy 2020, 45, 1343–1358. [Google Scholar] [CrossRef]
- Project Foreship. Engineering Considerations for Fuel Cell installation. In Proceedings of the Maritime Hydrogen & Fuel Cells Conference, Bergen, Norway, 3 September 2019. [Google Scholar]
- Lisowski, E.; Lisowski, F. Study on thermal insulation of liquefied natural gas cryogenic road tanker. Therm. Sci. 2019, 23, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Derwent, R.; Simmonds, P.; O’Doherty, S.; Manning, A.; Collins, W.; Stevenson, D. Global environmental impacts of the hydrogen economy. Int. J. Nucl. Hydrog. Prod. Appl. 2006, 1, 57. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Li, Y.; Zhu, K.; Ma, Y.; Wang, J. Cooling behaviors of liquid hydrogen by helium gas injection. Heat Mass Transf. 2019, 55, 2373–2390. [Google Scholar] [CrossRef]
- SIGTTO. LNG Marine Loading Arms and Manifold Draining, Purging and Disconnection Procedure; SIGTTO: London, UK. Available online: https://www.sigtto.org/media/2384/lng-marine-loading-arms-and-manifold-draining-purging-and-disconnection-procedure.pdf (accessed on 31 May 2021).
- IMO—International Maritime Organization. IGF Code—International Code of Safety for Ships Using Gases or Other Low-Flashpoint Fuels; IMO Publishing: London, UK, 2016; Available online: https://www.imo.org/en/OurWork/Safety/Pages/IGF-Code.aspx (accessed on 3 June 2021).
- Aarskog, F.G.; Hansen, O.R.; Strømgren, T.; Ulleberg, Ø. Concept risk assessment of a hydrogen driven high speed passenger ferry. Int. J. Hydrogen Energy 2020, 45, 1359–1372. [Google Scholar] [CrossRef]
- Schinas, O.; Butler, M. Feasibility and commercial considerations of LNG-fueled ships. Ocean Eng. 2016, 122, 84–96. [Google Scholar] [CrossRef]
- European Commission. EU Commission Charts Path towards 100% Renewable Hydrogen; Brussels, Belgium, 9 July 2020. Available online: https://www.euractiv.com/section/energy/news/eu-commission-charts-path-towards-100-renewable-hydrogen/ (accessed on 20 July 2020).

| Property | Dimension | LNG/NG | LH2/H2 | Ref. LNG/NG | Ref. LH2/H2 |
|---|---|---|---|---|---|
| Mol. weight | g/mol | 16.5–18.9 | 2.0 | [11] | [12] |
| Density | kg/m3 | 450 (LNG) | 71 | [13,14,15] | [16] |
| Vapor density at standard cond. (1 atm., 15 °C) | kg/Sm3 | 0.7–0.9 | (0.08) 1 | [17] | [16] |
| Vapor density at normal cond. (1 atm., 0 °C) | kg/Nm3 | (0.7–0.9) 2 | 0.08 | [17] | [16] |
| Vapor density at 200 bar | kg/m3 | 182 | N/A | [18] | |
| Vapor density at 250 bar | kg/m3 | 215 | N/A | [17] | |
| Vapor density at 350 bar | kg/m3 | N/A | 23 | [19] | |
| Vapor density at 700 bar | kg/m3 | N/A | 38 | [19] | |
| Boiling temperature LNG, 1 atm. | °C | −163/−161 | −253 | [2]/[13] | [12] |
| Liquid phase temperature at 1–10 bar | °C | −163 to −130 | −253 to −240 | [9] | [20] |
| ΔT in processing equipment (Amb. temp. 17 °C) | °C | 180 | 270 | [9] | [20] |
| Energy content of liquefied gas 3 | GJ/m3 | (23–24) | (9–10) | [13,21] | [16,22] |
| Higher heating value | MJ/kg | 54 | 142 | [13,21] | [22] |
| Lower heating value | MJ/kg | 50 | 120 | [13] | [22] |
| Higher heating value of gas | MJ/Nm3 | 43 | 13 | [23] | [16,22] |
| Lower heating value of gas | MJ/Nm3 | 39 | 11 | [23] | [16,22] |
| Heat capacity at constant pressure | kJ/kgK | 2.09 | 14.3 | [23] | [24] |
| Heat capacity at constant volume | kJ/kgK | 1.61 | 10.2 | [23] | [25] |
| Heat of vaporization | kJ/kg | 502–508/500 (LNG/CH4) | 451 | [12,26] | [27] |
| Lower flammability limit in air (LFL) | % | 5 (CH4) | 4 | [14] | [28] |
| Upper flammability limit in air (UFL) | % | 15 (CH4) | 75 | [14] | [28] |
| Minimum ignition energy | mJ | 0.28 (CH4) | 0.02 | [29] | [29] |
| Auto-ignition temperature | °C | 599 (CH4) | 560 | [14] | [28] |
| Maximum laminar flame speed in air | m/s | 0.374 | 2.933 | [30] | [30] |
| Abbrev. | Fuel Cell Technology | Fuel | Operating Temp. (°C) | Operating Pressure Range of H2 | Efficiency 1 (%) |
|---|---|---|---|---|---|
| PEMFC | Proton Exchange Membrane Fuel Cell | H2 (g) [44] | 50–70 [45] | 1–4 atm. [45,46] | 40–60 [47] |
| DMFC | Direct Methanol Fuel Cell | Methanol [44] | 0–80 [44] | 1 atm. [48] | 35–40 [47] |
| SOFC | Solid Oxide Fuel Cell | Pure H2 or hydrogen-rich fluid [44] | 600–900 [44] | 1–8 bar [49] | 45–55 [47] |
| AFC | Alkaline Fuel Cell | H2(g) [44] | 40–150 [50] | 1–4 atm. [50] | 45–60 [47] |
| PAFC | Phosphoric Acid Fuel Cell | H2(g) [44] | 160–220 [51] | 0–4 atm. [51] | 35–45 [47] |
| PBI | Polybenzimidazole Fuel Cell | H2(g) [44] | 120–180 [52] | 1–3 atm. [52] | - |
| MCFC | Molten carbonate fuel cell | Pure H2 or hydrogen-rich fluid [44] | 650 [53] | 1–2 bar [53] | 45–60 [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerheim, A.R.; Æsøy, V.; Holmeset, F.T. Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems? J. Mar. Sci. Eng. 2021, 9, 743. https://doi.org/10.3390/jmse9070743
Nerheim AR, Æsøy V, Holmeset FT. Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems? Journal of Marine Science and Engineering. 2021; 9(7):743. https://doi.org/10.3390/jmse9070743
Chicago/Turabian StyleNerheim, Ann Rigmor, Vilmar Æsøy, and Finn Tore Holmeset. 2021. "Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems?" Journal of Marine Science and Engineering 9, no. 7: 743. https://doi.org/10.3390/jmse9070743
APA StyleNerheim, A. R., Æsøy, V., & Holmeset, F. T. (2021). Hydrogen as a Maritime Fuel–Can Experiences with LNG Be Transferred to Hydrogen Systems? Journal of Marine Science and Engineering, 9(7), 743. https://doi.org/10.3390/jmse9070743





